Meiofaunal Adaptation to Environmental Variability and Human Trampling of Tropical Sandy Beaches at Goa, India
SM Parvez Al-Usmani and ZA Ansari*
Multi-Faculty college, Goa
Submission:November 02, 2020;Published:January 08, 2021
*Correspondence author: ZA Ansari, Multi-Faculty college, Dharbandora, Goa
How to cite this article:SM Parvez Al-U, ZA Ansari. Meiofaunal Adaptation to Environmental Variability and Human Trampling of Tropical Sandy Beaches at Goa, India. Oceanogr Fish Open Access J. 2020; 13(1): 555851. DOI:10.19080/OFOAJ.2021.13.555851
Abstract
Physical disturbances have direct impact on sandy beach ecosystem. The old hypothesis of meiofauna as indicator of disturbance was tested for the assessment of health of sandy beaches. Two sandy beaches in Goa were sampled for meiofauna and sediment properties covering three seasons of the year. There were differences in physical and chemical properties of the sediment of two beaches. The meiofaunal density varied from 386 to 1222⁄ 10 cm2 (mean 712) at Caranzalem and 185 to 662⁄ 10 cm2 (mean 387) at Candolim. The meiofaunal assemblage revealed similar seasonal pattern but significant variation (p<0.01) in total abundance at two beaches. Percent composition of taxa differ at two beaches. The nematodes were the most dominant group with contribution of > 50% followed by harpacticoida polychaeta and turbellaria. General linear model of correlation matrix parameter revealed different relation on two beaches. The N:C ratio suggested its usefulness in disturbance study. The differences in meiofaunal abundance of the two beaches, could be attributed to variability in the physical environment. The trampling has led to changes in relative abundance of meiofaunal taxa at Candolim beach. This study further demonstrated the use of meiofauna in the health assessment of sandy beaches.
Keywords: Sandy beach; Morpho dynamic; Recreational activity; Meiofauna Ecology
Introduction
Sandy beaches are valuable ecosystem and are main attractant all over world. Beach management focuses largely on maximizing the recreational activities for more beach users at commercial scale. Many beach users have the impression that the compact beach sand is unbreakable and in good health. However, the fact is that numerous influences on sandy beaches like mechanical beach cleaner, trampling and beach fisheries pose serious threat to the ecology of sandy beaches [1,2]. The impact of human recreational activities is more severe than we think [3]. The destructive effect of trampling on larger macrobenthic organisms od sandy beaches have been documented [4,5]. Beach recreation and trampling is reported to lower abundance and diversity of macro benthic invertebrates [6]. However, studies on the effect of anthropogenic activities on smaller metazoans is still rare and negligible [7]. The, knowledge of the effects of different intensities of anthropic activities is essential for the management, conservation, and sustainable tourism policies [8]. The short life span of smaller metazoans, high numerical abundance and remarkable physiological adaptation to extreme conditions make them more suitable for the assessment of climate change and anthropogenic disturbances such as beach trampling [9].
Goa is endowed with beautiful sandy beaches all along its 101 km long coast. These beaches are considered as avenues for its economic potential and hence are maintained to attract tourists. Some of the beaches of Goa are crowded by tourists round the year. The increased tourism and the recreational beach activities pose increasing threat to the infauna and biodiversity of sandy beaches [10]. The consequences of human action need to be understood to mitigate its potential impact on the health of an ecosystem in the long run. Several methods using benthos have evolved to check the ecological status and health of marine ecosystem [11]. In this communication we have used the meiofauna as sentinel and indicator of environmental disturbance for health assessment of sandy beaches in Goa, India.
Material and Method
The study was carried out during 2018, covering three seasons, pre-monsoon, monsoon, and post-monsoon, prevailing in this region. Two beaches selected for this study are (1) Caranzalem which is westward extension of famous Miramar Beach and (2) a more urbanized and crowded beach at Candolim (Figure 1). The two beaches differ morpho-dynamically. The Caranzalem beach is a dissipative shelter estuarine beach with least disturbance while the Candolim is bustling with activities water sports and always crowded. The two beaches were covered for systematic environmental data on physical, chemical, and biological parameters. The beach hydrodynamic feature was carried out according to Emery [12]. A horizontal sampling was planned to study the effect of trampling. The sediment samples for grain size analysis were collected at each tidal level on only one transect at each beach. Each transect was divided into 3 equidistance sectors from its drift line up to the water line. The sediment temperature was measured with an ordinary thermometer of 0.1-degree accuracy.

The interstitial water was collected for physical and chemical parameters. Samples of sediment for meiofauna were collected at each tide level of both beaches using a 5 cm long Perspex glass tube covering an area of 10 cm2. Replicate sampling was done at highest High Tide Level (HTL) Mid Tide Level (MTL) and Low Tide Level (LTL) below the swash line. On each sampling date sediment and core samples were collected. The core samples were fixed in 5% buffered formalin Rose Bengal sea water solution and the meiofauna was extracted by decantation and sieving technique in the laboratory. A 62micron sieve was used to separate the meiofauna. The sediment was analyzed for organic carbon and grain size distribution [13,14]. The chemical and biological parameters were analyzed by the method given in APHA [15]. The software program of PRIMER V6 was used for univariate and multivariate analyses of data.
Results
Beach Characteristics
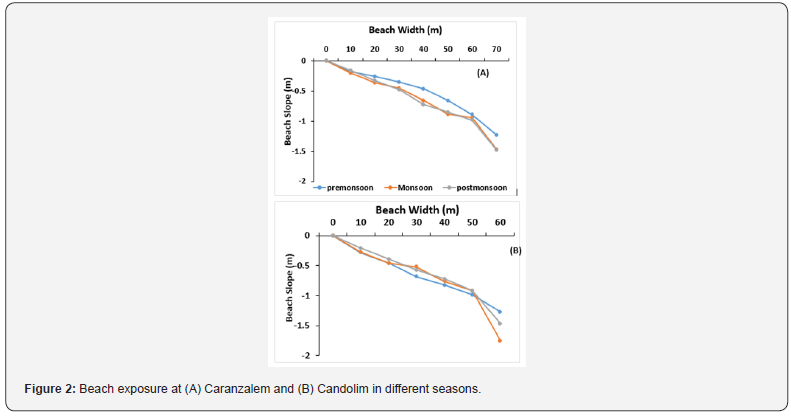
The profile of the two beaches is given in Figure 2. The intertidal expanse at Caranzalem was large and varied from 60-70m. The slope of the beach was gentle and gradual. Some changes during monsoon at lowest low tide level were clear. The expanse of more exposed Candolim beach was shorter and varied from 40 to 60m. The slope was sharper and got steeper during the monsoon season due to direct high wave action of the sea. The lowest low tide of the two beaches showed a shift during monsoon season. The temperature of the beach was in the range of 30.5 to 31.9 oC during pre-monsoon¸ 25.4 to 26.4 oC during monsoon season and 29.4 to 34.2 oC during post monsoon season the sediment temperature at Candolim was in the range of 31.4 to 32.8 oC during pre-monsoon 25.7 to 29.4 during monsoon and 27.2 to 31.5 oC in the post monsoon season (Table 1).
Relatively low temperature recorded during monsoon and there were temperature differences from high to low tide. The sediment at both beaches consisted of poorly sorted silty sand with high admixture of fine-grained silt and medium to coarse sand particles. The mean grain size at Caranzalem was in the range of 0.35 to 0.38 mm during pre-monsoon season; 0.28 to 0.47 mm during monsoon and 0.28 to 0.47 mm during postmonsoon season. The sediment tended to increase in grain size towards the LTL mark. The mean grain size at Candolim beach was in the range of 0.47 to 0.55 mm during pre-monsoon season, 0.62 to 0.79 mm, and 0.46 to 0.73 mm during post monsoon season. The sediment showed tendency towards fine sand particles at supra littoral zone and coarser towards infra littoral zone at both beaches. There were changes in the percentage composition of silt clay at all tide levels the sediment organic carbon (mg̷ g) at Caranzalem was in the range of 2.43 to 4.12 during pre-monsoon’ 1 .98 to 2.95 during monsoon and 1.82 to 3.38 during the post monsoon season.
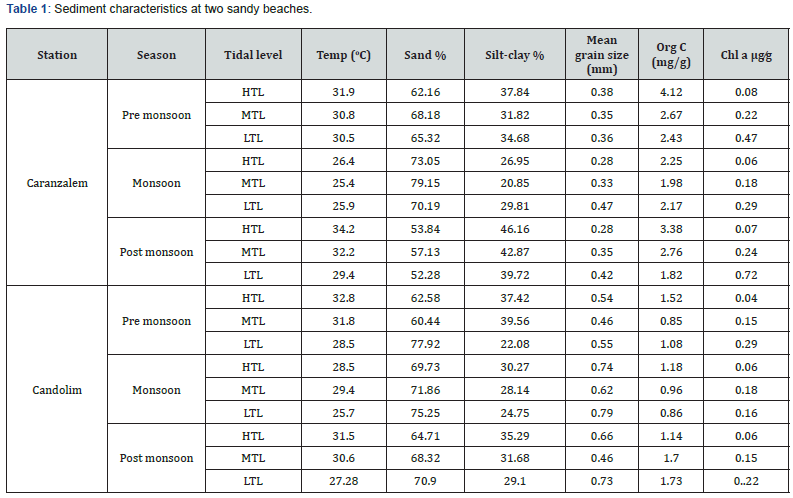
The organic carbon (mg⁄g) at Candolim ranged between 0.85 and 1.52 during pre-monsoon 0.85 to 1.18 during monsoon and 1.14 to 1.73 during post monsoon. The average chlorophyll a content (μg⁄g) of the sediment at Caranzalem beach was 0.08 to 0.47 in pre-monsoon‚ 0.06 to 0.29 in monsoon and 0.07 to 0.72 in the post-monsoon. Similarly, the values at Candolim were 0.04 to 0.29 in pre-monsoon‚ 0.06 to 0.18 in monsoon and 0.06 to 0.22 μg⁄g in post-monsoon season. The values were high at LTL and high concentration was recorded at caranzalem beach. The result of the analysis of interstitial water of the two beaches is given in Table 2. The temperature was in the range of 25.6 to 30.8 oC and salinity varied between 22.2 and 31.2 PSU at two sites. The dissolved oxygen was moderately high, and values ranged between 4.18 and 5.70 mg/l. The pH values were towards alkaline side. There was seasonal variation in the physical, chemical, and morphological properties of the two beaches.

Meiofaunal Distribution
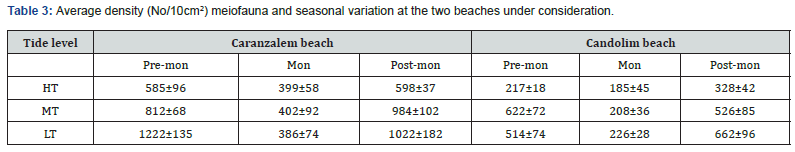
The meiofaunal distribution at two sites are presented in Table 3. At Caranzalem Beach the total density (number⁄10cm2) was in the range of 399 to 598⁄ 10 cm2 at HTL‚ 402 to 984/10cm2 at MTL and 386 to 1222/10cm2 at LTL with 712/10cm2 as annual average. The density at Candolim Beach was 186 to 328 at HTL, 208 to 622 at MTL and 206 to 662/10cm2 at LTL with an annual average of 387/10cm2. The monsoon season recorded the lowest density on both beaches while post-monsoon the highest density at both sites. The numerical count was significantly influenced by high number of nematoda‚ a feature of tropical beaches. Generally higher average total meiofaunal densities were recorded at Caranzalem Beach. The relative abundance (percentage) of dominant meiofaunal taxa at two beaches is illustrated in Figure 3a and 3b.
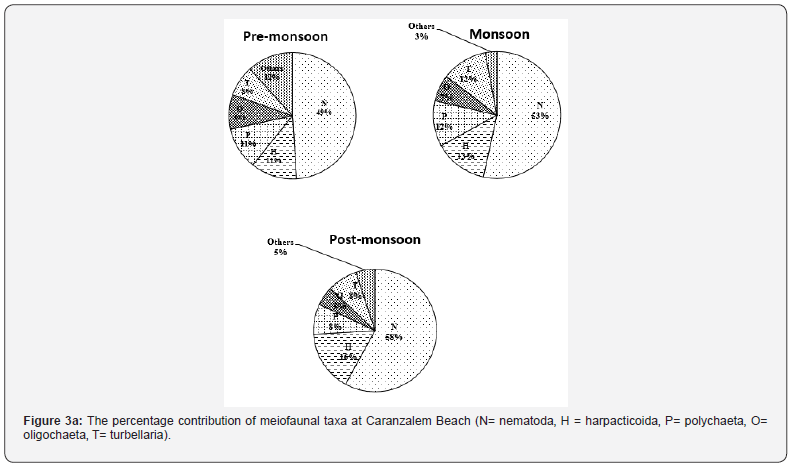

The nematodes were the most abundant taxon with 50 to 58% contribution followed by harpacticoid copepod (12-16%), polychaeta (8-12%) oligochaeta (5-9%) and turbellaria (8- 12%). The Other group comprising of Halacarida‚ Gastrotricha‚ Kinorhynca‚ Foraminiferans and crustacean nauplii formed the least group. The distribution of meiofauna in the present and the different taxa was by no means uniform and the order of domination of taxa varied at different tide levels in different season. The poor presence of benthic copepod‚ turbellarians‚ foraminifera and Oligochaeta at the HTL and MTL of tourist beach at Candolim was noticed. There was consistency in the occurrence of nematode at all tide levels. The difference in the overall abundance of meiofauna at tidal level of the two beaches was due to the most abundant group the nematoda.
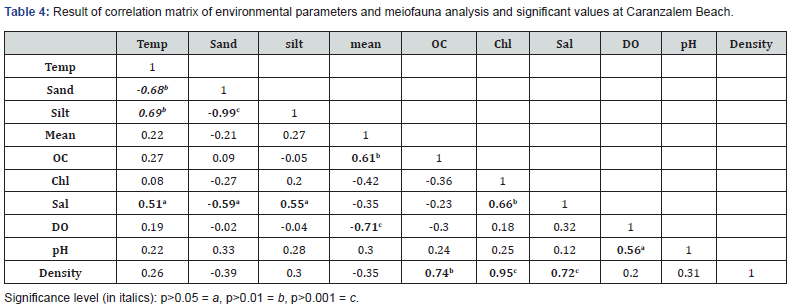
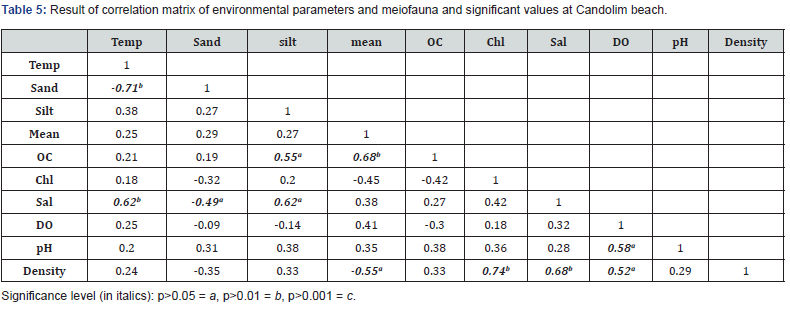
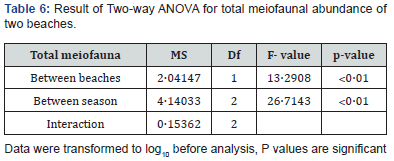
The correlation matrix is a presentation of the result of spearman correlation coefficient (r) between sets of variables. It allows us to see which pair has the highest value. Each value is tested for its significance. The result is presented in Table 4 and 5. Significant positive correlation between salinity and temperature (p<0.05, r=0.51), salinity and chlorophyll (p<0.01, r=0.66), salinity and density (p<0.001, r=0.72) and density and chlorophyll (p<0.001, r=0.95) at Carasnzalem was recorded. Similarly, at Candolim the positive significant correlation resulted between salinity and temperature (p<0.01, r=0.66), salinity and chlorophyll (p<0.01, r=0.62), density and chlorophyll (p<0.001, r=0.84), density and dissolved oxygen (p<0.01, r=0.62). The univariate parameters differed significantly between the two beaches and between different season (p.˂0.01) (Table 6).
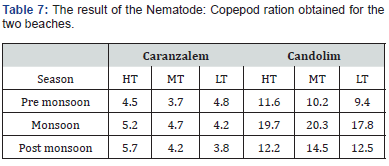
The meiofaunal taxa recorded on the two beaches showed similarity. The nematodes showed dominance at both beaches while the crustaceans in particular harpacticoid copepod looked dim and showed vulnerability towards supra littoral zone. The controversial nematode: copepod (N:C) ratio has been used in the past as indicator of organic pollution [16]. We have used the ratio to check its suitability for the assessment of health of sandy beaches under stressed condition. The result is given in Table 7. The values showed high degree of variability between two beaches and between seasons. The ratio produced high values (>9) at Candolim as compared to Caranzalem. The values ranged between 9.4 and 20.3 at Candolim and 3.7 and 5.7 at Caranzalem.
Discussion
The beach is occupied by different types of organisms at supralittoral, littoral and infralittoral zone. Many beach users consider sandy beaches as unbreakable and so is the beach ecosystem. Beach nourishment is an important factor for the infauna of sandy beaches [17]. The impact of nourishment varies from case to case, but the influence is direct on the resident organisms. The beach disturbances will affect the nourishment directly and mortality of infauna [1]. The physical and chemical features of the two beaches were normal and followed regular seasonal cycle as reported earlier [18].
Relative coarseness in the size of the sand particle at low tide during the monsoon season at both beaches is a manifestation of wave action. The beach slope at Candolim tended to be steeper than the Caranzalem. The morpho dynamic and sediment granulometary of the two beaches varied [19]. The fine-grained particles generally have large surface area and thus adsorbs more organic carbon and nutrient. This was also evident in the present study. Finer particle with high organic carbon and chlorophyll supported large population of interstitial fauna [20]. The changes in interstitial water properties are related to drainage capacity of capillary water and the evaporation of interstitial water in the surface layer. It effects the dissolved oxygen and pH and plays a role in the migration and mortality of interstitial fauna of the marine biome [20].
Studies on the effect of human trampling have reported different response by benthic organisms of sandy beaches [21,22]. Macrofaunal response have been found different than meiofauna. Moffett et al [5] reported that different trampling intensities gave varied response by different macrofaunal species. Rossi et al [23] have indicated that trampling clearly modified the abundance and population dynamics of the clam Macoma balthica (L.) and the cockle Cerastoderma edule (L.). There was a negative impact on adults of both species, probably because footsteps directly killed or buried the animals, provoking asphyxia. Available accounts on the effect of trampling associated to the digging for bait collection report a general decrease in the numbers of tube-dwelling, subsurface deposit-feeders and deep-burrowing forms [24].
In a manipulative trampling study Martinez et al. [25] reported that trampled plots showed negative shift in invertebrate community as compared to controlled plots of the coralline sandy surface. Similarly, significant effect of trampling on the occurrence of meiofauna taxa and their relative abundance have been reported on the other sandy beaches. Gheskiere et al. [26] has reported that tourism related activities have negative effect on sandy beach meiofauna. Significant reduction in meiofaunal density of a sandy beach due to cyclone was reported recently [27] with fast recovery subsequently. Sarmento et al.‚ Sun et al, Santos et al. [28-30] has also reported negative effect with significant reduction in numerical abundance of a few meiofaunal taxa due to human trampling of beaches. The disturbances have led to change in the taxa and their relative abundance in this study. The result of this study supports the severity and negative impact of trampling reported by others.
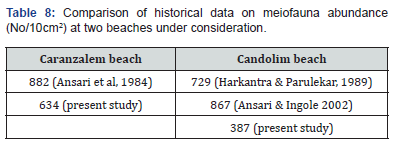
The factors responsible for the distribution of organisms in the intertidal zone of sandy beaches has been well studied and reported [2,31]. The anthropogenic impact has a general negative effect on meiofauna [9]. The fluctuation in the meiofauna of tropical beaches is a natural phenomenon [32]. The meiofaunal distribution in the present study revealed the pattern and faunal dominance observed by others on the sandy beaches of the central west coast of India [18,33]. The population density at Caranzalem was higher than that of Candolim. A comparison of faunal abundance with old data revealed that at Caranzalem no noticeable changes found while at Candolim significant lowering in number was recorded (Table 8).
While studying the pollution effect of petroleum hydrocarbon, (TPH) on intertidal benthos of Sinquerim beach at Goa, Ingole et al. [34] reported significantly low density of meiofauna (22-64 /10 cm2) initially which increased subsequently to 1057/10cm2 with parallel reduction in the concentration of sediment petroleum hydrocarbon. In another study significant impoverishment in meiofauna number of sandy beaches due to oil spillage and anthropogenic disturbance on west coast of India was reported [35]. The meiofauna are known for high resilient nature and have shown rebuilding capacity in a short time after any environmental disturbance [36]. Candolim also appeared to have undergone changes in benthic life due to disturbance caused by regular mechanical cleaning and heavy trampling. The ecological resilience of meiofauna of such beaches with high trampling is in question [37]. The changes in benthic life and the recovery rates are mediated by a combination of physical, chemical, and biological factors that differ in their relative importance in different habitats [2].
The effect of persistent human trampling could have a negative impact on meiofauna of sandy beaches like Candolim beach of the present study as compared to a non- disturbed beach at Caranzalem. The differences recorded in the metazoans of the two beaches may be the result of both environmental variation and beach disturbance caused by anthropogenic pressure. The severity of the trampling on meiofauna discussed earlier in this paper agrees with the reports of earlier studies made elsewhere. It has been demonstrated that N⁄C ratio is useful bioindicator of organic pollution of sandy beaches [16]. In a recent study Santos et al [30] have reported high N⁄C ratio (more than 10) of a sandy beach having large human trampling at sandy beaches in Brazil. The elevated N:C ratio at Candolim during all season of the present study is support the use of controversial N:C ratio in the health assessment of beaches and agrees with earlier result [30, 36].
High mortality in harpacticoid due to their sensitive nature led to reduction in number which is responsible for higher N:C values at more disturbed beach of Candolim. Nematodes are known for high physiological adaptation and can live under low oxygen. The sensitive nature of meiobenthos anthropogenic pressure is also demonstrated by others [20,26]. The total population showed significant seasonal variation (Two-way ANOVA, p<0.01, F=26.7). It may be attributed to natural changes. The extensive environmental disruption caused by annual monsoon resulting in heavy mortality of benthic organism is reported from the beaches of central west coast of India [18,38]. Such changes are regularly occurring and not limited to anthropogenic beach disturbances.
The present study supports the hypothesis that meiofauna are good indicator of anthropogenic pressure and should be included in the health assessment studies of sandy beaches. The result of the present study differs in many respects from others. The meiofaunal community appeared to be more related to the physical environment and recreational activities of the beach. It suggests that trampling, in the absence of any pollution, may have caused reduction in the fauna and mortality of some benthic organisms as reported earlier [26,29]. In Goa, the tourism related industries are doing well but the damage caused is not ascertained fully. The increase in water sports, disturbance of sensitive habitats, beach driving, beach litter and sanitation are playing their role in effecting the native fauna adversely. Such pressure is expected to alter the ecological functioning and beach dynamic [6]. This will affect the beach life at individual and community level. The recovery will depend on disturbance persistence and its intensity [39-42].
Conclusion
The environmental variables are fundamental in the interpretation of faunal distribution. Meiofauna offer several advantages in the study of anthropogenic impacts and should be a part of all studies related to environmental disturbances of sandy beaches. The physical disturbance and littering of the beaches with persistent trampling may cause severe impoverishment of fauna. The nematode: copepods are valuable taxonomic component of environmental effects. The management and conservation strategies for the development of beach is essential and all studies should invariably include the impact on meiofauna. A more detailed annual study and monitoring of sandy beaches using meiobenthos is suggested in future to mitigate the impact and save the biodiversity of these ecotones. Our understanding of the tourism as a safe and clean industry needs to be considered with caution under beach aesthetic and safety consideration. we re-emphasize the usefulness of meiobenthos in the health assessment of sandy beaches.
References
- SousaW P (1984) The role of disturbance. Annual Review of Ecology and Systematics 15: 353-391.
- McLachlan A, AC Brown (2006) The ecology of sandy shores. In: Academic press, Burhington, USA, Pp. 373.
- Defeo C, A McLachlan, DS Schoeman, T Schlacker, J Dugan, et al. (2008) Threat to sandy beach ecosystem.Estuarine coastal Shelf Science 81(1): 1-12.
- Liddle MJ (1997) Recreational Ecology: the ecological impact of outdoor recreation and ecotourism Chapman and Hall, London.
- Moffett M D‚ A McLachlan‚ Winter PED, AMC De Ruyck (1998) Impact of trampling on sandy beach fauna.Journal Coastal Conservation 4:87-90.
- Schlacher T and L Thompson (2012) Beach recreation impacts benthic invertebrates on ocean-exposed sandy shores. Biological conservation 147: 123-132.
- Brown A C, A McLachlan (2002) Sandy shore ecosystem and the threat facing them: Some prediction for the year 2025. Environmental Conservation 29: 62-77.
- Mota P‚ CS Marjorie‚ L CostaI, R Zalmon (2017) Tourism impacts on benthic communities of sandy beaches.Marine Ecology 38(4): e12440.
- Zeppilli D, Sarrazin J, Leduc D (2015) Is the meiofauna a good indicator for climate change and anthropogenic impact? Marine Biodiversity 45: 505-535.
- Chandrasekara W U, C L J Frid (1996) Effect of human trampling on tidal flat infauna. Aquatic conservation: Marine and Freshwater Ecosystems 6(4): 299-311.
- Sivadas SK, R Nagesh, GVM Gupta, U Gaonkar, I Mukherji, et al. (2019) Testing the efficiency of temperate indices in assessing the ecological status of a tropical ecosystem. Marine Pollution Bulletin 106: 62-76.
- Emery KO (1961) A simple method of measuring beaches profiles. Limnology Oceanography 6: 90-93.
- El Wakeel S K, P J Riley (1956) The determination of organic carbon in marine muds.ICES Journal of Marine Science 22: 180-183.
- Folk R L (1968) Petrology of sedimentary rocks. Cambridge university press, Texas, US, p. 170.
- APHA (2005) Standard method forthe estimation of water and wastewater. American Public Health Association Washington, DC, p. 1193.
- Amjad S, JS Gray (1983) Use of Nematode-Copepod ratio as index of organic pollution. Marine Pollution Bulletin14(5): 178-181.
- Speybroeck J, D Bonte, W Courtens, T Ghjeskiere, P Grootaest, et al. (2005) How may beach nourishment affect the sandy beach ecosystem? The case of Belgian Beaches. In: Herrier J L, J Mees, A Salman (Eds) Proceeding: Dunes and Estuaries, International Conference on Nature Restoration Practices inEuropean Coastal Habitats, Belgium, Pp. 557-568.
- Harkantra S N, AH Parulekar(1989) Population distribution of meiofauna in relation to some environmental factors in a sandy intertidal region of Goa, west coast of India. Indian Journal Marine science 8:259-264.
- Ansari Z A‚ A Chatterji‚ A HParulekar (1984) Effect of domestic sewage on sand beach meiofauna at Goa ‚ India. Hydrobiologia 111: 229-233.
- Priyalakshmi G and NR Menon (2014) Ecology of interstitial faunal assemblage from the beaches along the coast of Kerala India.International journal Oceanography 1-9.
- Jaramillo E, Contreras H, Quijo NP (1996) Macrofauna and human disturbance in a sandy beach of south-central Chile. RevistaChilena de Historia Natural 69:655-663.
- Jenyffer VV, AB Carlos, L Luciano, GC Fabiano (2012) Human impact on benthic macrofauna of two beach environments with different morphometric characteristics in southern Brazil. Brazilian journal Oceanography 60: 135-148.
- Rossi F R M‚ F Forster‚ M Montserrat‚ A Ponti, T Terlizzi, et al. (2007) Human trampling as short-term disturbance on intertidal mudflats: effects on macrofauna biodiversity and population dynamics of bivalve. Marine Biology 151: 2077-2092.
- Wynberg RP‚ GM Branch (1994) Disturbance associated with bait collection for Sand prawns (Callianassakraussi) and Mud prawns (Upogebiaafricana): Long-term effects on the biota of intertidal sand flats. Journal of Marine Research 52(3):523-558.
- Martinez M J R, MCR Delgado, JES Moyano, FJG Garcia (2015) Response of sandy beach macrofauna to human trampling: An urban vs. natural beach system approach. Marine Environmental Research 103: 36-45.
- Gheskiere T‚ M Vincx, JM Weslawski‚ F Scapini‚ S Degraer (2005) Meiofauna as descriptor of tourism-induced changes at sandy beaches. Marine Environmental Research 60(2): 245-265.
- Sugumaran J, R Padmasai R, K Altaff (2019) The effect of tropical cyclone Gaja on sandy beach meiofauna of Pak Bay, India. Regional study in Marine Science 31.
- SarmentoV C‚ AFS Barreto‚PJP Santos (2011) The response of meiofauna to human trampling on coral reefs.Scientia Marina 75: 559-570.
- Sun X, H Zhou, E Hua, S Xu, B Cong, et al. (2014) Meiofauna and its sedimentary environment as an integrated indication of anthropogenic disturbance to sandy beach ecosystem. Marine Pollution Bulletin 88(1-2): 260-267.
- Santos G‚R Correa‚L de Lima, AMartins,RCardoso‚ et al. (2016) Can meiofauna beusedto trampling effects on sandy beachesA casestudy from Carioca’s beaches.Poster presentation Research Gate.
- Bally R (1983) Factors affecting the distribution of organisms in the intertidal zone of sandy beaches. Sandy beach as ecosystem. 19: 391-403.
- Alongi DM (1987) Intertidal zonation and seasonality of meiobenthos in tropical mangrove estuaries. Marine Biology 95: 447-458.
- Ansari, Zakir Ali, B Ingole (2002) Effect of an oil spill from M.V. Sea Transporter on intertidal meiofauna at Goa, India.Marine Pollution Bulletin 44(5): 396-402.
- Ingole B, S Sivadas, R Goltekar, S Clement, M Nanajkar, et al. (2006) Ecotoxicological effect of grounded MV River Princess on the intertidal benthos organisms off Goa. Environment International 32(2): 284-291.
- Sahoo G, ZA Ansari, S Sukumaran, SN Gajbhiye (2017) Defaunation of meiofauna in Mumbai Bay – a severely polluted area. Regional study in Marine Science 16: 95-108.
- Sugumaran J and R Padmasai (2019) A reliability assessment of Nematode: Copepod Ratio for the polluted sandy beaches of Palk Bay, India. International J Pharmacy and Biological Sciences 9: 1244:1252.
- Jones A, T Schlacher, J Dugan, M Lastra, D Schoeman, et al. (2008) Sandy beach ecosystem: Vulnerability, resilience and management.17th NSW coastal conference, Australia.
- McLusky DS, SA Nair, A Stirling, R Bhargawa(1975) The ecology of a central westIndian beach with particulalr reference to Donax incarnates. Marine Biology 30:267-276.
- Dernie K M‚ M J Kaiser, R M Warwick (2003) Recovery rate of benthic communities following physical disturbance. Journal Animal Ecology 72(6): 1043-1056.
- Raffaelli DG, CF Mason (1981) Pollution monitoring with meiofauna using ratio of nematodes to copepods. Marine Pollution Bulletin 12: 158-163.
- Sawkar K,LNoronha, A Mascaranhas, OS Chauhan, S Saeed (1998) Tourism and the environment: Case studies on Goa, India and the Maldives, Stock No. 37134, The World Bank, Washington,US,p.36.
- Zielinski S, Botero CM, Yanes Andrea (2019) To clean or not to clean? A critical review of beach cleaning methods and impacts. Marine Pollution Bulletin 139: 390-401.






























