Heavy Metals Profile Assessment in the Liver and Muscle Tissues of Nile Tilapia at The End of Production Cycle
Emad Mohamed Zidan1 and Samy Yehya El-Zaeem2*
1Animal Husbandry and Animal Wealth Development, Alexandria University, Egypt
2Animal and Fish Production Department, Alexandria University, Egypt
Submission:September 07, 2020; Published:October 19, 2020
*Correspondence author: Samy Yehya El-Zaeem, Animal and Fish Production Department, Faculty of Agriculture (Saba-Basha), Alexandria University, Egypt
How to cite this article:Emad M Z, Samy Yehya El-Z. Heavy Metals Profile Assessment in the Liver and Muscle Tissues of Nile Tilapia at The End of Production Cycle. Oceanogr Fish Open Access J. 2020; 12(4): 555844. DOI: 10.19080/OFOAJ.2020.12.555844
Abstract
The present study was conducted to evaluate the heavy metals profile in liver and muscle tissues of Nile tilapia at the end of the production cycle. To determine the concentration of 10 heavy metals in liver and muscle tissues of Nile tilapia (Oreochromis niloticus) cultured in natural and tap water, the samples of fish cultured for ten months were randomly collected and analyzed for Cadmium, Zinc, Lead, Copper, Manganese, Iron, Nickel, Chromium, Mercury, and Arsenate by Spectrophotometric method. The results revealed that the concentrations of heavy metals in fish samples collected from natural water were higher than that of samples collected from tap water. This concluded that, the concentrations of heavy metals in liver and muscle tissues of Nile tilapia reflect their concentrations in culture water and confirm its possibility to be an aquatic friendly environment.
Keywords: Nile tilapia; Heavy metals; Liver; Muscle; Water
Introduction
The high protein content, essential amino acids, vitamins, and minerals make fish a very good candidate as a human food [1,2]. Unfortunately, the aquatic life is at a continuous risk of exposure to pollutants such as heavy metals, pesticides, and other organics that leak to their water habitat [3]. Disposal of dredge spoil, sewage sludge, and industrial effluents in water bodies introduces various pollutants such as metals to the aquatic ecosystems. Deposition of metals suspended in air, leaching, runoffs, agriculture, industries, and other anthropogenic activities continuously elevate the levels of heavy metals at a frightening rate in the environment and the aquatic ecosystems in particular causing a global problem [4]. Most elements even essential trace elements are not toxic unless they reach a concentration high enough to cause pathological changes [5]. Due to their non-biodegradable nature, those metals accumulate in high concentrations in the bodies of aquatic organisms causing toxicity.
The persistence of heavy metals in the food chain and the difficulty of their elimination from the environment is the major problem [5]. Minimal concentrations of Mercury (Hg), Cadmium (Cd), Arsenic (As), Chromium (Cr), and Lead (Pb) normally exist in the environment and even act as essential micronutrient for plants, animals, and humans, but they cause toxicity at high levels. Copper, Nickel, Manganese, Lead, Cadmium, and Iron at high levels lead to deleterious effect on human body such as hypertension, nausea, sporadic fever, and renal injury [6]. Determination of heavy metals is useful and important in the environmental pollution. World Health Organization (WHO) set maximum permissible levels of heavy metal in the environment that should not be exceeded.
Fish are at the top of aquatic food chain and can accumulate high concentrations of heavy metals from water which can easily ascend through the food chain to higher organisms like human beings [7]. Therefore, it is essential to assess heavy metals concentration in fishery products from various environments to guarantee that they are safe for human consumption. Owing to its capacity to store heavy metals and other organics, fish are believed to be a fascinating bio-indicator [8]. Oreochromis niloticus is an important species in commercial fisheries that readily responds to environmental alterations. The present study was conducted to use Nile tilapia as a bioindicator for evaluating the water resources in terms of heavy metals.
Materials and Methods
Fish Collection and Area of Study
Twenty fish of Nile tilapia (Oreochromis niloticus) were randomly collected from circular plastic tanks supplied with tap water in the fish farm that belongs to the Faculty of Veterinary Medicine, Alexandria University and from hapas in an earthen pond supplied with natural water located at Edko, Beheira Governorate, Egypt after ten month of growth in tanks and hapas. Growth period began in September 2015 to June 2016. The muscle and liver tissues of Nile tilapia were isolated and transported in ice box to the laboratory. Water samples were manually collected in triplicates from each of the studied areas at 30 cm underneath the surface of the water and stored at 4°C in sterile glass bottles, preserved with concentrated hydrochloric acid (HCl).
Detection of Metals in Fish Organs and Water
The muscle and liver samples were extracted by digestion using a modified method described by Seymore et al. [9]. Briefly, 1 g of fresh tissue, 20 ml nitric acid, and 5 ml perchloric acid were placed in a 200 ml flask. The samples were heated at 225°C for 12 h (The temperature was gradually increased) on a hotplate and evaporated to ~5 ml. Upon forming a clear liquid, 0.2 ml lanthanum chloride (100 g La/L solution) was added. 2% HNO3 was then added to make the volume up to 20 ml. The same procedure was used to make a reagent blank. Atomic absorption spectrophotometer (AAS) Analyst 800 (Perkin Elmer Instruments, USA) with an acetylene flame (Cu and Zn) or an argon non-flame (Cr, Hg, Cd, Pb, Fe, Mn, Cr, As and Ni) was used to measure the concentration “ Cadmium (Cd); Zinc (Zn); Lead (Pb); Copper (Cu); Manganese (Mn); Iron (Fe); Nickel (Ni); Chromium (Cr); Mercury (Hg) and Arsenate (As)” after preparation of the calibration standard. The concentrations of heavy metals were expressed as μg/gram dry weight for fish organs. Determination of heavy metals in water was measured by the same method, but concentrations of all heavy metals in water are expressed as mg/l (ppm).
Statistical Analysis
Data were analyzed by Student’s “t” test to compare the different parameters by SPSS (2010) statistical package version. The data values were presented as mean ± standard error of mean (SEM).
Results
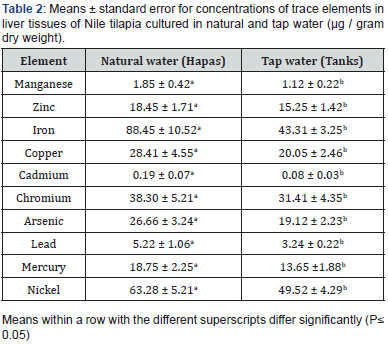
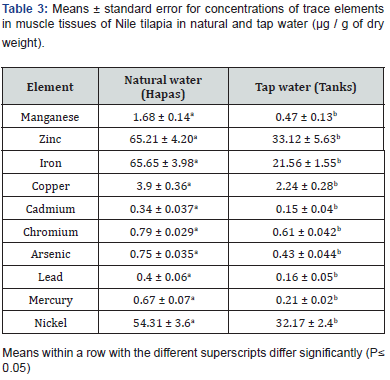
Heavy metal concentration in water samples collected from natural water showed significantly increased compared to tap water (Table 1). Concentrations of heavy metals in liver tissues had the following order Fe > Ni > Cr > Cu > As > Hg> Zn > Pb > Mn and Cd. Heavy metal concentration in liver tissues of fish culture in natural water revealed significantly higher compared to fish cultured in tap water (Table 2). Heavy metal concentration in muscle tissues of fish culture in natural water revealed significantly higher compared to fish cultured in tap water (Table 3). Level of heavy metal residues was assessed in edible muscles and nonedible liver of fish obtained from natural and tap water; results are illustrated in Table 2 and 3. The recorded data confirmed the presence of heavy metals traces in all fish tissues under the study. In addition, the prevalence of each element was not the same in the two sampling sites.

Discussion
Nowadays, water pollution is regarded as a major environmental and public health issue facing Egypt and the Middle East. Oreochromis niloticus is important species in commercial fisheries that is readily affected by environmental modifications. Owing to the disparity in content of metal and chemical characteristics of water from which fish are obtained, their ecological needs, feeding patterns, and metabolism, heavy metals in freshwater fish appear in various concentrations in different studies [10-12], Heavy metals diffused through the gills or the gut wall. The data of the present study indicated the accumulation of ten heavy metals in muscle tissues of O. niloticus in the order of Fe >Zn > Ni > Cu >Mn > Cr > As > Hg > Cd > Pb for samples of fish and liver tissues in the order of Fe > Ni > Cr > Cu > As > Hg> Zn > Pb > Mn and Cd. Meanwhile, the heavy metals concentration in water showed that Ni had the highest mean level in natural water, followed by Cr, Fe, Pb, Zn, As, Hg, Cd, Mn, and Cu.
Iron (Fe) is a naturally occurring element in the soil, but agricultural activities add up to the iron level found in the sampled fish. Iron level in the samples was below the permissible level of 107 μg/g set by World Health Organization [13] thus not posing any threat to fish consumers. The drop of iron level in human body results in iron deficiency anemia. Zinc (Zn) is a naturally occurring fundamental element in organisms and in the earth crust and is clearly not dangerous to fish or its shoppers. Fish diet and its water habitat are the main source of zinc accumulated in fish tissues [14]. Its concentration in the samples was below the National Contaminant Bio-monitoring Program (NCBP) maximum contaminant level of 34.2: g/g. High levels of zinc cause death, reproductive impairment, and growth retardation [15]. The interaction between zinc and various elements is antagonistic, additive, or synergistic [16].
Nickel (Ni) concentration in the samples was below the dangerous level of 0.7 g/g thus not jeopardizing the health of fish consumers [16]. Excess copper (Cu) is toxic to fish though the exact effect is not yet well [17]. Once combined with ammonia, mercury, and zinc, its toxicity increases [18]. The concentration of copper (3.9 μg / g dry weight in hapas and 2.24 μg / g dry weight in tanks) in the fish samples slightly exceeded the maximum contaminant level as reported by Schmitt and Brumbaugh [19] probably due to leaching and runoff of naturally occurring copper in the soil. It is an essential micronutrient but may cause nausea, vomiting, and diarrhea when approaching high concentrations. Hepatic and renal damage even death is reported with very high levels of copper [20].
Manganese (Mn) in fish came from naturally occurring manganese in the soil, leaks through leaching and runoff, or released from Agro chemicals as herbicides and pesticides. In this study, its concentration was below the permissible level of 5 μg/g in hapas and tanks according to the World Health Organization [13]. It’s a fundamental trace element whose deficiency may lead to skin diseases, neurological symptoms, or coagulation disorders. High level may cause nerve damage, hallucinations, forgetfulness, pulmonary embolism, bronchitis, and Parkinsonism. Chromium (Cr), though essential, is lethal to fish and wildlife at high levels [21]. No maximum level of chromium in fish and wildlife was set as a guideline value [22]. In the current study, its concentration was far below (53.8: g/g) the level set by United States Environmental Protection Agency (USEPA) as contaminant to fish.
Naturally occurring arsenic (As) in the soil leaching into the water is the major source of arsenic in sampled fish. In this study, its level in hapas and tanks was below (0.5 g/g dry weight) which according to Walsh et al. [23] leads to deleterious effects on fish when exceeded. The recorded results of Mercury (Hg) level in the present study were lower than those obtained by Sohsah- Madiha [24] (0.81 ± 0.05 for Tilapia nilotica) while the concentration of Mercury in the present study was higher than the results recorded by El-Zahaby- Dina [25] (0.013 ± 0.001 ppm in Tilapia nilotica). Mercury is normally present in the environment in low concentrations, mainly because of industrial activities [26]. Methyl mercury is not excreted so it is more dangerous to human health than inorganic one. In addition, it acts as accumulative poison and can cross blood-brain barrier causing progressive and irreversible cerebral damage [27].
Land preparation and application of Agro-chemicals are believed to be the main source of Cadmium (Cd) in the sampled fish. Fertilizers as phosphate fertilizers which are annually applied on farmlands contain an average of 13.4 g/g of cadmium as reported by Modaihsh et al. [28]. Cadmium is a non-essential trace metal; it has potentially deleterious effect on most fish, wildlife, and freshwater organisms [21]. The highest cadmium levels in fish samples were below the 0.5g/g threshold but are still harmful to fish and other predators [23]. Automobile exhaust, industrial wastewater, wastewater sludge, and pesticides are the major sources of Lead (Pb) in the environment [29]. The global mean lead concentration in lakes and rivers ranges approximately between 1.0 and 10.0 μg/L [30]. Lead gained access to the 15 aquatic environments through erosion and leaching from soil, dust fallout, gasoline combustion, and industrial wastes, runoff of fallout deposit from streets and other surfaces as well as precipitation [9]. In this study, the concentration was lower than the estimated level in lakes and rivers [31-35].
Conclusion
The concentrations of heavy metals in muscle and liver tissues of Nile tilapia (Oreochromis niloticus) were higher in fish samples culture in natural water compared to fish collected from tap water, and this is due to nature of water resources where in tanks was tap water while in natural water was Nile water mixed with agriculture water drainage. This means that rearing and growth of fish in tap water is better than natural water for human health and human consumption, although these elements did not reach the toxic levels. Written culture where a letter d is added to this quantity and becomes cultured
References
- Gogus U, Smith C (2010) n-3 Omega fatty acids: a review of current knowledge. Int J Food Sci Technol 45(3): 417-36.
- Kim W, McMurray DN, Chapkin RS (2010) n-3 Polyunsaturated fatty acids-physiological relevance of dose. Prostaglandins Leukot Essent Fatty Acids 82(4-6): 155-158.
- Wood CM (2001) Toxic responses of the gill. In: Schlenk D, Benson WH (Eds.), Target Organ Toxicity in Marine and Freshwater Teleosts. Taylor and Francis, London, UK Pp. 1-89.
- Malik N, Biswas AK, QureshiTA, Borana K,VirhaR (2010) Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environ Monit Assess 160(1-4): 267-267.
- Dimari GA, AbdulrahmanFI, AkanJC, GarbaST (2008) Metals concentrations in tissues of tilapia Gallier, CrariasLazeraand Osteoglossidae Caught from Alau Dam, Maiduguri, Borno State, Nigeria. American Journal of Environmental Sciences 4(4): 373-379.
- NRC (National Research Council) (1999-2000) Dogfish liver (Dolt-2), Dogfish Muscle (Dolt-2), Non-Defatted lobster Hepatopancreas (LUTS-1) and Marine Lobsters Hepatopancreas (Tort-2) Reference materials for Trace Metals. In: Chemical Metrology, introduction to NRC Certified Reference Materials (CRMS)-Institute for National Measurement standard (INMS). Catalogue Number and Date sheet: DOLT-2 INMS CRMs. Institute for Marine Biosciences, Halifax, Government of Canada.
- Rauf JM, UbaidullahM(2009) Heavy Metal Levels in Three Major Carps (CatlaCatla, LabeoRohita and CirrhinaMrigala) from the River Ravi, Pakistan. Fisheries Research Farms.
- Ahmed AK, Shubaimi-Othman (2010) Heavy metal Concentration in Sediments and Fishes from Lake Chini, Pahang, Malaysia. Journal of Biological Sciences 10(2): 93-100.
- DWAF (Department of water Affairs and forestry) (1996). South Africa Water Quality Guidelines. Aquatic Ecosystems. DWAF, Pretoria.
- Javed M, Hayat S (1998) Fish as a bio indicator of freshwater contamination by Metal. Pakistan Journal of Agricultural Sciences 35: 11-15.
- Chattopadhyay B, Chatterjee A, Mukhopadhyay SK (2002) Bioaccumulation of metals in the East Calcutta wetland ecosystem. Aquatic Ecosystems Health Management 5: 191-203.
- Papagiannis I, Kagalou I, Leonardos J, Petridis D, Kalfakaou V (2004) Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece). Environ Int 30(3): 357-362.
- World Health Organization (WHO) (1986) Environmental health criteria 108. International Programmed on Chemical Safety World Health Organization.
- Eisher R (1993) Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. Uinted States Fish Wildllife Service 85: 90-102.
- Sorenson EM (1991) Metal Poisoning in Fish. IN: CRC Press Inc, Boca Raton, Florida, USA, Pp: 119-174.
- Baumann PC, May TW(1984) Nickel residues in fish from inland waters of the United States. Workshop Proceedings, the Effects of Trace Elements on Aquatic Ecosystems. Electr Pow Res Instit Palo Alto7: 1-16.
- Woodward DF, Brumbaugh WGA, Deloney J, Little EE, Smith CE (1994) Effect of contaminant metals on fish in the Clark Fork River in Montana. Journal of American Fisheries Society 123: 51-62.
- Rompala JM,Rutosky FW, Putnam DJ(1984) Concentrations of Environmental Contaminants from Selected Waters in Pennsylvania. Fish Wild Serv 102.
- Schmitt CJ, Brumbaugh WG(1990) National Contaminant Bio Monitoring Program, Concentrations (NCBP) of arsenic, cadmium, copper, lead, mercury, selenium and zinc in U.S. freshwater fish, 19761984. Archives of Environmental Contamination and Toxicology 19: 731-747.
- ATSDR (Agency for Toxic Substances and Disease Registry) (2011) Toxic Substances Portal-Copper.
- Robertson SM, LR Gamble, TC Maurer (1992) Fish and Wildlife Service, Contaminant Survey of La Sal Vieja, Corpus Christi, Texas, US.
- Akan JC, Abdulrahman FI, OA Sodipo, AkanduPI(2009) Bioaccumulation of some heavy metals of six freshwater fishes caught from Lake Chad in Doron Buhari. Journal of Applied Sciences in Environmental Sanitation 4(2): 103-114.
- Walsh DF, BergerBL, Bean JR(1977) Mercury, arsenic, lead, cadmium and selenium residues in fish: 1971-1973-national pesticide monitoring program. PesticMonit J 11(1): 5-134.
- SohsahMadiha AM (2009) Studies on some heavy metal residues in freshwater fish with special reference to water environmental pollution. PhD Thesis, Fac Vet Med, Benha Univ, Egypt.
- El-Zahaby-Dina I M (2007) Microbiological and chemical studies on some fish and fish products in Menoufia Governorate. M.V. Sc., Thesis. (Meat Hygiene), Fac Vet Med, Menoufia Univ, Sadat Branch, Egypt.
- Clarkson TW (2002) The three modern faces of mercury. Environmental Health Prespectives 110(1): 11-32.
- Clark RB (1989) Marine pollution. In: (2nd Edn) Oxford Science publications, Clarendon press, UK, Pp. 130-140.
- Modaihsh AS, MS Al-Swailem, MO Mahjoub (2004) Heavy metals content of commercial inorganic fertilizers used in the Kingdom of Saudi Arabia. Journal of Agricultural Marine Science 9(1): 21-25.
- Balba A, Shibiny G, El-Khatib E (1991) Effect of Lead Increments on the Yield and Lead Content of Tomato Plants. Water, Air, and Soil Pollution 57: 93-99.
- Weiner E R, (2008) Application of Environmental Aquatic Chemistry. Taylor and Francis 109.
- AOAC (1990)The Association of Official Analytical Chemists. Official Methods of Analysis Atomic Absorption Method for Fish, Washington, USA
- Brown A, Halls JD, Taylor A (1986) Atomic spectrometry update-clinical materials, foods and beverages. Journal of Analytical Atomic Spectrometry 1: 21-35.
- Mason C (1991) Biology of freshwater pollution. Longman Scientific and Technical, Harlow, England.
- In: Parasad AS,OberleasD (Eds), (1976) Trace elements in Human Health and Disease, vol I and II, Academic press, New York.
- Zehra I,Kauser T, Zahir E, Naqvi II (2003) Determination of Cu, Cd, Pb and Zn Concentration in Edible Marine Fish Acanthopagurusberda(DANDYA) Along Baluchistan Coast-Pakistan. International journal of agriculture & biology (1): 80- 82.






























