Habitat Ecology and Biological Characteristics of a Hypersaline Ciliate, Fabrea salina from Solar Salterns of Mumbai Coast, India
Bam Deo Pandey1* and S G Yeragi2
1Udai Pratap College, India
2K J Somaiya College of Science & Commerce, India
Submission:April 06, 2020; Published:August 03, 2020
*Correspondence author: Bam Deo Pandey, Udai Pratap College, Varanasi, India
How to cite this article:Bam Deo P, S G Yeragi. Habitat Ecology and Biological Characteristics of a Hypersaline Ciliate, Fabrea salina from Solar Salterns of Mumbai Coast, India. Oceanogr Fish Open Access J. 2020; 12(2): 555833. DOI: 10.19080/OFOAJ.2020.12.555833
Abstract
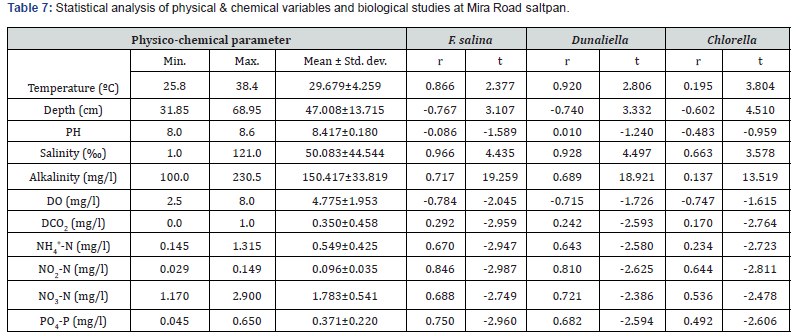
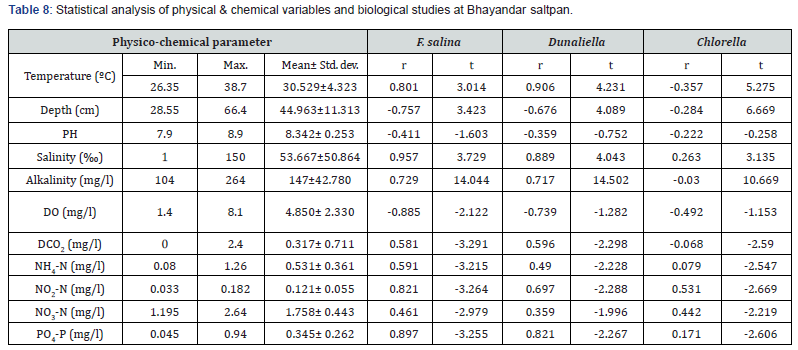
The ecology of a hypersaline ciliate, Fabrea salina was studied in two saltpans along the Mumbai coast, India. There was an apparent trend of its seasonal abundance being maximum (up to 58 x 103 cells L-1 in May) during late post- monsoon to summer months and complete disappearance during monsoon period. Being the most dominant species in microzooplankton community, it had an average annual density of 18 x 103 cells L-1. It flourishes well under higher temperature (30-39ºC) and salinity (40-150 ‰) conditions. Among phytoplankton, Dunaliella was the dominant one with a highest density of 58 x 103 cells mL-1 in April, followed by Chlorella with up to 42 x 103 cells mL-1, in March. The ANOVA test for physical and chemical variables has revealed significant difference (P=0.05) in their values in different months. Except in water temperature and NO2-N, no significant difference was observed at various stations as the case with phytoplankton and zooplankton. There was strong positive correlation of Fabrea with water temperature (r=0.866, 0.801), salinity (r=0.966, 0.957), total alkalinity (r=0.717, 0.729) and PO4-P (r=0.750, 0.897) while negative correlation with water depth (r=-0.767, -0.757) and pH (r=-0.086, -0.411). Fabrea varies widely in its total length (60-600 μm) and cyst diameter (70-180 μm). The average length of body cilia is 12 μm and the width of each adoral zone of membranelle (AZM) is 10 μm
Keywords: Fabrea salina; Hypersaline ciliate; Solar salterns; Ciliate ecology
Introduction
Ciliates are the most specialized and perhaps most widely distributed and diverse group of protozoa having representatives in virtually all kinds of freshwater to marine environments, often in extremely high densities [1]. They form an important component of estuarine as well as coastal marine ecosystems as they feed upon bacteria and in turn serve as food for metazoans [2,3]. However, the ecology and biology combined with factors controlling the distribution of protists in tropics have received very little attention [4]. Many hypersaline environments are inimical to macroscopic life but are the preferred habitats of a variety of microorganisms. The heterotrichous ciliate, F. salina , reported from several diverse environments such as salt marshes, hypersaline lakes and solar salterns [5-7], has received much attention in the recent years primarily due to its potentiality as live food source for maricultural purposes [8-11] and also as an experimental animal in basic research of applied value in eukaryotic microbiology [12-16]. Though fairly a good amount of literature is available on the hydrobiology of estuaries and backwaters in India, the information on the plankton ecology in inland saline lakes and solar salterns is very scanty. The present study deals with the ecology of F. salina in two saltpans near Mumbai, West Coast of peninsular India, for a period of one year, January 1998 to December 1998.
Study Area
The study areas, Mira Road and Bhayandar saltpans, the parts of Thane District, Maharashtra, India, are located at 19º16’N Lat & 72º51’E Long and 19º19’ Lat & 72º51’E Long, respectively. The former relates to Manori creek while the later with Bassein creek, along the Mumbai coastline. Two sampling stations in each saltpan, denoted as MR1 & MR2 at Mira Road and BH1 & BH2 at Bhayandar were selected in the present study. The region has typical tropical climate.
Methods
Phytoplankton and Zooplankton
For phytoplankton analysis, one-liter water was directly collected whereas zooplankton samples were taken by filtering 50 L of water through plankton net of 40 μm mesh size. The samples were preserved with Lugol’s solution. After three days of stagnation, the phytoplankton samples were concentrated to 100 mL volume by decanting the supernatant. Except Dunaliella that was counted by haemocytometer, all the plankton were enumerated using Sedgwick-Rafter cell counter (50mm x 20mm x 1mm). For each month, the average density of Fabrea, Dunaliella and Chlorella were taken for statistical purposes.
Hydrological and Soil-Quality Parameters
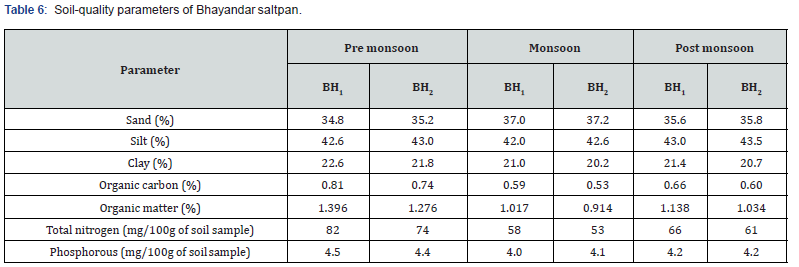
Both ambient and water temperatures were measured using thermometer with 0.1ºC accuracy while water depth by a meter scale. Salinity and pH were recorded at the site using Salinity Refractometer (S/Mill- E, Atago) and portable pH meter (Model No. E Merck 325). Other water quality parameters such as dissolved oxygen, dissolved free carbon dioxide, total alkalinity, ammonium- nitrogen (NH4+-N), nitrite-nitrogen (NO2-N), nitratenitrogen (NO3-N) and phosphorus (PO4-P) were analyzed following Standard Methods (APHA, 1992) monthly. The values of various parameters obtained at both the stations of each saltpan were summed up and average values are used in data analysis. The soil-quality parameters viz. percentage of sand, silt, clay, organic carbon, organic matter and total nitrogen and phosphorus (mg/100 g of soil sample) were analyzed following Ghosh et al. [17] during pre- monsoon, monsoon, and post-monsoon periods.
Encystment and Excystation
To validate the existence of F. salina in encysted form during the periods of non- availability of its free-swimming trophozoites in nature, the sun-dried scum- mat with some soil of salt-pans was immersed at 5 g L-1 in saline water (2 L) of six different salinities i.e. 30, 40, 50, 60, 80 and 100 ‰ provided with mild aeration. For encystment, the salinity of culture medium (5 L) was raised gradually from 65 to 110 ‰. After harvesting, the cysts were subjected to hatching under different salinities as indicated above. These experiments were carried out at ambient and water temperature of 34±1ºC and 31±1ºC, respectively.
Results
Phytoplankton and Zooplankton
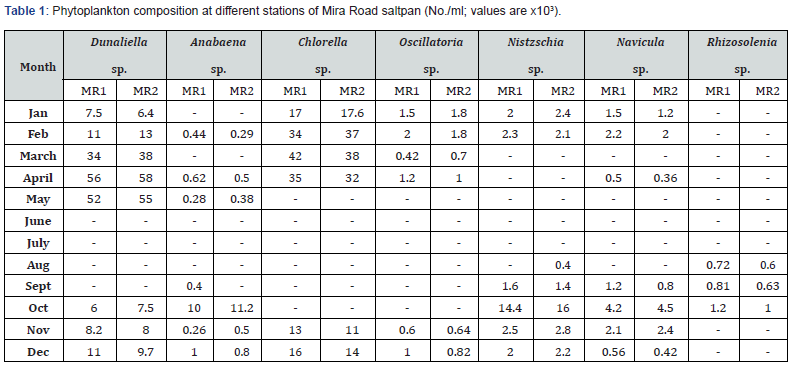
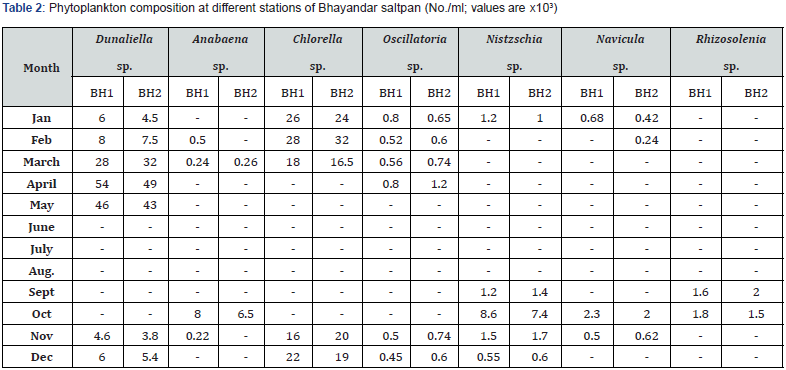
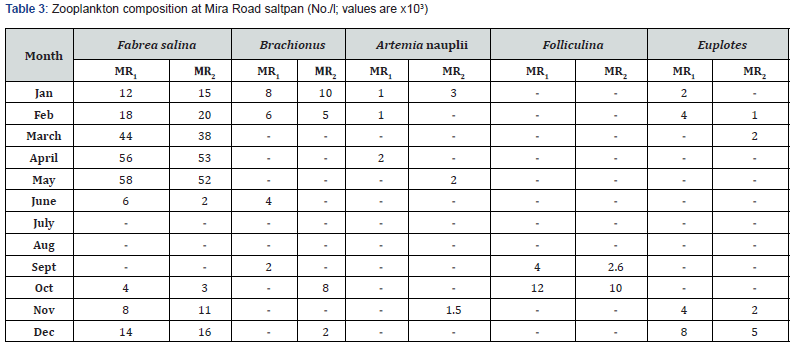
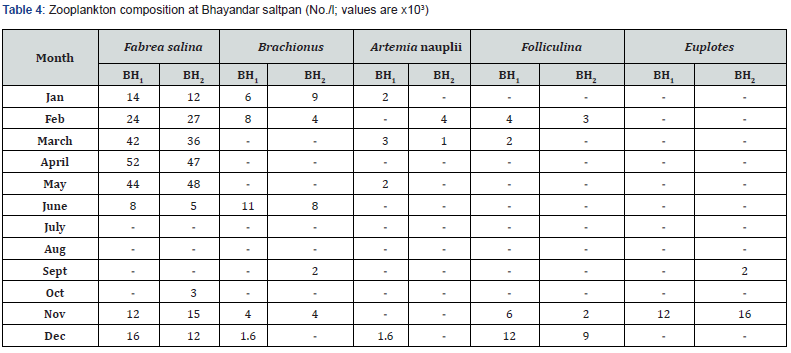
Phytoplankton and zooplankton were in abundance during pre- and post- monsoon months while their density was quite low in monsoon periods (Table 1-4). Zooplankters were completely absent during July and August months. Dunaliella, the most abundant species noted, was with a maximum density of 57 x 103 cells mL-1 during April. Chlorella, the second largely available plankton, had the highest density of 40 x 103 mL-1 in March. This was followed by the occurrence of Nitzschia sp., Navicula sp., Anabaena, Oscillatoria and Rhizosolenia.
Physical and chemical variables
The highest water temperature (39.0ºC) was recorded at Bhayandar saltpan in May. The water pH was alkaline throughout the year, varying from 8.0 to 8.6. Salinity was very low during monsoon months, varying from nil to 16 ‰ contrary to its higher values (up to152 ‰) prevailing during late post- monsoon and pre- monsoon months. The levels of total alkalinity were generally low during monsoon months being 100 mg L-1 in July. The low DO levels (1.4- 3.4 mg L-1) were recorded during pre- and postmonsoon months. Generally, the dissolved free carbon dioxide was nil throughout the year. The values of NH4 +-N, NO2-N, NO3-N and PO4-P were less during monsoon months and higher in postmonsoon periods. There was no wide variation in soil quality parameters at both the saltpans (Table 5,6). The ANOVA has revealed significant difference (P=0.05) in the values of all the 12 physical and chemical variables studied in various months. In addition, water temperature and NO2-N values showed significant difference at various stations too. There was strong positive correlation of Fabrea with water temperature, salinity, total alkalinity, and PO4-P while negative correlation with water depth and pH (Table 7,8).

Population of Fabrea salina
F. salina was the most dominant species in microzooplankton community. Its density varied from zero in monsoon months to 55 x 103 cells L-1 in May. The population abundance was in strong correlation with salinity, temperature, alkalinity, water-depth, DO and NO2-N. Fabrea flourishes well under the higher temperature (30-39ºC) and salinity (40-150 ‰) conditions. Dunaliella acts as natural food for Fabrea. The bloom of Dunaliella was noticed during March to May with the concurrent abundance of Fabrea.
Cyst Hatching
It is evident that 40 to 50 ‰ salinities are suitable for cyst hatching. No significant hatching occurred beyond 60 ‰ salinity.
Discussion
Phyto and Zooplankton
Dunaliella salina has best growth in 120 ‰ salinity with a tolerance limit of 350‰ [18]. Like Dunaliella, diatoms are also ubiquitous inhabitants of hypersaline environments, but they never appear to dominate. The present findings are in conformity with the occurrence of diatoms in solar salterns having salinity up to 129 ‰ in the Great Salt Lake [19]. Nitzschia sp. and Navicula sp. are represented in all these aquatic environments. The probable factors restricting the eukaryotic algae from many hypersaline environments include their inability to osmoregulation under prevailing conditions and to assimilate nutrients that may be scarce coupled with periodic habitat desiccation.
Physical and Chemical Variables
The saltpans are exposed typically to a wide range of environmental stress and perturbations. On solar heating of brines having halobacterial colouration, a maximum temperature of 46ºC is attained by the densely colored brine, while the clear brines could reach up to 39ºC [20]. Not only do gases diffuse more slowly as brine density increases, the capacity to hold them also becomes poor. Further the low DO levels in the present study were also probably due to bacterial consumption of oxygen that diffuses from the atmosphere or produced by microalga Dunaliella. The low pH levels indicate the high levels of CO2 and alkalinity in water bodies as observed in the present study. The noteworthy trend in salinity values were due to influx of freshwater during monsoon while prevailing higher temperature, excessive evaporation, and low water-depths in the summer months.
Population of F. salina
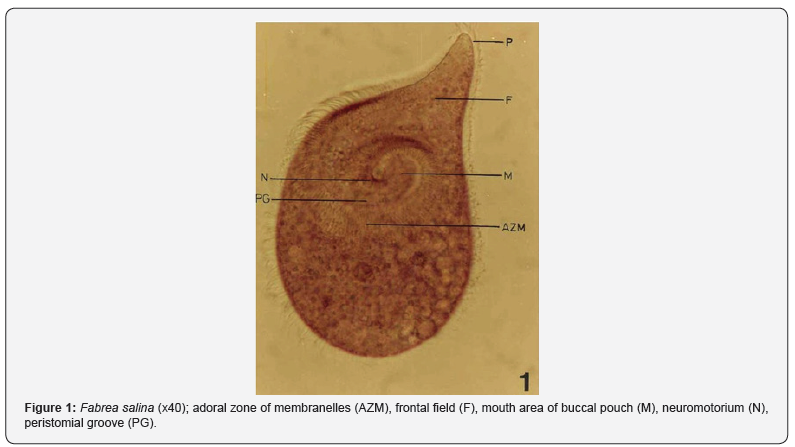
The present study reveals great ability of Fabrea to withstand wide ranges of environmental variables. Its better growth has been obtained at 6 x 106 and 8 x 106 Dunaliella cells mL-1 [9]. As observed, in solar salterns, it feeds voraciously upon Dunaliella cells (Figure 1). However, it appeared that Fabrea, in extremely saline conditions (>240 ‰) when Dunaliella is not available, survives on halobacterial and it subsists on bacteria during food scarcity [21]. In marine planktonic realm, nearly all phytoplankton produced are consumed, primarily by microzooplankton [22]. Protists are capable of sensing the biochemical properties of their prey cells [23,24], and both ciliates and flagellates have been seen to feed preferentially on more nutritious phytoplankton species [25,26] indicating why Dunaliella is preferred by Fabrea. Fabrea disappears from saltpans in monsoon months as it does not thrive well in brackish water. It forms cyst and resumes normal active form and life activities on the return of suitable conditions during mid of November [9].
The positive correlation with nitrogen and phosphate is an indication of demand-supply of nutrients to sustain its higher densities in the month of April and May. The optimal production of solar salt requires a well-established balance between primary and secondary producers, with Artemia grazing on phytoplankton constitutes the major interaction [27]. Artemia also tolerates very high salinities [28]. It is surmised, therefore, that Fabrea too contributes to solar salt manufacture. Very sparse, heterogeneous distribution and above all almost vanished populations of Artemia from majority of saltpans along the Mumbai coastline make Fabrea as a predominant inhabitant of these solar saltworks [29]. The present knowledge of the ecology of Fabrea in its natural habitat and effective management practices can be applied for its controlled production on commercial scale in solar salt- beds [30]. The euryplasticity, easy acceptability of a variety of live and inert feeds, short generation period and biochemical composition make F. salina as an appropriate animal for studying microbiology of hypersaline environments.
References
- Lee J J,Hutner S H and Bovee E C (1985) An illustrated guide to the protozoa. Society of Protozoologists, Allen Press,Kansas, US, Pp: 629.
- Stoecker D K and Cappuzzo J M (1990) Predation on protozoa, its importance to zooplankton. Journal of Plankton Research12: 891-908.
- Sanders RW and Wickham S A (1993) Planktonic protozoa and metazoa:production, food quality and population control. Mar Microb Food Webs:7:197-223.
- Smetacek V (1991)Trophic behaviour-Session Summary. In: Philip C R, Carol M T and Peter H B (eds), Protozoa and their role in marine processes. Ecology Science25: 195-203.
- Post F J, Borowitzka L J, Borowitzka M A, Mackay B and Moulton T (1983) The protozoa of a Western Australian hypersaline lagoon. Hydrobiologia105: 95-113.
- Yufera M (1985) The population of Fabreasalina(Ciliata: Heterotrichida) in the Salterns of Cadiz bay. Invest PesqBarc49: 493-500.
- Pandey B D and Yeragi S G (1998) Fabreasalina: Live food for use in aquaculture.Fish Chimes18: 17-18.
- Pandey B D and Yeragi S G (2000) The importance of live feeds in aquatic seed production. Infofish International4: 31-36.
- Pandey B D(2001) Ecology, biology, and culture aspects of Fabreasalina. University of Mumbai, India.
- Pandey B D and Yeragi S G (2003) Preliminary and mass culture aspects on a heterotrichous ciliate, Fabreasalina. Aquaculture232(1-4): 241-253.
- Pandey B D, Yeragi S G and Pal A K (2004) Nutritional value of a heterotrichous ciliate, Fabreasalinawith emphasis on its fatty acid profile. Asian-australasian Journal of Animal Sciences17(7): 995-999.
- Marangoni R, Puntoni S, Favati L and Colombetti G (1994) Phototaxis in Fabreasalina. I. Action spectrum determination. Journal of Photochemistry and Photobiology B: Biology23: 149-154.
- Marangoni R, Batistini A, Puntoni S and Colombetti G (1995) Temperature effects on motion parameters and phototactic reaction of the marine ciliate Fabreasalina. Journal of Photochemistry and Photobiology B: Biology30: 123-127.
- Marangoni R, Gobbi L, Verni F, Albertini G and Colombetti G (1996) Pigment granules and hypericin- like fluorescence in the marine ciliate Fabreasalina. Acta Protozoologica35: 177-182.
- Marangoni R, Preosti G and Colombetti G (2000) Phototactic orientation mechanism in the ciliate Fabreasalina, as inferred from numerical simulations. Journal of Photochemistry and Photobiology B: Biology54:185-193.
- Puntoni S, Maramgoni R, Gioffre and Colombetti G (1998) Effects of Ca 2+ and K+ on motility and photomotility of the marine ciliate Fabreasalina. Journal of Photochemistry and Photobiology B: Biology43: 204-208.
- Ghosh A B, Bajaj J C, Hasan R and Singh D (1983) Soil and water testing methodsA laboratory manual. Indian Agricultural Research Institute, New Delhi, India
- Leoblich L A (1972) Studies on the brine flagellate Dunaliellasalina. University of California. San Diego, US, P. 142.
- Felix E A and Rushforth S R (1979) The algal flora of the Great Salt Lake, Utah, USA. Nova Hedwigia31: 163-194.
- Javor B J (1983) Planktonic standing crop and nutrients in a saltern ecosystem. Limnology and Oceanography28(1): 153-159.
- Repak A J (1983) Suitability of selected marine algae for growing the marine heterotrich ciliate Fabreasalina. The Journal of Protozoology30: 52-54.
- Strom S (2002) Novel interactions between phytoplankton and microzooplankton: their influence on the coupling between growth and grazing rates in the sea. Hydrobiologia480: 41-54.
- Monger B C, Landry M R and Brown L S (1999) Feeding selection of heterotrophic marine nanoflagellates based on the surface hydrophobicity of their picoplankton prey. Limnology and Oceanography44: 1917-1927.
- Strom S L, Wolfe G V and Slajer A (2001) Phytoplankton DMSP release: a possible chemical defense against protist grazers?, Amer Soc Limnol Oceanogr.
- Stoecker D K, Cucci T L, Hulburt E M and Yentsch C M (1986) Selective feeding by Balanion (Ciliata: Balanionidae) on phytoplankton that best support its growth. Journal of Experimental Marine Biology and Ecology 95: 113-130.
- Buskey E J (1997) Behavioural components of feeding selectivity of the heterotrophic dianoflagellateProtoperidinium pellucidum. Marine Ecology Progress Series153: 77-89.
- Tackaert W and Sorgeloos P (1993) The use of brine shrimp Artemiain biological management of solar saltworks. In: Seventh Symposium on Salt. Elsevier Science Publishers BV, Amsterdam, NetherlandsPp: 617-622.
- Ludwig C Naegel and Sonia AR (2002) Ecological observations and biomass proximate composition of the brine shrimp Artemia(Crustacea: Anostraca) from Pichilingue, Baja California Sur, Mé Hydrobiologia, 486: 185-190.
- APHA (1992) Standard Methods for The Examination of Water and Wastewater.Washington, DC.
- Demar-Gervais C,Genermont J (1971) Donneesexpeimentales sur le mecanisme de l’eclosion des kystes de Fabreasalina. Protistologica7:421-433.






























