Some Aspects of Reproductive Biology of Common Pandora (Pagellus Erthrinus) Collected from the Coast off Benghazi, Libya
Amina Elmajedeb, Houssein Elbaraasi*, Fatma Altomi and Hussein Jenjan
Faculty of Science, Department of Zoology, University of Benghazi, Libya
Submission:May 14, 2019; Published: May 30, 2019
*Correspondence author: Houssein Elbaraasi, Faculty of Science, Department of Zoology, University of Benghazi, Libya
How to cite this article:Amina Elmajedeb, Houssein Elbaraasi, Fatma Altomi, Hussein Jenjan. Some Aspects of Reproductive Biology of Common Pandora (Pagellus Erthrinus) Collected from the Coast off Benghazi, Libya. Oceanogr Fish Open Access J. 2019; 10(1): 555778. DOI: 10.19080/OFOAJ.2019.10.555778
Abstract
The reproductive biology of Common Pandora (Pagellus erthrinus) collected from the coast off Benghazi, Libya were investigated. A total of 225 specimens were collected throughout a year of 2011-2012. The sex ratio was 0.22: 0.13: 0.65 for males to hermaphrodites to females. First maturation size for males were (24.5 cm), for hermaphrodite were (25.0 cm), and for females were (22.0 cm). In general, GSI in females group were higher than that found in males group. For the fecundity, the highest means values were recorded in April (244344 ± 104158) and in October (222794 ± 73720). Nevertheless, the lowest fecundity values were recorded in February (4699 ± 6356) and in September (15504 ± 20055).
Keywords: Common Pandora (Pagellus erthrinus), Reproductive, Benghazi, Libya.
Introduction
Understanding the reproductive biology of fish is the most important feature to provide a scientific suggestion for fisheries management and fish culture. Common Pandora Pagellus erythrinus is one of the most important commercial fish species in Libyan fishery production. It is an omnivorous species, but feed mostly as carnivorous. It is usually distributed in the Mediterranean Sea and along the European and African coasts of the Atlantic Ocean [1,2]. Moreover, P. erythrinus is a protogynous hermaphrodite, that is matures at first as a female and changes to a male after two years of age or wherever attaining a body length of 17–18 cm [3,4]. Furthermore, it spawns from late spring to late summer on northern part of Mediterranean, at depths of 60 – 800 m, wherever the temperature of water is approximately 16–21 °C [5-7]. Consequently, the current study aimed to understanding some of the aspects of the reproductive biology of Pagellus erythrinus collected from coast off Benghazi, Libya
Material and Methods
A total of 225 samples of Pagellus erythrinus (Mean total length 21.73 ± 2.57 cm and mean body weight 138.75 ± 54.30 g) were monthly collected from December 2011 until November 2012 off the coast of Benghazi, Libya using gill nets with 40mm stretched mesh size. The total body length and body weight were measured. Twenty scales were removed from different place of fish body to determine fish age. Fish was dissected and gonads were removed and weighted. The sex was determined by gonads morphology. The sex ratio (SR) was expressed as a percentage and determined according to Hossucu & Cakir, [8] by the formula:
The Gonado-Somatic Index (GSI) were determined according to Micale & Perdichizzi, [9] by the fallowing equation:
Fecundity was estimated for each maturing ovary by counting all ripening eggs. Eggs were separated and put in normal saline solution (0.9 % Na Cl) for 24 hours and counted under light microscope at magnification of 40X. Fecundity was calculated according to Gaikwad et al. [10] by the fallowing equation:
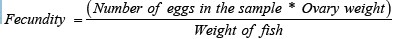
Oocytes were collected randomly from each ovary (left and right) and eggs diameter were measured using ocular micrometer using light microscope at 100X.
Results
Sex Ratio
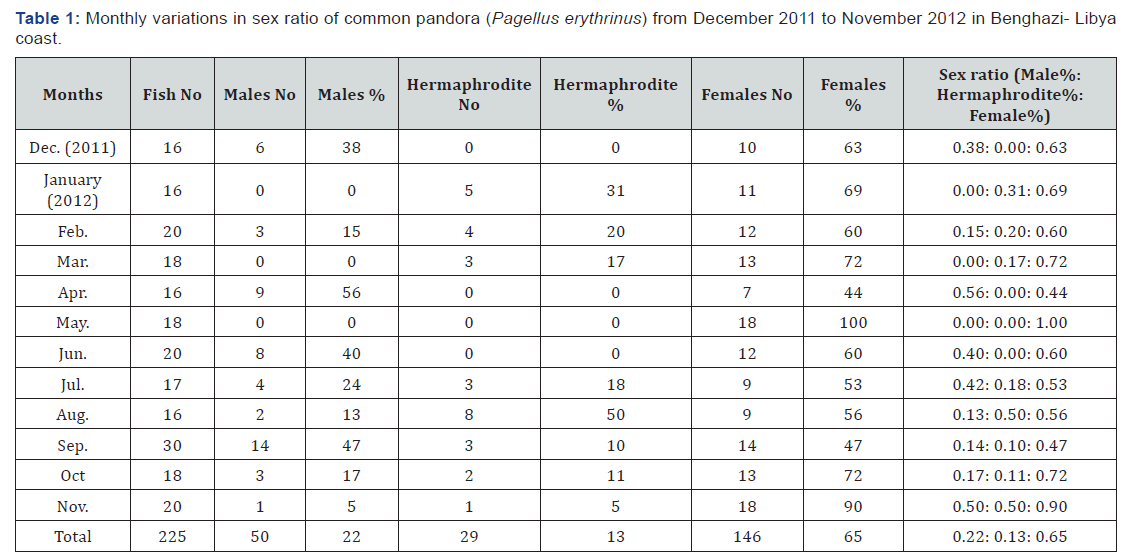
The overall sex ratio was 0.22: 0.13: 0.65 for males to hermaphrodites to females (Table 1). Furthermore, the sex ratio of male to female was (1: 2.92) and male to hermaphrodites was (1: 0.58). The sex ratio was not regular during the different months and the number of males was smaller than the sex ratio of males in all months except in April. the sex ratio of males was larger than females. However, the sex ratio of hermaphrodite group was smaller than the sex ratio that in females’ group, but larger than males’ group in January, February, March and August. In general, the maximum percentage of males was recorded in April (56 %), but in females found in May (100 %) and in hermaphrodites the maximum percentage was in August (50 %).
Length at the first Sexual Maturity
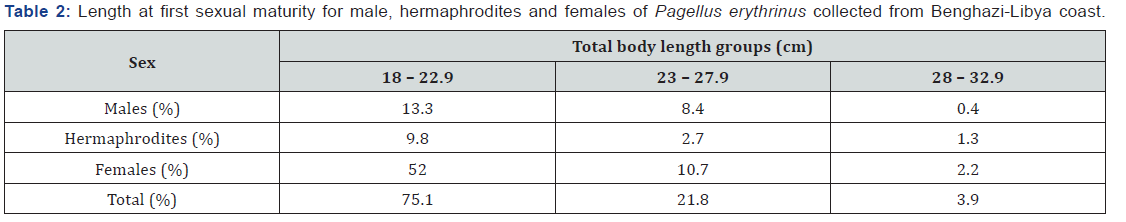
The percentage of sexes at different total body length for males, hermaphrodites and females showed in Table 2. First maturation size for males were (24.5 cm), for hermaphrodite were (25.0 cm), and for females were (22.0 cm) (Table 2).
Sex at Different Ages
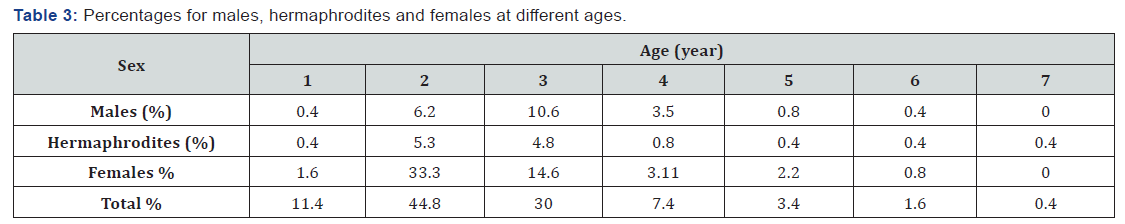
The percentages for males, hermaphrodites and females at different ages are showed in Table 3. For all groups the percentages at different ages were 11.4% at 1st year, 44.8% at 2nd year, 30.0% at 3rd year, 7.4% at 4th year, 3.4% at 5th year, 1.6% at 6th year and 0.4% at 7th year. Furthermore, females’ group were youngest in mean age (Table 3).
Gonado-Somatic Index (GSI)
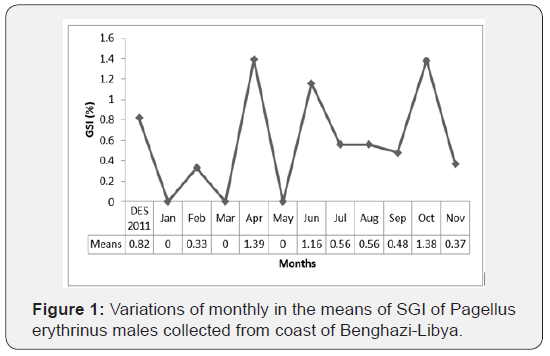
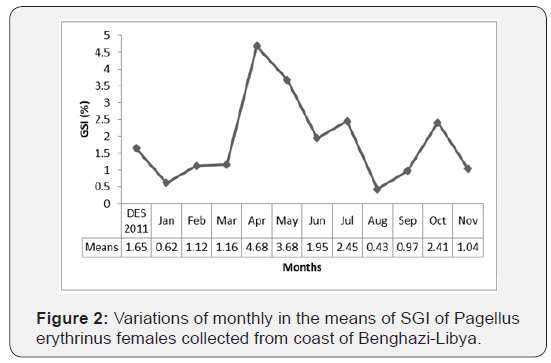
The monthly variation of the GSI are shown in Figures 1 & 2. For all groups (males and females) of P. erytherinus, the highest mean values of GSI observed in April, however, the lowest mean values have been found in August. In general, GSI in females’ group were higher than that found in males’ group. In males, GSI increased in April (1.39 ± 0.30) and June (1.16 ± 0.45) afterward started to decrease in July (0.56 ± 0.12) to September (0.48 ± 0.30) but returned to increase in October (1.38 ± 0.70). Although, in females’ group, GSI start increasing from February (1.12 ± 0.51) to July (2.45 ± 0.15) but decline in August (0.43 ± 0.20) and returned to increase in October (2.41 ± 1.32), then decreased in November (1.04 ± 0.43).
Fecundity and Eggs Diameters
٭ = The fecundity was difficult to measure
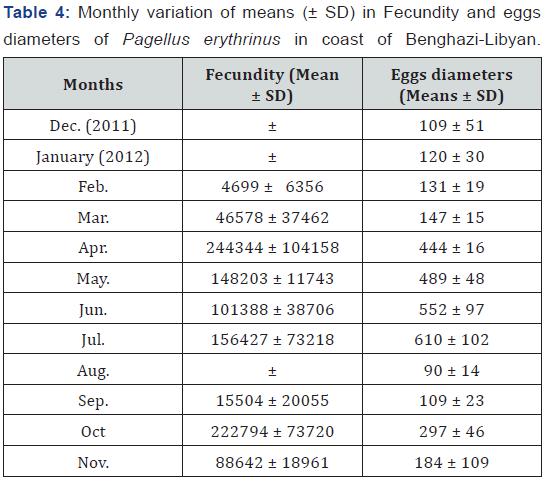
Monthly variation of means (± SD) for fecundity and eggs diameter of Pagellus erythrinus showed in Table 4. For the fecundity, the highest means values were recorded in April (244344 ± 104158) and in October (222794 ± 73720). Nevertheless, the lowest fecundity values were recorded in February (4699 ± 6356) and in September (15504 ± 20055). Furthermore, significantly differences (p < 0.05) were found between months, except between April and October and between May and July were no significantly differences (p > 0.05). For the egg’s diameters, the highest mean values were found in July (610 μm ± 102). However, the lowest mean value was found in August (90 μm ± 14) (Table 4).
Discussion
The common pandora is a demersal fish belonging to the Actinopterygii, sparidae family. This fish is extensively distributed in continental shelf of the Mediterranean Sea. Therefore, the aims of this present study were to investigate some features of the reproductive biology of common pandora Pagellus erythrinus collected from Benghazi, Libya coast. The common pandora is a protogynous hermaphrodite, that is matures first as female and changes to male after two years of age or when attaining a body length of 17-18 cm [11]. The sex ratio is an important parameter for understanding reproductive pattern and fish culture [4]. In the current study the sex ratio of males to females of common pandora was 1:2.92, and these results are like results of common pandora in Al-Khoma, Libyan coast. Hossucu & Cakir, [8] resulted that, the sex ratio of common pandaora females to males was 1:3.16. The sex ratio is not regular thought the diverse months, mainly through the reproduction season of each fish species [12]. The sex ratio of males to females was low, this could connect to protogynous hermaphroditism. Furthermore, the females are heavy and obtain caught in the gear in great number, consequential in an unhinged sex ratio. In the current study, the size of maturation for males were 22.34 cm and for females were 21.36 cm. These results are like results have recorded by Pajuelo & Lorenzo, [5] and Sweelem, [13]. This result indicated that, males of common pandora longer than females. These could be related to sex changes from male to female and temperature of water [7,4].
In the current study, the GSI values of females were higher than those of males, and this like study by Hossucu and Cakir (8) and Mtin et al., [4] in the same species from different body water and geographical location. The highest values of GSI were during spring for whole samples, but reproductive season of common pandora happening mostly in the late spring and early summer with a peak in spawning activity in April and May in Coast of Benghazi. This result confirmed with results of Valdes [14] and results of Sweelem [13] on common pandora. The spawning period of common pandora is differ in deferent study area. For examples, the reproductive season of common pandora in Canary Island extended from April to September, with a peak in spawning activity in June and July, and in Portugal extends from March to July, with a peak during May to June. The present study showed that the highest fecundity was through spawning season (from April to July and October). Also, eggs diameters were gradually increased toward spawning season. This result confirmed with results of Yueh & Chang [15] on black porgy (Acanthopagrus schlegeli). The raise in diameters of egg could be related to the deposition of large quantity of proteins and lipids that arrives from food and water temperature through the developing eggs [1,12].
References
- Klimogianni A, Koumoundouros G, Kaspiris P and Kentouri M (2004) Effect of temperature on the egg and yolk-sac larval development of common pandora Pagellus erythrinus. Marine Biology 145(5): 1015-1022.
- Froese R and Pauly D (2010) Fish Base.
- Klaoudatos SD, Lakovopoulos G and klaoudatos DS (2004) Pagellus erythrinus (common pandora): a promising candidate species for enlarging the diversity of aquaculture production. Aquaculture International 12(3): 299-320.
- Mentin G, Llkaz AT, Soykan O and Kinacigil HT (2011) Biological characteristics of the common pandora, Pagellus erythrinus (Linnaeus, 1758), in the central Aegean Sea. Turkish Journal of Zooology 35(3): 307-315.
- Pajuelo G and Lorenzo M (1998) Population biology of the common pandora Pagellus erythrinus (Pisces: Sparidae) of the Canary Islands. Fisheries Research 36(2-3): 75-86.
- Somarakis S and Machias A (2002) Age, growth and bathymetric distribution of red pandora on the Cretan shelf (eastern Mediterranean). Journal of Marine Biology Association of UK 82(1): 149-160.
- Coelho R, Bentes L, Correia C, Goncalves JMS, Lino PG, et al. (2010) Life history of the common pandora, Pagellus erythrinus (L, 1758) (actinopterygii: sparidae) from southern Portugal. Brazilian Journal of Oceanography 58(3): 233-245.
- Hossucu B and Cakir DT (2003) Some parameters about population biology of the common pandora (Pagellus erythrinus, L, 1758) (Sparidae) in the Edremit Bay (Turkey). Journal of Fisheries and Aquatic Sciences 20(3-4): 329-336.
- Micale V and Perdichizzi F (1990) Gonadal responsiveness to photoperiod extension in captivity born Sparus aurata (L.) during the male phase. Bolletino di Zoologia 57(1): 21-26.
- Gaikwad MV, More VR, Shingare SM, Hiwarale DK and Khillare YK (2009) Study on Gonado-Somatic and Fecundity relationship in air breathing fish Ghanna gachua (Ham) from Godavari near Aurangabad. African Journal of Basic and Applide Sciences 1(5-6): 93-95.
- Klaoudatos SD and klaoudatos DS (2004) Brood stok formation of the hermaphrodite finfish species Pagellus erythrinus (common pandora) from fish reared in captivity. Mediterranean Marine Science 5 (1): 5-17.
- El-Mor M, Moftah SAM, Abdalafid YKA and Abdulnabi BM (2016) Some aspects of reproductive physiology of the Flathead grey mullet Mugil cephalus (Linnaeus, 1758) in Benghazi coast, eastern Libya. International Journal of Bioassays 5(4): 4996-4999.
- Swelem MA (2010) Biological study on common pandora (Pagellus erythrinus) at Al-Khoms coast, Libya. Faculty of Science, El-Marghib University.
- Valdes P, Garcia-Alcazar A, Abdel I, Arizcan M, Suarez C (2004) Seasonal changes on gonadosomatic index and maturation stages in common pandora Pagellus erythrinus (L.). Aquaculture International 12(4-5): 333-343.
- Yueh W and Chang C (2000) Morphological changes and competence of maturing Oocytes in the Portandrous Black Porgy, Acanthopagrus schlegeli. Zoological Studies 39(2): 114-122.






























