Effect of Selection for Increasing Body Weight at Harvest on Immune Response Parameters in Second Generation of Nile Tilapia (Oreochromis Niloticus)
Emad M Zidan1 and Samy Y El-Zaeem2*
11Faculty of Veterinary Medicine, Alexandria University, Egypt
22Animal and Fish Production Department, Alexandria University, Egypt
Submission: May 08, 2019; Published:May 28, 2019
*Correspondence author: Samy Y El-Zaeem, Animal and Fish Production Department, Faculty of Agriculture (Saba-Basha), Alexandria University, Egypt
How to cite this article:Emad M Zidan, Samy Y El-Zaeem. Effect of Selection for Increasing Body Weight at Harvest on Immune Response Parameters in Second Generation of Nile Tilapia (Oreochromis Niloticus). Oceanogr Fish Open Access J. 2019; 10(1): 555777. DOI: 10.19080/OFOAJ.2019.10.555777
Abstract
This work was conducted to investigate the effect of selection for increased body weight on immune response parameters in second generation of Nile tilapia, Oreochromis niloticus by collecting eight fish from selected full-sib families and eight fish from control breed line. Blood samples were collected from the fish caudal peduncle of the two groups, and immune response parameters were measured as total protein, albumin, and globulin, ALT, AST, Urea, and creatinine, lysozyme activity, bactericidal activity, total lipid, trigylcerol, total cholesterol, and hematological parameters. The results revealed that the immune response parameters were higher in selected breed line than control breed line and this concluded that selection for increased body weight improved immune response and functions which play a role in disease resistance.
Keywords: Selection; Body weight; Immune; Oreochromis niloticus
Introduction
Selection for improved immunity in applied breeding programs may decrease mortality rates in fish farming. The basis for selection is genetic difference in improved quantitative traits such as immunity and disease resistance [1]. Therefore, the value of selective breeding depends on the level of genetic difference to a disease within the population in question, being most advantageous when the variation is relatively large [2]. Selection may be done directly based on survival records under farming conditions, in challenge tests or indirectly using correlated traits as indicators. As the immune system is mostly responsible for cover against disease, immunological markers are being used as indirect measures of disease resistance.
The markers showed wide genetic difference, correlate with disease resistance as well as being simple and quick to be quantified from large populations of live fish [3]. Both physiological and biochemical mechanisms conferring resistance to micro parasites can have a strong genetic basis [4]. Previous work showed that potential correlation exists between disease resistance of fish and nonspecific immune parameters viz., serum lysozyme, complement, hemolytic, and bactericidal activities, which affect inherent capacity of fish to resist pathogens prior to generation of a specific immune response [5-9].The objective of the present investigation was to determine the specfic and non specific immune parameters between selected and non-selected breed line of the second generation of full-sib Nile tilapia, Oreochromis niloticus.
Material and Methods
Fish Origin and Culture Condition
Nile tilapia used in this work was the second generation of selection program for growth. Sixteen fish were randomly collected from tanks (circular fiber glass tanks with capacity of 500 liter) and each tank represents a full-sib family. Sixteen full-sib families were used. Eight fish were collected from selected line, and the average body weight was 35 ± 1.5. Also, eight fish were collected from control line, and their body weights were 28 ± 1.1 g. These tanks were in laboratory at faculty of Veterinary Medicine, Alexandria University at period from 30 September 2015 to 30 May 2016. At the end of the experiment, blood samples were collected from the fish caudal peduncle of the two groups and the following parameters were measured.
Serum Samples
Samples were collected without anti-coagulant. Samples were kept at room temperature for 15-20 min, in order to clot then the clot is loosened from the wall and the tubes are placed in the incubator for half an hour to enhance retraction of the clot, then put in the refrigerator at 4C overnight, and the sera were separated by centrifugation. The serum samples were kept in deep freezer at 20-30C until being used [10].
Clinico-Biochemical Analysis
At the end of the experiment, blood samples were collected from the fish caudal peduncle of the different groups. Adequate amounts of whole blood in small plastic vials containing heparin were used for the determination of hemoglobin (Hb) by using commercial kits (Diamond Diagnostic, Egypt) and the hematocrit (PCV %) was measured according to Stoskopf [11]. Also, total erythrocytes (RBCs), platelets and total leukocytes (WBCs) were counted according to Dacie & Lewis [12] on an Ao Bright – Line Haemocytometer model (Neubauer improved, Precicolor HBG, Germany). Other blood samples were collected and transferred for centrifugation at 3500 rpm for 15 min to obtain blood plasma for determination of total protein (TP) [13]; albumin (Al) [14]; globulin (Gl) by difference [15] and total cholesterol [16] which were assayed following commercial test kits using a spectrophotometer (model 5010, Germany).
Determination of (S. GOT) and (S. GPT)
The activity of serum aspartate aminotransferase (S. AST), normally known as glutamic oxalacetic transaminase (S. GOT), and serum alanine aminotransferase (S. ALT), usually known as glutamic pyruvic transaminase (S. GPT), were estimated according to Bohnert et al., [17] using commercial kits made by Pasteur Lab. Estimation of serum alkaline phosphatase: Serum alkaline phosphatase was determine according to modified method of Aoki et al., [18] using commercial kits produced by Pasteur Lab. Lysozyme activity and Serum bactericidal activity: Serum lysozyme activity was estimated through the turbidimetry described by Hultmark et al., [19]. Serum bactericidal activity to Aeromonas hydrophila strain (Standard strain ATCC obtained from Dept. Avian and aquatic Animal Med., Fac. Med. Alexandria University) was determined according to Rainger and Rowley [20].
Determination of IgA, IgG and IgM
Immunoglobulin an ELISA Kit (Saliva) - Salimetrics Assays, 1-1602. Chicken IgG ELISA Cat. No. KT-619 PRODUCT the K-ASSAY Ò Chicken IgG ELISA is an enzyme immunoassay for the quantitative determination of IgG in chicken serum plasma. Chicken IgM ELISA Cat. No. KT-621PRODUCT the K-ASSAY Ò Chicken IgM ELISA is an enzyme immunoassay for the quantitative determination of IgM in chicken serum plasma. IgM was measured according to Ohta et al. [21], Nevens et al. [22] & Wendelborn et al. [23].
Total Cholesterol and Triglycerides Determination
Cholesterol was measured enzymatically in serum or plasma in a series of coupled reactions that hydrolyze cholesteryl esters and oxidize the 3-OH group of cholesterol. One of the reaction byproducts, H2O2 was measured quantitatively in a peroxidase catalyzed reaction that produces a color. Absorbance was measured at 500 nm. Triglycerides was measured enzymatically in serum or plasma using a series of coupled reactions in which triglycerides are hydrolyzed to produce glycerol. Glycerol is then oxidized using glycerol oxidase, and H2O2, one of the reaction products, was measured as described above for cholesterol. Absorbance is measured at 500 nm. High density lipoprotein (HDL) cholesterol Low serum concentrations of HDL-cholesterol was associated with increased risk for CHD. Coronary risk increases markedly as the HDL concentration decreases from 40- to 30 mg/dL. A low HDL-cholesterol concentration was a value below 35 mg/dL, and high HDL, >60 mg/dL. HDL-cholesterol values were also used in the calculation of LDL-cholesterol Low density lipoprotein (LDL) cholesterol: Most of the circulating cholesterol was found in three major lipoprotein fractions: very low-density lipoproteins (VLDL), LDL and HDL. [Total chol] = [VLDL-chol] + [LDL-chol] + [HDL-chol]. LDL-cholesterol was calculated from measured values of total cholesterol, triglycerides and HDL-cholesterol according to the relationship: [LDL-chol] = [total chol] - [HDL-chol] - [TG] /5. (Bachorik and Albers 1986). Where [TG]/5 estimated of VLDLcholesterol and all values are expressed in mg/dL.
Statistical Analysis
Data were analyzed by Student’s “t” test to compare the different parameters using SPSS (2010) statistical package version. The data values were presented as mean ± standard error of mean (SEM).
Results and Discussion
Mean values of immune parameters were compared between selected bred line and non-selected bred line of the second generation of Nile tilapia, Oreochromis niloticus. The highest means of total protein, albumin, globulin, alpha, beta, gama, ALT, AST, Total lipid, Triglycerol, cholesterol, HDL, LDL, Lysozyme activity, Bactericidal activity, IgA, IgG, IgM, TWBCS, TRBCS, HB, PCV, Lymphocytes, monocytes, Basophil, Eosinophil, Neutrophil, and Heterophil, showed significant superiority for selected bred line compared to those of non-selected bred line of Nile tilapia. While, the means of urea, creatinine, and Alkaline phosphate showed significant decrease for selected bred line compared to non-selected bred line (Table1).
Experimental selection for increasing body weight had a large and significant effect on immune functions. The results of selection for increased immune functions are mixed, however. Significant overall effect was found. Pinard-Van Der Laan [24] found no consistent difference between the arms of the immune system, either when comparing the effects of selection for growth on different immune system components, or when comparing the effects of selection for growth on different components of the immune system. This is not unexpected because the effectiveness of different arms of the immune system need not be genetically correlated. However, the effects of selection for growth were consistent with respect to the aspect of immune function that was measured, while the variation in growth response to immune function selection was independent of the type of immune response that was selected. Variation in innate immune response was examined for fish collected randomly from tanks. The selected fish were obtained from the second generation of selection program of Nile tilapia for growth showed significant superiority compared to non-selected bred line.
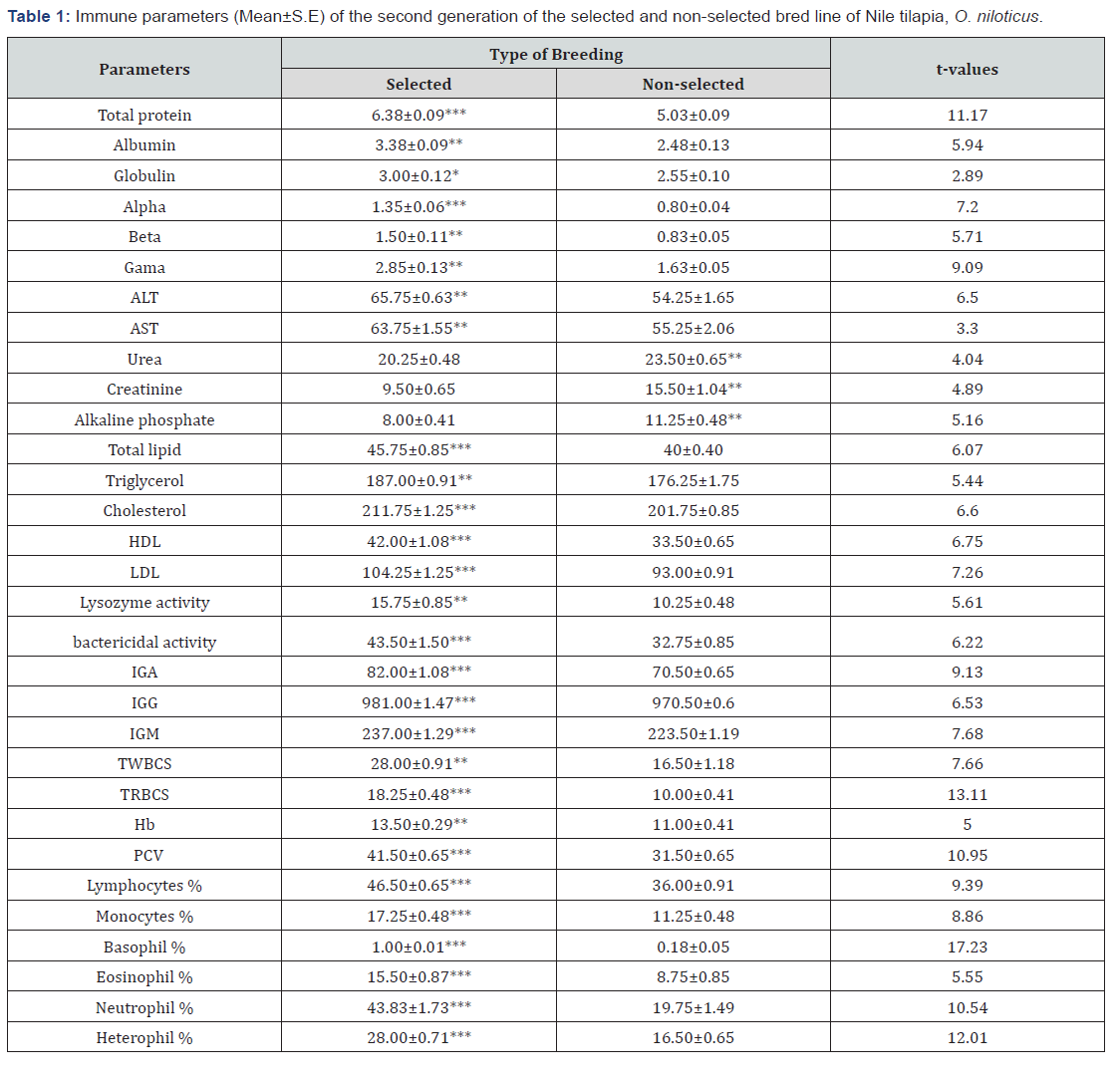
* Significant at (P < 0.05), ** Significant at (P < 0.01), *** Significant at (P < 0.001)
El-Zaeem [25], reported that serum total protein and globulin of genetically modified Tilapia zillii were significantly enhanced compared to non-genetically modified fish. Moreover El-Zaeem & Assem [26] and Assem & El-Zaeem [27], stated that serum total protein, globulin and IgM of both genetically modified Oreochromis niloticus and Tilapia zillii were improved significantly compared to non-genetically modified fish. The results of the present work are consistent with these findings. RBC and WBC levels were enhanced, and there was also variation in Hb and PCV levels between selected breed line and control breed line. The amounts of hemoglobin and hematocrit are a function of RBC changes [28]. Previous studies have also reported that fish selected for increased body weight show increased WBC and RBC levels and the cellular innate immune response is enhanced [29]. Selected breed line fish show a significant increase in cholesterol and triglyceride levels compared to non-selected breed line. In the contrary Wang et al., [30] reported declining pattern of triglycerides and total serum cholesterol levels in tilapia fed with feed containing Bacillus cereus. However, levels of total protein were significantly higher in selected breed line fish compared to the control breed line fish
In general, an increase in serum protein level is an indicator of innate immunity, which is considered important in invertebrates and a fundamental defense mechanism of fish [31]. Such mechanisms form a series of essential functions that keep host cells alive, healthy, and protected from pathogens [32]. The two liver enzymes of ALT and AST increased in selected breed line fish group compared to the control line. This is in accordance with the results of Lin & Luo [33]. Among the innate immune parameters, lysozyme activity was found to rise significantly in the selected fish compared to the control. Similar results were observed in the study of Mohapatra et al., Bandyopadhyay et al., [34,35]. Lysozyme is found in fish mucus, serum and ova, which helps in degrading peptidoglycan layer of the cell wall of bacteria. It also promotes phagocytosis by activitating polymorphonuclear leucocytes and macrophages. The relatively large variation observed in the levels of lysozyme in selected and control bred line reflects the variation in their ability to destory bacteria. Such variation in lysozyme levels was also observed in Atlantic salmon and rainbow trout by Roed et al., [5,7] selected for disease resistance.
The enhancement of immune response parameters with selection breeding program decreased mortality rate in Nile tilapia there by protecting the fish against different diseases by increasing the immunity of fish. For our analysis we found usable data for fish selection lines, but data from other taxa do indicate that selection for production traits had adverse effects on metabolic, reproduction and health traits [36]. Furthermore, productivity and immune function were negatively associated in sheep and pigs [37,38]. Studies involving commercial poultry lines were excluded from our analyses because the selection criteria are unknown and control lines are absent, but their results support our findings. Modern commercial broilers had a decreased humoral response compared with older, lighter lines [39]. Furthermore, differences reported between broiler and layer chicken lines are consistent with our results. A comparative study between a broiler and a layer line suggested that broilers are more specialized in mounting strong short-term humoral immune responses, while layers employ in a long-term humoral immune and cellular response
The effect of selection for immune function on growth differed between selection lines, while there was no significant overall effect. This contrasts with the effects of selection for growth on immune function, which were uniform across selection lines and immune traits. There are several possible explanations for the difference in results between selection for growth or immune function, which are not mutually exclusive. First, the costs of growth are likely to be high in comparison to immune function [40]. Therefore, when growth is selected for, there is likely to be less leeway when compared with selection for a less resource demanding process such as immune function, and this asymmetry may cause the different selection effect. The potential effect of an increase in immune function on growth is substantially smaller than the potential effect of an increase in growth on immune function, which may explain why selection for growth affected immune function while selection for immune function did not affect growth. Secondly, because selection was for single immune traits rather than for a more comprehensive measure of immune function. It is possible that the selection success was achieved at the expense of other arms of the immune system, or through the selection of a more specific response. In this way it is possible that selecting for an increase in components of immune function did not result in an increase in the resource allocation to all the immune function, and hence no effect on growth [41-44].
Conclusion
In selection for increased body weight of Nile tilapia at harvest increased immune response parameters in selected breed line than non-selected breed line, and this play important role in disease resistance and improve immunity against diseases
References
- Fjalestad KT, Carr WH, Lotz J, Sweeney JN and Gjedrem T (1999) Genetic variation and selection response in body weight and disease resistance in Pacific white shrimp (Penaeus vannamei). Aquaculture 204: 198.
- Marsden M J, Freeman L C, Cox D and Secombes CJ (1996) Nonspecific immune responses in families of Atlantic salmon, exhibiting differential resistance to furunculosis. Aquaculture 146: 1-16.
- Lund T, Gjedrem T, Bentsen HB, Eide DM, Larsen HJS, et al. (1995) Genetic variation in immune parameters and association to survival in Atlantic salmon. J Fish Biol 46: 748-758.
- Chevassus B and Dorson M (1990) Genetics of resistance to disease in fishes. Aquaculture 85: 83-107.
- Roed KH, Larsen HJS, Linder RD and Refstie T (1993a) Genetic variation in lysozyme activity in rainbow trout. Aquaculture 109: 237-244.
- Roed KH, Brun E, Larsen HJ and Refstie T, (1990) The genetic influence on serum hemolytic activity in rainbow trout. Aquaculture 85: 109-117.
- Roed KH, Fjalestad KT and Stromsheim A (1993b) Genetic variation in lysozyme activity and spontaneous hemolytic activity in Atlantic salmon. Aquaculture 114: 19-31.
- Roed KH, Brun E, Larsen H J and Refstie T (1992) Genetic variation in serum hemolytic activity in Atlantic salmon. Fish Biol 40: 739-750.
- Sarder MRI, Thompson KD, Penman DJ and MacAndrew BJ (2001) Immune responses in Nile tilapia clones: Non- specific responses. Dev. Comp Immunol 25(1): 37-46.
- Lied E, Gezerde Z and Braskhan DR (1986) Simple and rapid technique for repeated blood sampling in Rainbow trout. J of Fish Res Board of Canada 32 (5): 699-701.
- Stoskopf MK (1993) Fish Medicine. In: WB Saunders Comp, Philadelphia, US.
- Dacie JV and Lewis SM (1995) Practical Haematology. In: 8th (edn.), Churchill Livingston, Scotland, US.
- Gornall AG, Bardawill GJ and Parid MM (1949) Method of determination protein in serum blood. J Biol Chem 177: 751.
- Weichsebum TE (1946) Method for determination of albumin in serum blood. Amer J Clin Pathol 16-40.
- Doumas BT and Biggs HG (1972) Determination of serum albumin. Standard Method of Clinical Chemistry 7: 175-188.
- Ellefson RD and Caraway WT (1976) Fundamentals of clinical chemistry. In: Tietz NW (ed.), p. 506.
- Bohnert M, Baumgartner R and Pollak S (2000) Spectrophotometric evaluation of the colour of intra- and subcutaneous bruises. Int J Legal Med 113(6): 343-384.
- Aoki T, Arai T and Egusa S (1977) Detection of R plasmids in naturally occurring fish-pathogenic bacteria, Enterobactereacea. Microbial Immunol 21 (2): 77-83.
- Hultmark D, Engström A, Andersson K, Steiner H, Bennich H, Boman H G (1983) Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. The EMBO Journal 2(4): 571-576.
- Rainger G E and Rowley A F (1993) Antibacterial activity in the serum and mucus of rainbow trout, Oncorhynchus mykiss, following immunisation with Aeromonas salmonicida. Fish & Shellfish Immunology 3(6): 475-482.
- Ohta M, Okada M, Yamashina I, Kawasaki T (1990) The Mechanism ofcarbohydrate-mediated complement activation by the serummannan-binding protein. J Biol Chem 265: 1980-1984.
- Nevens JR, Mallia AK, Wendt MW, Smith PK (1992) Affinitychromatographic purification of immunoglobulin M antibodies utilizingimmobilized mannan binding protein. J Chrom 597(1-2): 247-256.
- Wendelborn LA, Sommer CV, Larson CS, Nevens JR (1992) Purification of fish IgM using mannan-binding protein affinitychromatography. Poster presentation at the Autumn ImmunologyConference, Chicago, USA.
- Pinard-van der Laan MH (2002) Immune modulation: the genetic approach. Veterinary Immunology and Immunopathology 87: 199-205.
- El-Zaeem SY (2001) Breeding studies in Tilapia, Faculty of Agriculture (Saba-Basha), Alexandria University, Egypt.
- El-Zaeem SY, Assem SS (2004) Application of Biotechnology in fish breeding: II. production of highly immune genetically modified Nile Tilapia, Oreochromis niloticus with accelerated growth by direct injection of shark DNA into skeletal muscles. Egypt J Aquat Biol Fish 8(3): 67-92.
- Assem SS, El-Zaeem SY (2005) Application of biotechnology in fish breeding: II. Production of highly immune genetically modified redbelly tilapia, Tilapia zillii. Afr J Biotechnol 5: 449-459.
- Soltanzadeh S, Esmaeili Fereidouni A, Ouraji H (2016) Growth performance, body composition, hematological, and serum biochemical responses of beluga (Huso huso) juveniles to different dietary inclusion levels of faba bean (Vicia faba) meal. Aquacult Int 24(1): 395-413.
- Kumar R, Mukherjee SC, Prasad KP and Pal AK (2006) Evaluation of Bacillus subtilis as a probiotic to Indian major carp Labeo rohita (Ham.). Aquaculture Res 37: 1215-1221.
- Wang M, Liu G, Lu M, Ke X, Liu Z, Gao F, et al. (2016) Effect of Bacillus cereus as a water or feed additive on the gut microbiota and immunological parameters of Nile tilapia. Aquac Res 48(6): 3163-3173.
- Ellis AE (1990) Lysozyme assays. In: JS Stolen, TC Fletcher, DP Anderson, BS Roberson and Van WB Muiswinkel (eds.) Techniques in fish Immunology, SOS Publications, Bangladesh pp. 101-103.
- Kamgar M and Ghane M (2012) Evaluation of Bacillus subtilis effect as probiotic on Hematological parameters of rainbow Trout, Oncorhynchus mykiss (INaIbaum) following experimental infection with Streptococcus iniae. J Fish Aquat Sci 7: 422-430.
- Lin S and Luo L (2011) Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus × O. aureus. Anim Feed Sci Technol, 168(1): 80-87.
- Mohapatra S, Chakraborty T, Prusty AK, PaniPrasad K and Mohanta KN (2014) Beneficial Effects of Dietary Probiotics Mixture on Hemato-Immunology and Cell Apoptosis of Labeo rohita Fingerlings Reared at Higher Water Temperatures. PLoS ONE 9(6): e100929.
- Bandyopadhyay P, Sarkar B, Mahanty A, Rathore RM and BC Patra (2015) Dietary Administered Bacillus sp. PP9 Enhances Growth, Nutrition and Immunity in Cirrhinus mrigala (Hamilton). Proc Natl Acad Sci India Sect B Biol Sci 85(3): 759-766.
- Rauw WM, Kanis E, Noordhuizen-Stassen EN and Grommers FJ (1998) Undesirable side effects of selection for high production efficiency in farm animals: a review. Livestock Production Science 56: 15-33.
- Clapperton M, Glass EJ and Bishop SC (2008) Pig peripheral blood mononuclear leucocyte subsets are heritable and genetically correlated with performance. Animal 2(11): 1575-1584.
- Greer AW (2008) Trade-offs and benefits: implications of promoting a strong immunity to gastrointestinal parasites in sheep. Parasite Immunology 30(2): 123-132.
- Kramer J, Visscher AH, Wagenaar JA, Cornelissen J and Jeurissen SHM (2003) Comparison of natural resistance in seven genetic groups of meat-type chicken. British Poultry Science 44: 577-585.
- Klasing KC (1998) Nutritional modulation of resistance to infectious diseases. Poultry Science 77: 1119-1125.
- Wijga S, Parmentier HK, Nieuwland MGB and Bovenhuis H (2009) Genetic parameters for levels of natural antibodies in chicken lines divergently selected for specific antibody response. Poultry Science 88: 1805-1810.
- Bachorik PS, Albers JJ (1986) Precipitation methods for quantification of lipoproteins. Methods Enzymol 129: 78-100.
- Diab AS, John G, Abd El Hady Y and Fathy M (2004) Evaluation of Nigella Sativa L. (Black Seeds, Barka), Allium Sativum (Garlic) & Blogen as feed additives in Fish cluture O. niloticus. SCMJ 2: 557-562.
- FAO (2005) Aquaculture production, Yearbook of Fishery Statistics. Food and Agriculture organization of the United Nations, Rome, Italy.






























