Growth and Hematology of Juvenile Piaractus Mesopotamicus Stocked At 10 Up To 40kg/m3 For Twenty-One Days
Raul Machado-Neto*, Wiolene Montanari Nordi, Mariana Caroline Furian Pontin, Jéssica Pampolini and Débora Botéquio Moretti
Animal Science Department, University of São Paulo, Brazil
Submission: April 04, 2018; Published: August 02, 2018
*Correspondence author: Machado-Neto R, Animal Science Department, Luiz de Queiroz College of Agriculture, University of São Paulo, Av. Pádua Dias 11, 13418-260, Piracicaba, Brazil.
How to cite this article: R Machado-Neto, Wiolene M N, Mariana C F P, Jéssica P, Débora B M. Growth and Hematology of Juvenile Piaractus Mesopotamicus Stocked At 10 Up To 40kg/m3 For Twenty-One Days. Oceanogr Fish Open Access J. 2018; 8(2): 555733. DOI:10.19080/OFOAJ.2018.08.555733
Abstract
Pacu Piaractus mesopotamicus, a freshwater fish, has great potential for intensive commercial production, showing resistance to adverse conditions, such as high stocking density. Growth performance and hematological condition were evaluated in juvenile pacu stocked at high density. Juveniles were distributed into different stocking densities: 10, 20, 30 and 40kg of fish/m3 (n=4) and fed with a commercial feed for 21 days. Growth data were collected at 0 and 21 days and blood samples every 7 days. Differences were not detected in performance of pacu under different stocking densities (P > 0.05). Feed conversion rate (3.9±0.5) and specific growth rate (4.0±0.1%/ day) were worse than normally observed at this stage of life, indicating an unsuitable condition. Hematocrit of juveniles stocked at 30 and 40kg/m3 showed higher values than ones at 10kg/m3. Thus, stocking density from 10 up to 40kg/m3 did not affect growth and hematology of pacu after 21 days. However, the low growth rates indicate that all stocking densities could have determined an adverse condition to fish. Considering the high stocking density used in the present work and the intensive management without mortality, we suggest that pacu is very resistant and a promising species for fisheries.
Keywords: Teleost; Omnivorous; Performance; Red blood cells indices
Introduction
Intensive fish farming involves a great challenge for the producer, that is, the concentration of large populations of animals within confined areas that increases predisposition to bacterial and parasitic diseases [1,2]. This problem is especially critical in the early larval stages of teleost, since in this period they depend on maternal components, such as nutritional and immunological factors, for their survival. According to Falcon et al. [3] and Baldwin [4] (2010), handling, biometry, vaccination and stocking density in the intensive culture system induce changes in the physiological responses interfering with health, behavior status, adaptation and welfare of fish determining situations of constant stress. Falcon et al. [3] also point out that the change in temperature may be one of the main causes of losses in production due to the drop in resistance and greater susceptibility to diseases.
Stressful conditions determine the release of cortisol and the consequent depression of the immune system, making fish more susceptible to infectious diseases [5]. The understanding of the causative factors and inhibitors of stress make possible the development of strategies that attenuate this physiological condition.
The influence of stocking density in fish growth parameters has not had an explicit adverse trend in some fish species, showing contradictory results. While S. senegalensis exhibits satisfactory growth rate at high stocking density, survival and development were not affected in Siganus rivulatus and were compromised in Oreochromis niloticus [6-8]. Although the occurrence of chronic stress caused by high stocking densities can depress the immune system, dietary supplementation represents a valuable tool to neutralize the oxidative damages in freshwater fish [9,10]. The levels of lipids and some vitamins, such as vitamins E and C, and minerals in the diet also influence antioxidant defenses and the oxidative state of fish [11]. The main features of pacu that endorse it as a commercial species are related to its high adaptability to rearing in ponds and fish nurseries, resistance to pathogens and low demands on water quality, resulting in a species of high rusticity [12]. Thus, the objective of the present work was to evaluate the effects of high stocking density of 10 up to 40kg/m3 for twenty-one days in the performance and hematological parameters of juvenile pacu.
Material and Methods
Experimental procedures
Plastic blue cages of 40 l were placed inside tank with continuous aeration and water recirculating system with controlled conditions (temperature 25.4±0.2 ºC; pH 7.9±0.2, dissolved oxygen 4.0±0.5mg l-1; dissolved ammonia:<0.05mg> l-1), seeking to guarantee the same condition for all juveniles. Farm-raised, feed-conditioned juvenile pacu (120.2±7.5g and 14.6±0.4cm) were randomly distributed into 16 cages with four different stocking densities (10, 20, 30 and 40kg of fish per m3, 4 cages per stocking density). Juveniles were handfed to apparent satiety twice a day (08h 30 and16h 30) with commercial diet (Agromix 3.3mm: 360g kg-1 of CP, 68g kg-1 of fat, 110g kg-1 of minerals and 35g kg-1 of fiber) for 21 days. Growth data was collected in the beginning and end of experiment period for performance evaluation and blood samples every 7 days (3 collections) for hematological and biochemistry analysis. Juvenile pacu were kept, maintained and treated according to accepted standards for the humane treatment of animals (ESALQ/USP ethics committee).
Growth performance
Juveniles were anesthetized with benzocaine (0.05g l-1), measured and weighed at the beginning and end of the experimental period. The following parameters were evaluated:
I. Relative weight gain - RWG (%) = 100 x (final biomass weight - initial biomass weight)/initial biomass weight;
II. Feed conversion ratio – FCR = feed intake per cage (g) / WG (g);
III. Specific growth rate – SGR (% day-1) = 100 x [(ln final biomass weight - ln initial biomass weight) days-1].
Hematological and biochemistry parameters
Blood samples were collected by puncture of the caudal vessel using syringes previously moistened with disodium ethylenediaminetetraacetic acid (Na2EDTA) solution (3%). Red blood cell indices (RBC) were evaluated following the procedures described by Ranzani-Paiva et al. [13].
Blood aliquots were diluted (1:200) in sodium formaldehyde solution and red cells counted in Neubauer’s chamber under an optical microscope. The result was expressed as number of erythrocyte μl-1. The hematocrit value was determined by the microhematocrit procedure and expressed as a percentage of red cells in relation to whole blood. The hemoglobin (Hb) was determined by the hemoglobin cyanide method (HiCN), a colorimetric endpoint reaction system and the results were expressed as g dl-1. The red blood cell indices mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were determined according to Wintrobe [14] by the following calculations:
a. MCV = (Hematocrit x 10) erythrocyte-1
b. MCH (pg) = (Hemoglobin x 10) erythrocyte-1
c. MCHC (g dl-1) = (Hemoglobin x 100) hematocrit-1
Plasma glucose was determined by enzyme-colorimetric test using glucose oxidase, which catalyzes the oxidation of glucose into glycolic acid and hydrogen peroxide. Total protein was determined in samples of blood collected without anticoagulant by the biuret test.
Statistical analyses
Statistical analysis was performed using the SAS (v 9.1) program package (SAS Institute, Cary, NC, USA). The performance data was analyzed based on a completely randomized design, considering different stocking densities (10, 20 30 and 40Kg/ m3) the main effects. The hematological variables were analyzed as a repeated measure-over-time design, considering stocking density, 10, 20, 30 and 40kg/m3 as main effects. The cage effect was considered random, and the other effects were considered fixed in the model. After the data was confirmed for normal distribution with the Shapiro-Wilk test, a variance analysis was performed using the PROC MIXED (SAS Institute Inc., 2008). If the F value was significant, the Tukey test was used for multiple comparisons between pairs of means at 5% of probability.
Results
Growth performance
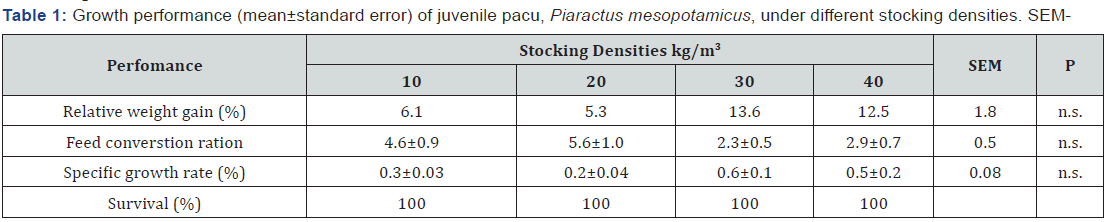
standard error mean; n.s. – non-significant, P > 0.05.
The different stocking densities did not induce differences in growth performance of juvenile pacu (P>0.05), Table 1. Independent of stocking density, the overall mean of RWG, FCR and SGR were 9.6±1.8 %, 3.9±0.5 and 0.4±0.1%.
Hematological parameters
Only hematocrit value was influenced by different stocking density (P<0.05>), Table 2. Juvenile pacu maintained at 30 and 40kg/m3 showed higher hematocrit value than juveniles maintained at 10kg/m3. Effect of sampling time was observed for all variable analyzed (P<0.05>), except for VCM.
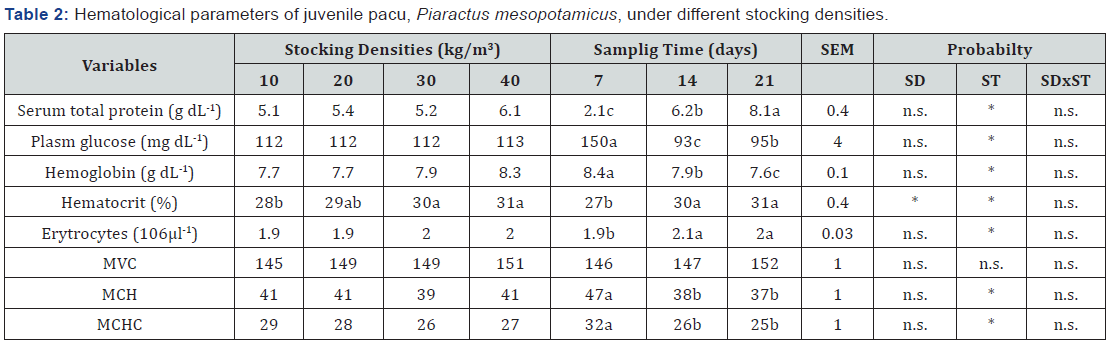
SEM- standard error mean; SD – stocking density effect; ST – sampling time effect; SDxST – interaction between SD and ST. n.s. – non significant, P>0.05; * significant, P<0.05> using Tukey’s test.
Discussion
Intensive culture is a cost-effective production system; however, high stocking density can trigger chronic stress in fish, since there is competition for food and swimming movements are limited. Considering performance and animal health, the ideal capacity of stocking needs to be adequately determined, since each species presents different responses in the same stocking density. In the present work, pacu had unaffected growth parameters under different stocking densities. In Siganus rivulatus, stocking density at 5kg/m3 also did not affect growth of juveniles [6]. In Oreochromis niloticus, in turn, the stocking density at 13kg (m3)-1 decreased development and survival [8]. Bittencourt et al. [15] observed that stocking densities at 5.6; 8.5; 11.36kg/m3 do not influence erythrocyte parameters, glucose level or carcass yields of Piaractus mesopotamicus; however, density of 5.6kg/m3 results in higher final weight. Di Maggio et al. [16] suggest that densities of up to 0.5 fish l-1 (4.70kg/m3 appear to be conducive to the culture of juvenile Orthopristis chrysoptera. The above authors worked with densities much lower than in the present study. Merola & Souza [17] however, suggest that standing crop values of 45- 50kg/m3 might be approximating a critical level for growth of Piaractus mesopotamicus. Sadhu et al. [18] in turn, observed that fish stocked at 35m3 had significant decrease in growth and final mean weight compared to ones stocked at 14m3. Although parameters were not influenced by different stocking density, the present juveniles showed FCR and SGR (3.9±0.5 and 0.4±0.1% day-1, respectively) worse that normally observed for the species at this stage of life and much lower than expected for the period, indicating an unsuitable condition for juveniles. Machado-Neto et al. [19] observed better rates of relative weight gain and feed conversion (1246% and 1.2, respectively) in juvenile pacu with initial weight of 8.5±0.7g fed twice daily for 60 days with diet containing 320g kg-1 of crude protein and 21g MJ-1 of energy. Bicudo et al. [20], in turn, observed a FCR and SGR (% day-1) around 1.05 and 2.2, respectively, after 70 experimental days when dietary protein requirement of pacu juveniles are met. Tesser et al. [21], in turn, restricting the supply of diets to 2.5% of biomass per day, observed in pacu juveniles (initial weight of 4.3±0.1g) a FCR and SGR (% day-1) of 0.60 and 3.53, respectively.
Considering hematological parameters, only hematocrit value was influenced by different stocking density, with juvenile pacu maintained at 30 and 40kg/m3 showing higher hematocrit value than juveniles maintained at 10kg/m3. Hemoconcentration caused by stress raises hematocrit values in several freshwater species [22]. The elevated hematocrit values will aid in blood oxygenation and overcoming stress [23]. Urbinati et al. [1] evaluating loading and transport stress of juvenile matrinxã (Brycon cephalus, Characidae) at various densities, observed higher hematocrit values after loading the fish. The authors suggest that higher hematocrit values after loading is due to swelling of red blood cells.
Thus, in the present work, considering the experimental conditions used in this work, the stocking density from 10 up to 40kg/m3 did not affect growth and hematology of juvenile pacu, Piaractus mesopotamicus, for 21 days. However, the low growth rates indicate that all stocking densities could have determined an adverse condition to fish. Considering the high stocking density used in the present work and the intensive management, collecting blood every week, without mortality, we suggest that P. mesopotamicus is very resistant and a promising species for fisheries.
Acknowledgment
Authors are indebted to “Fundacão de Amparo à Pesquisa do Estado de São Paulo” (São Paulo State Research Foundation – FAPESP 2014/14937-7) and “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (Brazil National Council of Scientific and Technological Development - CNPq), for funding the research project yielding this publication and to Fish Culture Section, ESALQ/USP.
References
- Urbinati EC, Carneiro PCF (2004) Práticas de manejo e estresse dos peixes em piscicultura. In: Cyrino JEP, Urbinati EC, Fracalossi DM, Castagnolli N (Eds.), Tópicos especiais em piscicultura de água doce tropical intensiva. TecArt, São Paulo, Brazil, 6: 171-193.
- Urbinati EC, Gonçalves FD (2010) Pacu (Piaractus mesopotamicus). In: Baldisserotto B, Gomes LC (Eds.), Espécies nativas para a piscicultura no Brasil. UFSM, Rio Grande do Sul, Brazil, pp. 205-244.
- Falcon DR, Barros MM, Pezzato LE, Solarte WVN, Guimarães IG (2008) Leucograma da tilápia do nilo arraçoada com dietas suplementadas com níveis de vitamina C e lipídio e submetidas a estresse por baixa temperature. Cien Anim Bras 9(3): 543-551.
- Baldwin L (2010) The effects of stocking density on fish welfare. The Plymouth Student Scientist 4(1): 372-383.
- Diniz NM, Honorato CA (2012) Algumas alternativas para diminuir os efeitos do estresse em peixes de cultivo. Arq Ciênc Vet Zool 15(2): 149- 154.
- Saoud PI, Ghanawi J, Lebbos N (2008) Effects of stocking density on the survival, growth, size variation and condition index of juvenile rabbitfish Siganus rivulatus. Aquacult Int 16: 109-116.
- Salas-Leiton E, Anguis V, Martín-Antonio B, Crespo D, Planas JV, et al. (2010) Effects of stocking density and feed ration on growth and gene expression in the Senegalese sole (Solea senegalensis): Potential effects on the immune response. Fish Shellfish Immunol 28(2): 296- 302.
- Costa ÂAP, Roubach R, Dallago BSL, Bueno GW, McManus C, et al. (2017) Influence of stocking density on growth performance and welfare of juvenile tilapia (Oreochromis niloticus) in cages. Arq Bras Med Vet Zootec 69(1): 243-251.
- Belo MAA, de Moraes FR, Yoshida L, Prado EJR, Moraes JRE, et al. (2014) Deleterious effects of low level of vitamin E and high stocking density on the hematology response of pacus, during chronic inflammatory reaction. Aquac 422-423: 124-128.
- Maciel ECS, Feitosa KCO, Corrêa Neto CR, Macedo FF, Mattioli WO, et al. (2013) Desempenho produtivo e parâmetros fisiológicos de juvenis de pacu criados em tanques-rede em diferentes densidades de estocagem (Performance and physiological parameters of juvenile pacu reared in cages at different stocking densities) Rev Bras Saúde Prod Anim 14(1): 185-194.
- Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: Biotic and abiotic factors. Rev Fish Biol Fish 15(1-2): 75-88.
- Jomori RK, Carneiro DJ, Martins MIEG, Portella MC (2005) Economic evaluation of Piaractus mesopotamicus juvenile production in different rearing systems. Aquaculture 243(1-4): 175-183.
- Ranzani-Paiva MJT, Pádua SB, Tavares-Dias M, Egami MI (2013) Métodos para análise hematológica em peixes. EdUEM, Maringá, Brazil, pp. 135.
- Wintrobe MM (1934) Variations in the size and hemoglobin content of erythrocytes in the blood of various vertebrates. Folia haematol 51: 32-49.
- Bittencourt F, Feiden A, Signor AA, Boscolo WR, Lorenz EK, (2010) Densidade de estocagem e parâmetros eritrocitários de pacus criados em tanques- rede. R Bras Zootec 39(2): 2323-2329.
- DiMaggio MA, Ohs CL, Broach JS (2014) Effects of Stocking Density on Growth, Survival, and Stress Physiology of Pigfish. N Am J Aquac 76: 201-210.
- Merola N, Souza JH (1988) Preliminary Studies on the Culture of the Pacu, Colossoma mitrei, in Floating Cages: Effect of Stocking Density and Feeding Rate on Growth Performance. Aquac 68(3): 243-248.
- Sadhu N, Sharma SRK, Dube PN, Joseph S, Philipose KK (2015) First results of culture of Asian seabass (Lates calcarifer, Bloch) in open sea floating net cages in India: Effect of stocking density on survival and growth. Indian J Mar Sci 44: 1540-1544.
- Machado-Neto R, Moretti DB, Nordi WM, Cruz TMP, Cyrino JEP (2016) Growth performance of juvenile pacu (Piaractus mesopotamicus) and dourado (Salminus brasiliensis) fed with lyophilized bovine colostrum. Aquac Res 47(11): 3551-3555.
- Bicudo AJA, Sado RY, Cyrino JEP (2010) Growth performance and body composition of pacu Piaractus mesopotamicus (Holmberg 1887) in response to dietary protein and energy levels. Aquac Nut 16(2): 213– 222.
- Tesser MB, Terjesen BF, Zhang Y, Portella MC, Dabrowski K (2005) Free- and peptide-based dietary arginine supplementation for the South American fish pacu (Piaractus mesopotamicus). Aquac Nut 11(6): 443-453.
- McDonald G, Milligan L (1997) Ionic, osmotic and acid – base regulation in stress. In: Iwama GW, Pickering AD, Sumpter JP, Schreck CB (Eds.), Fish stress and Health in Aquaculture. University Press, Cambridge, England, pp. 119-144.
- Patterson SM, Matthews KA, Allen MT, Owens JF (1995) Stress-induced hemoconcentration of blood cells and lipids in healthy women during acute psychological stress. Health Psychol 14(4): 319-324.






























