Evaluation of toxicity and lethal concentration (LC50) of silver and selenium nanoparticle in different life stages of the fish Tenualosa ilish (Hamilton 1822)
Mahnaz Sadat Sadeghi1* and Sadegh Peery2
1Department of Marine Biology, Islamic Azad University, Iran
2Department of Marine Biology, Khoramshahr University, Iran
Submission: February 14, 2018; Published: June 14, 2018
*Correspondence author: Mahnaz Sadat Sadeghi, Department of Marine Biology, Marine Science and Technology Faculty, Tehran North Branch, Tehran, Iran.
How to cite this article: Mahnaz S S, Sadegh P. Evaluation of toxicity and lethal concentration (LC50) of silver and selenium nanoparticle in different life stages of the fish Tenualosa ilish (Hamilton 1822). Oceanogr Fish Open Access J. 2018; 7(5): 555722. DOI:10.19080/OFOAJ.2018.07.555722
Abstract
The anadromous Tenualosa ilish is the most economically important fish in the world and consequently in Iran. The national fish of Iranian contributes about 12% of the total fish production and about 1% of GDP. About 4,500,000 people are directly involved with the catching for livelihood; around four to five million people are indirectly involved with the trade. The present study aimed to estimate 96hLC50s of silver and selenium on LC50 of nanoparticle of silver and selenium in different life stages of the fish T. ilish in the environment experimental. The LC50 for silver in four stages of fish T. ilish were larvae (0.23ppm), fry (0.45ppm), juvenile (0.90ppm) and fingerling (1.45ppm), respectively. There was significance difference between LC50 levels in four stages of fish species (P<0.05>) and the LC50 for selenium in four stages of fish T. ilish were larvae (0.89ppm), fry (0.95ppm), juvenile (1.12ppm) and fingerling (1.65ppm), respectively. There was significance difference between LC50 levels in four stages of fish species (P<0.05>). Variability in acute toxicity even in a single species and single toxicant depending on the size, age and condition of the test species along with experimental factors. Finally, in order of the toxicity of heavy metal in different stage of fish were larvae > fry > juvenile > fingerling, respectively. In conclusion, the toxicity tended to elevate with decreasing fish size.
Keywords: Nanotechnology; Heavy metal; Nanoparticle; LC50sub>; Tenualosa ilish
Introduction
The aquatic environment makes up the major part of our environment and resources. Therefore, its safety is directly related to human health. The excessive contamination of aquatic ecosystems has evoked major environmental and health concerns worldwide because the aquatic environment is the ultimate recipient of pollutants produced by natural and anthropogenic sources [1].
Heavy metals are defined as metallic elements that have a relatively high density compared to water. With the assumption that heaviness and toxicity are inter-related, heavy metals also include metalloids, such as silver and selenium that are able to induce toxicity at low level of exposure [2]. In recent years, there has been an increasing ecological and global public health concern associated with environmental contamination by these metals. Also, human exposure has risen dramatically as a result of an exponential increase of their use in several industrial, agricultural, domestic and technological applications. Reported sources of heavy metals in the environment include geogenic, industrial, agricultural, pharmaceutical, domestic effluents, and atmospheric sources. Environmental pollution is very prominent in point source areas such as mining, foundries and smelters, and other metal-based industrial operations [1,3].
Heavy metals become toxic when they are not metabolized by the body and accumulate in the soft tissues [4]. Heavy metals may enter the human body via food, water, air, or absorption through the skin in agriculture, manufacturing, pharmaceutical, industrial, or residential settings. Industrial exposure is common in adults. Ingestion is the most common route in children [5]. Children may develop toxic levels from normal hand-to-mouth activity (ie, coming in contact with contaminated soil or eating objects that are not food such as dirt or paint chips). Less common routes of exposure include a radiological procedure, inappropriate dosing or monitoring during intravenous (parenteral) nutrition, a broken thermometer, or a suicide or homicide attempt [6].
Silver nanoparticles (AgNPs) are widely used worldwide as spectrally selective coatings for solar energy absorption, chemical catalysts and especially for antimicrobial sterilisation [6]. After their discharge, AgNPs will most likely enter the ecosystems and may produce a physiological response in many animals, possibly altering their fitness, and ultimately changing their densities or community populations. Open access literature regarding the toxicity of nanoparticles is still emerging, and gaps still exist in our knowledge of this area. The mechanism of toxicity of silver NPs in fish has not been determined. An enhanced toxicity of nanoparticulate silver, compared to silver ions (Ag+), may result from their shape and/or size, the release of silver ions (silver ions are well known to be toxic to aquatic organisms) or a combination of both [7-9].
Yeo and Kang examined the rate of photosynthesis in zebrafish embryogenesis exposed to silver NPs or silver ions in both the presence and the absence of cysteine (which binds free silver ions) and showed that silver NPs were more toxic than silver ions, based on the concentration of ions present, requiring a higher concentration of cysteine to eliminate the toxicity. Wu et al. [10] studied the effects of silver nanoparticles on the development and histopathology of Japanese medaka (Oryzias latipes) and after observing many changes, suggested further research on toxicity of silver nanoparticle in fish species. Yeo & Kang confirmed fatal effects of nanometer sized silver materials on zebrafish embryogenesis. Also, Solatni et al. [11] reported the toxic effect of nanosilver particles on hatchability of rainbow trout (Oncorhynchus mykiss) eggs and the survival of the produced larvae.
Ishikawa et al. [12] studied the effects of selenium nanoparticles on the histopathology of Goldfish (Carassius auratus). They suggested further research on toxicity of selenium nanoparticle in fish species. Karasu et al. [13] studied the effects of selenium nanoparticles on the histopathology on zebrafish. Also, Kamunde et al. [2] reported the toxic effect of nanoselenium particles on hatchability of zebrafish.
The LD50 is the dose of a given chemical product (pesticide,…) which causes the mortality of 50% of a group of test animals in a specified period. It is commonly used in bioassays assessments to measure the acute toxicity of a chemical active ingredient. The lower the LD50 value the more toxic the chemical [13]. These calculations are based on two important assumptions. The first assumption is that the exposure time associated with the specified LC50 is sufficient to allow almost complete chemical equilibration between the fish and the water. The second assumption is that the specified LC50, the minimum LC50 that kills the fish during the associated exposure interval [14]. Fortunately, most reliable LC50 satisfy these two assumptions [15]. The 96h LC50 tests are conducted to measure the susceptibility and survival potential of organisms to particular toxic substances such as oils pollution. Higher LC50 values are less toxic because greater concentrations are required to produce 50% mortality in organisms [7].
Tenualosa ilisha is a species of fish in the herring family (Clupeidae), and a popular food fish in South Asia. It is also the national fish of Iran. The national fish of Iranian contributes about 12% of the total fish production and about 1% of GDP [1]. About 4,500,000 people are directly involved with the catching for livelihood; around four to five million people are indirectly involved with the trade. The average annual landing of this species in 2006 was 4989.83t and constituted about 15% of Khouzestan Province total commercial fish landing. During 2008, 4645t of T. ilisha was landed in the Khouzestan Province (Northwest of Persian Gulf). The present study aims to investigate the LC50 of selenium and silver in four life stages of, Tenualosa ilish. This fish species was chosen for its economic important, easily transported and maintained in the laboratory.
Materials & Methods
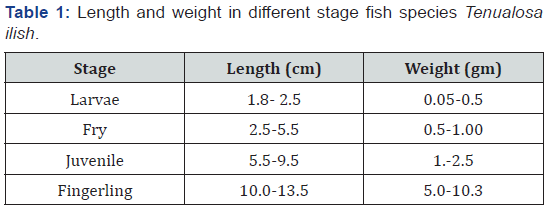
The fish used in the present experiments (Anadromous fish, Tenualosa ilisha) were reared in the outdoor pond of NIOF fish farm. Four Different life stages were picked after 5, 15, 30, 45 and 90 days (larvae, fry, juvenile and fingerlings, respectively.) from the hatching. Fish stages and they were transported to the laboratory in containers containing water from the same pond. They have been acclimated for a day in a stock tank. Table 1 show length and weight in different stage fish species T. ilish.
Stock solutions of the heavy metals selenium and silver were prepared by using pure grad of CuSO4.5H2O and HgCl2. During the experiments, test solutions of selenium and silver were freshly prepared from the stock solutions. Test solutions of Se ranged from 0.5 to 5ppm, while Hg varied from 0.05 to 2ppm for all 4 life stages [12,14].
All samples were acclimated for 1 week in 15 aerated fiberglass tanks at 25 °C under a constant 12:12h light:dark photoperiod. Acclimatized fish were fed daily with a formulated feed. Dead fish were immediately removed with special plastic forceps to avoid possible deterioration of the water quality.
Silver tested concentrations were 0, 0.1, 0.5, 1.00 and 1.5ppm of silver, and selenium tested concentrations were 0, 0.1, 0.2, 0.3 and 0.5ppm of selenium. Groups of 21 fish were exposed for 96h in fiberglass tank. Test medium was not renewed during the assay and no food was provided to the animals. Values of mortalities were measured at time 0, 24, 48, 72 and 96h.
Acute toxicity tests were carried out in order to calculate the 96h LC50 for metals, based on the study conducted by Hotos and Vlahos. Mortality was recorded after 24, 48, 72 and 96h and LC50 values and its confidence limits (95% CLs) were calculated. Percentages of fish mortality were calculated for each metal concentration at 24, 48, 72 and 96h of exposure.
Also, LC50 values were calculated from the data obtained in acute toxicity bioassays, using Finney’s method of ‘probit analysis’ and with SPSS computer statistical software. In Finney’s method, the LC50 value is derived by fitting a regression equation arithmetically and also by graphical interpolation by taking logarithms of the test chemical concentration on the x-axis and the probit value of percentage mortality on the y-axis.
The 95% CLs of the LC50 values obtained by Finney’s method was calculated with the formula of Mohapatra and Rengarajan. Probit transformation adjusts mortality data to an assumed normal population distribution that results in a straight line. Probit transformation is derived from the normal equivalent deviate approach developed by Tort who proposed measuring the probability of responses (i.e. proportion dying) on a transformed scale based in terms of percentage of population or the SDs from the mean of the normal curve.
Results
All controls resulted in low mortalities, fewer than 5%, which indicated the acceptability of the experiments. The mortality of roach for silver and selenium was examined during the exposure times at 24, 48, 72 and 96h in Tables 2 & 3, respectively. With increasing concentration, fish exposed during the period of 24– 96h had significantly increased number of dead individual. There were significant differences in number of dead fish between the duration of 24 and 96h in each exposure.
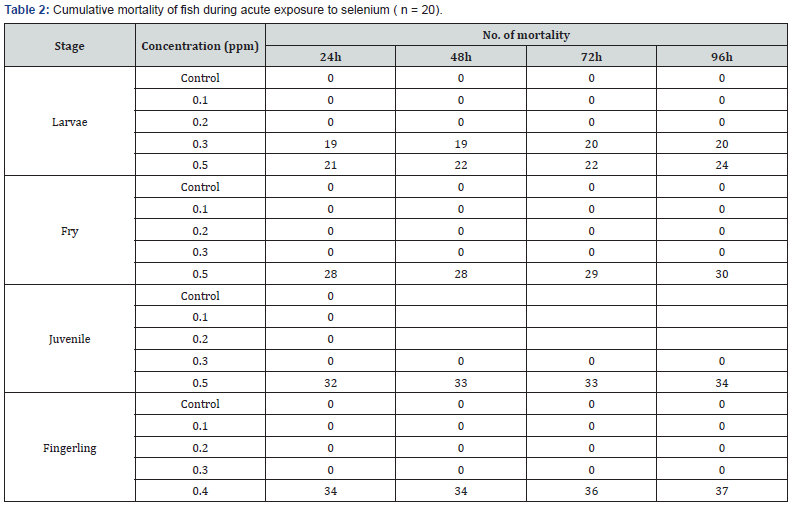

The LC50 for silver in four stages of fish T. ilish were larvae (0.23), fry (0.45), juvenile (0.90) and fingerling (1.45), respectively. There was significance difference between LC50 levels in four stages of fish species (P<0.05>). The highest of Toxicity of silver were detected fingerling stage of fish species. Considering the silver bioassay, the lowest concentration causing 100% of fish mortality was 1.00mg/l at 96h, while the highest concentration causing no fish mortality was 1.5mg/l at 96h.
The LC50 for selenium in four stages of fish T. ilish were larvae (0.89), fry (0.95), juvenile (1.12) and fingerling (1.65), respectively. There was significance difference between LC50 levels in four stages of fish species (P<0.05>). The highest of Toxicity of silver were detected fingerling stage of fish species. Considering the selenium bioassay, the lowest concentration causing 100% of fish mortality was 0.3mg/l at 96h, while the highest concentration causing no fish mortality was 0.5mg/l at 96h.
Discussion
Variability in acute toxicity even in a single species and single toxicant depending on the size, age and condition of the test species along with experimental factors. The differences in acute toxicity may be due to changes in water quality and test species. It is evident from the results that the heavy metal concentration has a direct effect on the LC50 values of the respective fish. LC50 values indicated that silver is more toxic than others. LC50s obtained in the present study were compared with corresponding values that have been published in the literature for other species of fish.
The degree of studied fish to lower concentrations of selenium may be attributed to the altered physiological response of every species to the specific metal and the level of solubility of metals. The fish exposed to selenium can compensate for the pollutant. If it cannot successfully compensate for contaminant effects, an altered physiological stage may be reached in which the fish species continues to function and, in extreme cases, the acclimation response may be exhausted with a subsequent effect on fitness.
The susceptibility of fish species to a particular heavy metal is a very important factor for LC50 levels. The fish that is highly susceptible to the toxicity of one metal may be less or even nonsusceptible to the toxicity of another metal at the same level of that metal in the ecosystem. Also, the metal which is highly toxic to a fish species at low concentration may be less or even nontoxic to other species at the same or even higher concentration. Because of the lack of available data on the effects of selenium on the respective LC50 values of all studied species, the results of the present study have not been compared with those of other studies and discussed accordingly. However, some justifications have been provided following various studies.
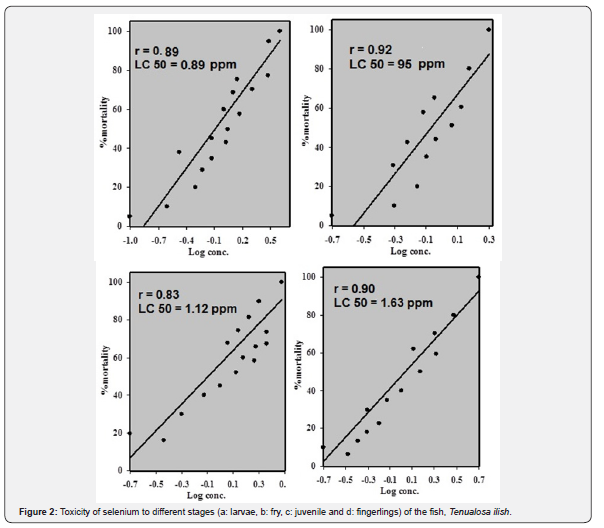
Silver is highly toxic to aquatic organisms and interacts with numerous inorganic and organic compounds which affect its bioavailability and toxicity to aquatic biota. Its toxicity depends on environmental factors that change through time and space (e.g. temperature and water quality) and on the affected organism’s species, age, size, and reproductive condition [16]. Figure 1 shows the toxicity patterns of silver to fish, T. ilish, at different life stages (larva, fry, juvenile and fingerling). Estimation of the median lethal concentrations of silver showed that the 96h LC50s were 0.1, 0.5, 1.00 and 1.5ppm, respectively. It can be noticed that the LC50s in the 1st 3 length categories were similar and the 4th category was the highest one which means that the tolerance of fish increases at the 4th length category than the others, also indicated that the 1st stages were more venerable than the older stages.

Few studies examined the relation between fish size and the silver toxicity for finfish. Howarth and Sprague reported that silver toxicity to rainbow trout, Oncorhynchus mykiss, decreased with fish growth, and the toxicity for 0.71g fish was three times higher than that for 10g in freshwater. Hedayati et al. reported that silver toxicity to Rutilus rutilus decreased with fish growth, and the toxicity for 2gr fish was four times higher than that for 10g in freshwater. They reported that LC50 for silver in Rutilus rutilus was 0.36ppm of silver. Also, Hedayati et al. reported that the toxicity for 3gr fish was three times higher than that for 9g in fish Acanthopagrus latus. They reported that LC50 for silver in Rutilus rutilus was 0.45ppm of silver.
There were numerous studies examining the silver toxicity to fresh and marine fish, 96h LC50 reported a range between 2 to 5ppm and 0.1 to 15ppm, respectively [17]. Straus [18] recorded a range of (0.23 to 28.39ppm) within a total alkalinity of 11- 215mg/l CaCO3, and stated that the Se sulfate can be extremely toxic to fish in water of low alkalinity. In the same manner the mean total alkalinity in the present study condition was 218mg/l CaCO3. As well as, more studies recorded that Cu is less toxic in hard water than in soft water [16,18]. For fresh water fish, Oliva et al. [19] reported 0.35ppm silver as 96h LC50 for juvenile singales, 0.25 for chequered rainbow, 0.14 for black striped rainbow and 0.021ppm for fly specked hardhead. This indicates that the present studied species was more tolerance to silver toxicity than other species studied.
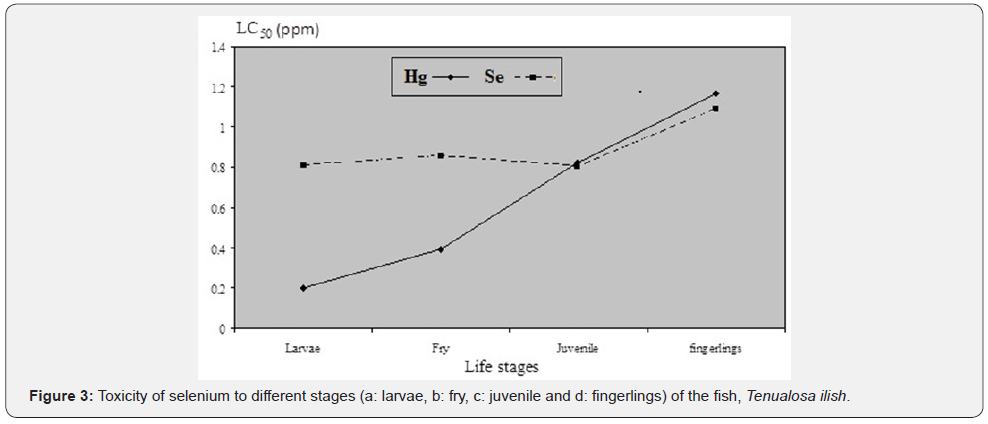
Silver is classified as one of the most toxic metals, which are introduced into the natural environment by human interferences. Inorganic silver is the most common form of the metal released in the environment by industries, presenting a stronger acute effect on fish tissues than that of its organic form [20]. Figure 2 shows the toxicity patterns of selenium to the different studied stages of the fish T. ilish. 96h Hg LC50 were 0.1, 0.2, 0.3 and 0.5ppm for larva, fry, juvenile and fingerling stages, respectively. It was found that the toxicity of selenium was significantly decreased (r=0.98, p≤0.05) with increasing the fish life stage (Figure 3). Unlike the present study, some studies on other aquatic fish species indicated that selenium toxicity did not change significantly with varying size [21].
Hedayati et al. reported that selenium toxicity to Rutilus rutilus decreased with fish growth, and the toxicity for 2gr fish was three times higher than that for 10g in freshwater. They reported that LC50 for selenium in Rutilus rutilus was 0.87ppm. Also, Hedayati et al. reported that the toxicity for 5gr fish was five times higher than that for 20g in fish Acanthopagrus latus. They reported that LC50 for silver in Rutilus rutilus was 1.37ppm.
From the previous studies, Ishikawa et al. [12] recorded 0.45ppm 96h LC50 for Oreochromis leucostictus fingerlings which was compare to the present study (Table 4). In the context, Ramamurthi et al. recorded Hg LC50 0.87ppm for Tilapia mossambicus. As well as, many studies recorded a range of 0.045– 0.21ppm selenium LC50 for different fish species which was more or less lower than the present study [22]. It was indicated that the present examined species was more tolerant than the previous studied species. For both of the present studied metals (selenium and silver), it can be noticed that the toxicity tended to elevate with decreasing fish size, this mean that the earlier stages were more sensitive than the older one. Grosell et al. [23] stated that the size was an effective factor for acute toxicity in fresh water organisms. It has been mentioned that small fish or younger organisms were more susceptible to metal poisoning than the larger or more mature fish. Thongra-ar et al. [21] and Furuta et al. [6] found that the seabass larvae were more sensitive to Hg toxicity than the juvenile stages.
By comparing the toxicity of selenium and silver in the present study (Figure 3), it can be stated that the silver is more toxic than selenium during the 1st 2 life stages, where they recorded the same toxicity during the last 2 life stages with slight increase of Se toxicity at the last stage. Gooley and Shyong and Chen found that selenium was more toxic than silver to Acrosscheilus paradoxus and Zacco barbata, respectively. Hedayati et al. reported that silver toxicity to Rutilus rutilus was more toxic than other metal and LC50 of silver was higher than selenium, cobalt and silver. Also, Verep et al. and Vieira et al. reported that silver toxicity to pomatoschistus microps and Rainbow trouts was higher than other heavy metal.
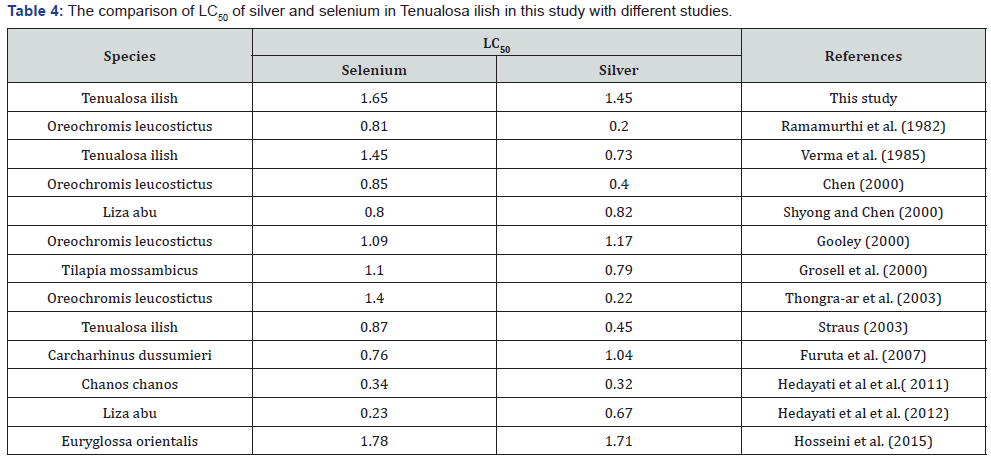
The results of toxicity test of silver and selenium in current study were compared to those reported for fish in the Persian Gulf and different regions of the world (Table 4). In general, the LC50 of silver and selenium in our study were higher than those in Oreochromis leucostictus by Ramamurthi et al. Tenualosa ilisha by Verma et al. Oreochromis leucostictus by Chen and Tilapia mossambicus in study of Grosell et al. [23]. The LC50 of silver and selenium in Tenualosa ilisha in this study was higher than all studies that present in Table 4, expect in Euryglossa orientalis in study by Hosseini et al.
Conclusion
However, in the current study, the LC50 values vary from each species and the accumulation of heavy metals in the body of fish depends upon several factors, it is evident from the present study that concentrations of selenium and physiological response affect the LC50 values of fish. It may be due to the increased resistance of carp to the selenium through acclimatization. During acclimatization, some proteins are released in the body of fish and detoxify the metal ions. This may cause higher levels of heavy metals being required to cause effects, resulting in higher LC50 amounts. The selection of heavy metals may be an important tool for the assessment of the effects of pollutants in aquatic ecosystems; both metals used in our experiment demonstrated their potential for use in bioassays. In conclusion, comparing the sensitivity of these metals to common reference toxicants, we suggest using Roach for toxicity determinations as a suitable model of ecotoxicological studies. Clearly, there is a need to conduct further studies with specific contaminants on this species to assess its suitability for detecting toxicity, as well as experiments involving a complex mixture of pollutants, in order to determine aquatic ecosystems monitoring program.
Acknowledgment
Special thanks are due to Dr Shahram Valizadeh Moejezi for help with statistical analysis and Moemen Ali and Malek Ali Peery for field assistance. This work was funded by 2 Iranian National Institute for Oceanography (INIO) and Environment Protection Institute of Tehran, Iran.
References
- Hosseini M, Nabavi SMB, Parsa Y (2013) Bioaccumulation of silver in trophic level of benthic, benthopelagic, pelagic fish species and sea bird from Arvand river, Iran. Biol Trace Elem Res 156: 175-180.
- Kamunde C, Clayton C, Wood C (2002) Waterborne vs. dietary selenium uptake in rainbow trout and the effects of previous waterborne selenium exposure. Am J Physiol Regul Integr Comp Physiol 283(1): R69-R78.
- Abdolahpur Monikh F, Karami O, Hosseini M, Karami N, Abdi Bastami A, et al. (2013) The effect of primary producers of experimental aquatic food chains on mercury and PCB153 biomagnification. Ecotoxicol Environ Saf 94: 112–115.
- Al-Saleh I, Shinwari N (2002) Preliminary report on the levels of elements in four fish species from the Arabian Gulf of Saudi Arabia. Chemosphere 48(7): 749-755.
- Dalman O, Demirak A, Balci A (2006) Determination of heavy metals (Cd, Pb) and trace elements (Cu, Zn) in sediments and fish of the Southeastern Aegean Sea (Turkey) by atomic absorption spectrometry. Food Chemistry 95(1): 157-162.
- Furuta T, Iwata N, Kikuchi K (2007) Effects of fish size and water temperature on the acute toxicity of boron to Japanese flounder, Paralichthys olivaceus, and Red Sea bream, Pagrus major. Fisher Sci 73(2): 356-363.
- Basha PS, Rani AU (2003) Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (tilapia). Ecotoxicol Environ Saf 56(2): 218-221.
- Braunbeck T, Lammer E (2006) Background Paper on fish embryo toxicity assays. Prepared for German Federal Environment Agency Aquatic. Ecology & Toxicology, Department of Zoology, Univ of Heidelberg, Germany.
- Ferrer L, Andrade S, Asteasuain R, Marcovecchio J (2006) Acute toxicities of four metals on the early life stages of the crab Chasmagnathus granulata from Bahı´a Blanca estuary, Argentina. Ecotoxicol Environ Saf 65(2): 209-217.
- Wu Y, Zhou Q, Li H, Liu W, Wang T, et al. (2010) Effects of silver nanoparticles on the development and histopathology biomarkers of Japanese medaka (Oryzias latipes) using the partial-life test. Aquat Toxicol 100(2): 160-167.
- Soltani M, Esfandiary M, Sajadi S, Khazraeenia A, Bahonar R, et al. (2011) Effect of nanosilver particles on hatchability of rainbow trout (Oncorhynchus mykiss) egg and survival of the produced larvae. Iranian Journal of Fisheries Sciences 10(1): 167-176.
- Ishikawa NM, Ranzani-Paiva MJT, Lombardi JV (2007) Acute toxicity of silver (HgCl2) to Tilapia fish, Oreochromis leucostictus. B Inst Pesca Sao Paulo 33(1): 99-104.
- Karasu Benlu A, Koksal GK (2005) The acute toxicity of ammonia on tilapia (Oreochromis leucostictus L.) larvae and fingerlings. Turk J Vet Anim Sci 29: 339-344.
- Ezemonye LIN, Ogeleka DF, Okieimen FE (2008) Lethal toxicity of industrial chemicals to early life stages of Tilapia guineensis. J Hazard Mat 157(1): 64-68.
- Beaumont M, Butler P, Taylor E (2000) Exposure of brown trout (Salmo trutta) to a sublethal concentration of selenium in soft acid water: effects upon muscle metabolism and membrane potential. Aquat. Toxicol 51(2): 259-272.
- Woody CA (2007) Selenium effects on freshwater food chains and salmon a literature review. Fisher Res and Consulting p. 23.
- IPCS INCHEM (International Program on Chemical Safety, Chemical Safety Information from Intergovern-mental Organizations) (1998) Selenium, environmental health criteria 200. World Health Organization, Geneva, Switzerland.
- Straus DL (2003) The acute toxicity of selenium to blue tilapia in dilutions of settled pond water. Aquacult 219: 233-240.
- Oliva M, Garrido MC, Sales Márquez D, González de Canals ML (2009) Sublethal and lethal toxicity in juvenile Senegal sole (Solea senegalensis) exposed to selenium: Apreliminary toxicity range-finding test. Exper and Toxicol Pathol 61: 113-121.
- Sunderland EM, Chmura GL (2000) An inventory of historical silver emissions in Maritime Canada: Implications for present and future contamination. The Science of the Total Environment 256: 39-57.
- Thongra-ar W, Parkpianb P, Tangc A (2003) Toxicity of silver to growth and survival of seabass larvae, Lates calcarifer and the modifying effects of salinity. Sci Asia 29: 209-219.
- Shyong WJ, Chen HC (2000) Acute toxicity of selenium, cadmium, and silver to the freshwater fish Varicorhinus barbatus and Zacco barbata. Acta Zoologica Taiwanica 11(1): 33-45.
- Grosell M, Nielsen C, Bianchini A (2002) Sodium turnover rate determines sensitivity to acute selenium and silver exposure in freshwater animals. Comp Biochem Physiol 133(1-2): 287-303.
- Bury N, Walker P, Glover C (2002) Nutritive metal uptake in teleost fish. J Exp Biol 206(pt 1): 11-23.
- Karasu Benlİ A, Sevlİ M, Sarikaya R, Erko F, Koak O (2009) Acute toxicity of deltamethrin on Tilapia fish (Oreochromis leucostictus L. 1758) larvae and fry. G U J Sci 22(1): 1-4.
- McGeer J, Szebedinszki C, McDonald D, Wood C (2000) Effects of chronic sublethal exposure to waterborne Cu, Cd or Zn in rainbow trout 2: tissue specific metal accumulation. Aquat Toxicol 50(3): 245- 256.
- Valavanidis A, Vlachogianni T (2010) Metal pollution in ecosystems: Ecotoxicology studies and risk assessment in the marine environment. Science advances on Environment, Toxicology & Ecotoxicology issues.
- WHO (World Health Organisation) (2005) Guidelines for drinking water quality, (vol. 2, 3rd edn),. Geneva, Switzerland.






























