Structural and Thermodynamic Properties of Tropomyosins from the Ordinary and Hybrid Tilapia
Ming-Chih Huang1*, Yoshihiro Ochiai2 and Shugo Watabe3
1Department of Biological Sciences and Technology, National University of Tainan, Taiwan
2Graduate School of Agricultural Science, Tohoku University, Japan
3Kitasato University School of Marine Biosciences, Japan
Submission: May 19, 2016; Published: June 08, 2016
*Corresponding author: Ming-Chih Huang, Department of Biological Sciences and Technology, National University of Tainan, 33. Sec 2, Shu-Lin St., Tainan, Taiwan, 700, R.O.C, Tel: +886-6-260-6123 ext 7730, Fax: +886-6-2606153; Email: mingchih39@mail.nutn.edu.tw
How to cite this article: Ming-C H, Yoshihiro O, Shugo W. Structural and Thermodynamic Properties of Tropomyosins from the Ordinary and Hybrid Tilapia. Fish & Ocean Opj. 2016; 1(1): 555555. DOI: 10.19080/OFOAJ.2016.01.555555
Abstract
Tilapia is a very common aquaculture species in Taiwan and both the so-called freshwater tilapia (Oreochromis niloticus) and seawater tilapia (O. mossambica) are available. In order to characterize muscle tropomyosin from these tilapia and hybrid ones (O. mossambica × O. niloticus ), the biochemical properties of tropomyosin among these tilapia species were analysed. The nucleotide sequences of tropomyosin genes were determined by cDNA cloning, and the amino acid sequences were deduced. Fast skeletal muscle tropomyosins were isolated by the combination of isoelectric point precipitation and salting out with ammonium sulfate. The thermodynamic properties of the purified tropomyosins were examined by differential scanning calorimetry (DSC) and circular dichroism (CD) spectrometry. The results showed that those tropomyosins were identical to each other including the deduced amino acid sequence, thermodynamic patterns as measured by DSC and CD, except the partial differences in the nucleotide sequence, namely, at 99th (A/G), 183rd (T/C) and 846th (T/C) positions in the open reading frame.
Keywords: Tilapia; Hybrid; Tropomyosin; cDNA cloning; Fast skeletal muscle; Thermal stability
Abbreviations: CD: Circular Dichroism; DSC: Differential Scanning Calorimetry; DTT: Dithiothreitol; PCR: Polymerase Chain Reaction; PVDF: Polyvinylidene Fluoride, SDS-PAGE: Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis; DCM: Dilated Cardiomyopathy
Introduction
Tilapia was first introduced to Taiwan in 1946 and has become an important aquaculture species in recent years. According to their culture environments, the fish can be categorized into “seawater tilapia” and “freshwater tilapia”. The seawater one is biologically Mossambica tilapia (Oreochromis mossambica), whereas freshwater one is Nile tilapia (O. niloticus). The hybrid tilapias have also been produced for selection breeding. The fishermen can select advantageous properties of different broodstock. Especially for tilapia, the quality or the market price depends on the characteristics of the edible portion, i.e., skeletal muscle.
The major muscle proteins in myofibrils are myosin, actin and tropomyosin. Among these muscle proteins, tropomyosin, in spite of its smallest quantities, is easy to be analyzed for the thermal stability due to its nearly homogenous structure all over the molecule. Tropomyosin belongs to a family of actin-binding proteins and is highly conserved throughout evolution [1]. It is found not only in muscle but also in non-muscle cells [2,3].
The tropomyosin molecule is made up of two subunits (each approximately 33,000 Da), and forms a coiled-coil structure consisting of two parallel α-helices [4,5]. The tropomyosin monomer, along its nearly entire molecule, contains a heptad repeat of seven amino acids, (designated abcdefg from the N-terminal side), in which generally large hydrophobic non-polar residues occur at the positions a and d, while the positions b, c, e, f and g are usually occupied by polar or ionic amino acids [2]. Tropomyosin associates with troponin and seven actin monomers on the thin filaments of striated muscle [1,6,7]. Overlapping of the molecules at the N- and C-termini makes this protein a fibrous polymer [8]. The role of tropomyosin is known in striated muscles, namely, in association with the troponin complex, it regulates the calcium sensitive interaction of actin and myosin [9,10]. Under resting intracellular calcium ion concentrations, the troponin-tropomyosin complex inhibits actin-activated myosin ATPase activity. When a stimulus induces increased concentrations of intracellular calcium ion, a conformational change is transmitted through the troponin-tropomyosin complex, releasing the inhibition of actin-activated myosin ATPase activity, resulting in muscle contraction.
As for fish skeletal muscle tropomyosin, the occurrence of isoforms has been reported [11-13], and the primary structures have been deduced for several species [14-16]. So far, some studies have revealed the stability and structural characteristics of fish muscle tropomyosins [15-18]. Tropomyosin is an excellent target protein to characterize the properties, especially the thermal stability of muscle proteins because of its simple structure and abundance in skeletal muscle. It is also of interest how this protein inherited in the hybrid, namely, hybrid tropomyosin is whether a paternal or maternal type.
In order to characterize the tropomyosin from hybrid tilapia, attempts were made to investigate tropomyosin gene and protein from freshwater, seawater and hybrid tilapia (O. mossambica × O. niloticus ). To compare the molecular relationship among the three tilapia species, we cloned the cDNA, and determined the nucleotides and deduced amino acid sequence and examined the phylogenetic relationship. Using differential scanning calorimetry (DSC) and circular dichroism (CD), their differences in thermodynamic properties of purified tropomyosins were compared.
Material and Methods
Materials
Live specimens of tilapia (the average body length 18 cm) including O. mossambica, O. niloticus and their hybrid tilapia were purchased at a Tainan traditional market and quickly killed by decapitation with a knife. Then the muscle was cut into small pieces and immediately preserved in RNAlater (ThermoFisher Scientific., Waltham, MA, USA), followed by storage at -200C until RNA preparation, as in our previous report [18]. The remaining muscle (fast skeletal muscle) was cut to 50 g blocks, packed into polyethylene bags and stored at -600C until used for protein preparations.
RNA preparation and first strand cDNAs synthesis
Total RNAs were isolated from tilapia fast skeletal muscle using ISOGEN solution (Nippon Gene, Tokyo, Japan) according to the manufacture’s protocol. First strand cDNAs were synthesized as follows. An aliquot of 2.5 μg total RNA was dissolved in 12 μL water, heated at 700C for 10 min, and quickly chilled on ice. The AP primer (5’-GGCCACGCGTCGACTAGTACT(16) -3’) was used at 50 μg/mL to initiate first strand cDNA synthesis with 0.5 U SuperScript III reverse transcriptase (ThermoFisher Sci. ) in 50 mM Tris-HCl (pH 8.3) containing 75 mM KCl, 3 mM MgCl2, 0.5 mM dNTP, and 10 mM dithiothreitol (DTT). The reaction was carried out at 420C for 50 min in a total volume of 20 μL and then the enzyme was heat-inactivated at 700C for 15 min.
Polymerase chain reaction (PCR)
PCR primers were designed based on the conserved regions of 5’ and 3’ ends, and the highly conserved regions in the amino acid sequences of several vertebrate tropomyosins [19,20].Forward primers: TMUF (5’-ATGGAYGCCATYAAGAAGAAGATG-3’) and TMF220 (5’-AAGCAAACAGCTTGAGGACG-3’), and reverse primers: TMUR (5’-TTAWATRGARGTCATGTCGTT-3’) and TMR916 (5’-TTGTACTTCAGTTTCTGGGC-3’) were used in PCR for the amplification of an expected cDNA fragment of approximately 900 nucleotides from the first strand cDNAs [17]. The PCR amplification was performed at 960C for 15 sec in denaturation, at 600C for 30 sec in annealing, and at 720C for 1 min in extension using a DNA thermal cycle model 9600 and GeneAmp kits (ThermoFisher Sci.). This procedure was carried out by 35 cycles, and the final extension step was performed at 720C for 7 min. The 100 μL of reaction medium contained 200 nM dNTPs, 50 pmol each set of forward and reverse primers, 2 units of AmpliTaq DNA polymerase (ThermoFisher Sci.), and 50 ng of first strand cDNA.
DNA and amino acid sequence analyses
The plasmid DNA extraction was performed as described by Sambrook and Russell [21]. The sequence was determined from at least three different clones encoding the open reading frame of cDNA. Sequencing was performed for both sense and antisense strands of subclones labeled with Dye Deoxy termination cycle sequencing kit, using a DNA sequencer model 373A (ThermoFisher Sci.) and open software BioEdit (http://www. mbio.ncsu.edu/bioedit/bioedit.html). A sequence homology search and calculation of molecular weight were performed using ExPASy (http://www.expasy.org/), BLAST and Compute pI/Mw tools. The amino acid sequence was deduced using open software Translate.
Phylogenetic analysis
Comparison of DNA nucleotide and deduced amino acid sequences was performed using the software Molecular Evolutionary Genetics Analysis 7 (MEGA7) (http://www. megasoftware.net/) (2013) [22]. The phylogenetic tree was constructed by the neighbor-joining method [23], using the Drawtree program Phylip package (http://bioweb.pasteur.fr/ seqanal/interfaces/drawtree.html).
The sequence data of fish muscle tropomyosins were collected from the database in National Center for Biotechnical Information (NCBI): walleye pollack Gadus chalcogrammus (GenBank accession no. BAC44994) [16], bluefin tuna Thunnus thynnus (BAD01050) [18], dogfish shark Scyliorhinus rotifer (AAK38348), zebrafish Danio rerio (AAA50021)[24], Atlantic salmon Salmo salar (AAB36559) [25], white croaker Pennahia argentata (BAB20881) [15], and tiger pufferfish Takifugu rubripes (BAC10576) [26,27], whereas the sequence of king prawn Melicertus latisulcatus tropomyosin (AGF86397) was used as an outgroup.
Preparation of tropomyosin protein
Acetone dried powder was prepared from the fast skeletal muscle as reported previously [15-18]. Purification of tropomyosin was based on the methods of Bailey [28] and Cummins and Perry [29]. Briefly, tropomyosin was extracted from the acetone dried powder with buffer (20 mM Tris- HCl, pH7.5 containing 1 M KCl and 10 mM mercaptoethanol), isoelectrically precipitated at pH 4.5 and fractionated between 50-60% ammonium sulfate saturation. The centrifugation was performed at 4°C and 20,000×g for 20 min to separate the tropomyosin precipitate from the supernatant containing contaminating proteins such as actin. Protein concentration was determined by the biuret method [30] using bovine serum albumin as the standard.
Electrophoretic methods
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli [31]. After electrophoresis, proteins were stained with 0.1% Coomassie Brilliant Blue R-250. Protein molecular weight markers (SDS6) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) consisted of isoelectric focusing for the first dimension and SDSPAGE (15% gel) for the second dimension. Immobiline dry gel strips (pH 4-7, GE Healthcare Life Sciences, Marlborough, MA, USA) were used for the isoelectric focusing.
N-terminal amino acid sequencing
Limited proteolysis of tropomyosin from three tilapia species were performed at 30oC using porcine α-chymotrypsin (Sigma-Aldrich Co.) with an enzyme-to-protein weight ratio of 1:700 (w/w). After electrophoresis, tropomyosin digests were eletroblotted in 0.1 M sodium phosphate (pH 7.0) onto polyvinylidene fluoride (PVDF) membranes. The N-terminal amino acid sequences of proteolytic fragments on the PVDF membranes were determined by the method of Matsudaira [32] using a protein sequencer (Applied Biosystems model 476A) with an on-line data processor system (model 610A).
Differential scanning calorimetry (DSC) and circular dichroism (CD) spectrometry
DSC was carried out using a differential scanning microcalorimeter (GE Healthcare Life Sciences, model VP-DSC) essentially according to Nakaya et al. [33,34]. At least three measurements were carried out for each sample. The solvent used was 10 mM sodium phosphate (pH 7.0) containing 0.1 M KCl, 1 mM EDTA, 0.01% NaN3, and 1 mM DTT. DSC scans were performed at 2.7 Pa at a temperature raising rate of 1oC/min in the temperature range from 50C to 800C. Protein concentration was 0.25 mg/mL (0.0038 mM tropomyosin). DSC data were analyzed for determination of transition temperature (Tm) using a software package (Origin) equipped with the apparatus. The molecular weight of tilapia muscle tropomyosin (dimer, 65,396) used for data analysis was calculated based on its deduced amino acid sequence [35,36].
CD spectra were determined at temperatures ranging from 5 to 800C in the same buffer used for DSC with a J-720 spectropolarimeter (Jasco, Tokyo, Japan). While constant N2 flux was employed, a jacketed cell of 0.2 mm optical path length was used and the temperature was controlled by circulating thermoregulated water. Wavelength and protein concentrations for measurement were in the ranges from 240 to 190 nm and from 1.2 to 1.5 mg/mL, respectively.
Results
Comparison of the primary structures
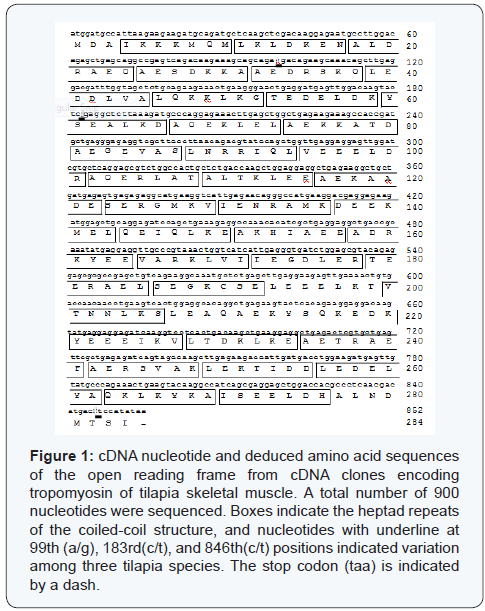
PCR with primers TMUF and TMUR gave an amplified product of approximately 900 bp which contained the open reading frame nucleotide sequence (1-852) encoding tropomyosins from the freshwater, seawater and hybrid tilapia. An open reading frame of tropomyosin cDNA from three tilapia species contained 852 nucleotides encoding 284 amino acid residues (Figure 1). The sequence of the α-chymotryptic fragment from Lys12 to Lys30 (KLDKENALDRAEQAESDKK) completely matched a corresponding part of the deduced amino acid sequence, suggesting the present cDNA clone was transcribed from the tropomyosin gene. Nucleotide sequence from the three species gave rise to three replacements at the 99th (A/G), 183rd (C/T) and 846th (C/T) positions (Figure 1). The replacements in the hybrid tilapia cDNA have succeeded from the maternal side (O. niloticus). Even though nucleotides were different in these positions, the amino acid sequence was identical for all the tropomyosins. The calculated molecular weight and isoelectric point of tilapia tropomyosin were 32,698 and 4.69, respectively.
Phylogenetic tree and structure simulation based on the amino acid sequence
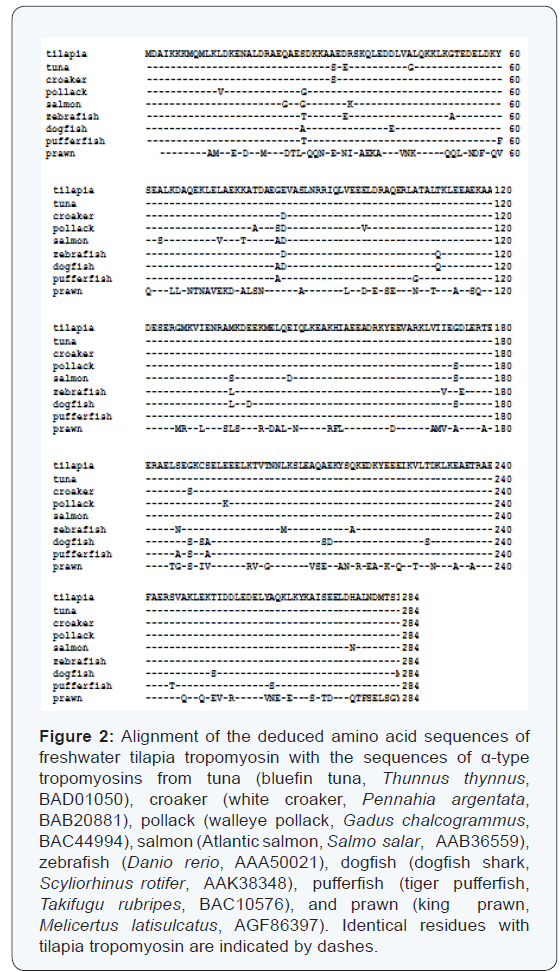
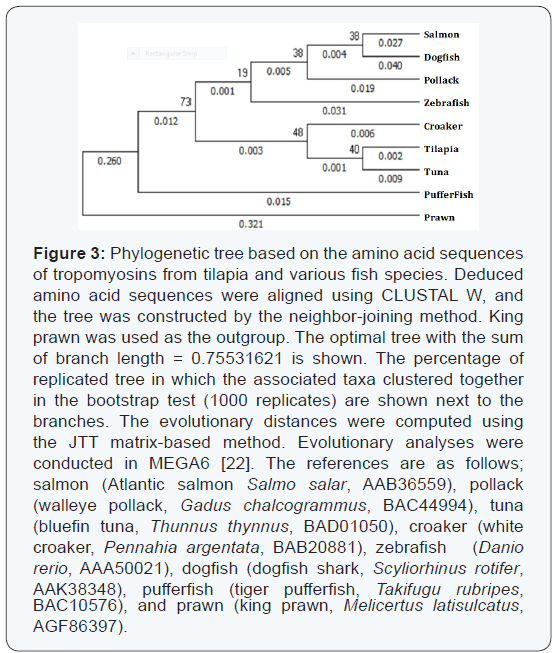
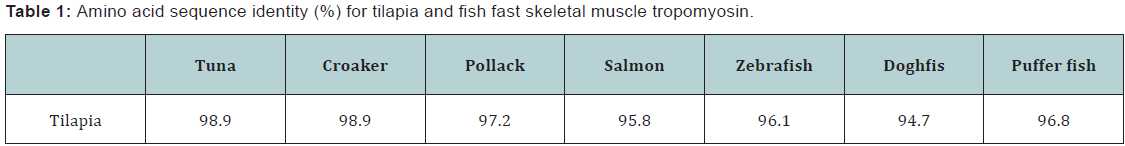
Sequence alignment was performed on fish skeletal muscle tropomyosins from atlantic salmon, walleye pollack, bluefin tuna, white croaker, zebrafish, dogfish shark, tiger pufferfish, and king prawn (Figure 2). The amino acid sequence identity was in the range of 95 to 99% (Table 1). The highest identity of α-tropomyosin was observed with those of bluefin tuna [18] and white croaker [15] (98.9%, three amino acid differences), whereas the lowest value of 94.7% (15 amino acid differences) was obtained with that of dogfish shark. In Figure 3, the phylogenetic tree is shown constructed by the neighbour-joining method. Bluefin tuna tropomyosin was found to have the closest relative with the tilapia counterpart among the species examined. Based on the phylogenetic analysis, fast skeletal tropomyosins from the three tilapia species were considered to be α-type by comparing with tropomyosins from fast skeletal muscles of many other fish species [11,15,16]
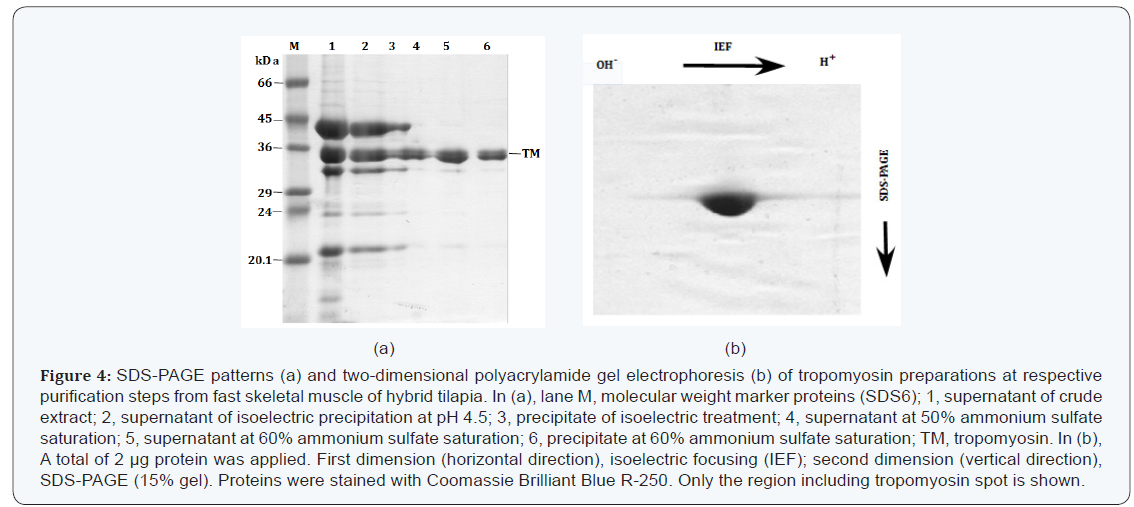
Tropomyosin purification and electrophoretic analyses
Tropomyosin was purified to homogeneity only by the repetition of isoelectric precipitation, high speed centrifugation and ammonium sulfate fractionation. The SDS-PAGE patterns of tropomyosin preparation from hybrid tilapia at respective purification steps are shown in Figure 4a. The patterns of seawater, freshwater and hybrid tilapia tropomyosins were almost the same (data not shown).
2D-PAGE pattern of hybrid tilapia tropomyosin is shown in Figure 4b, which was identical to those of freshwater and seawater tilapia tropomyosins and similar to that reported for walleye pollack [16], suggesting that tilapia fast skeletal muscle contained a single form of tropomyosin even in the case of hybrid.
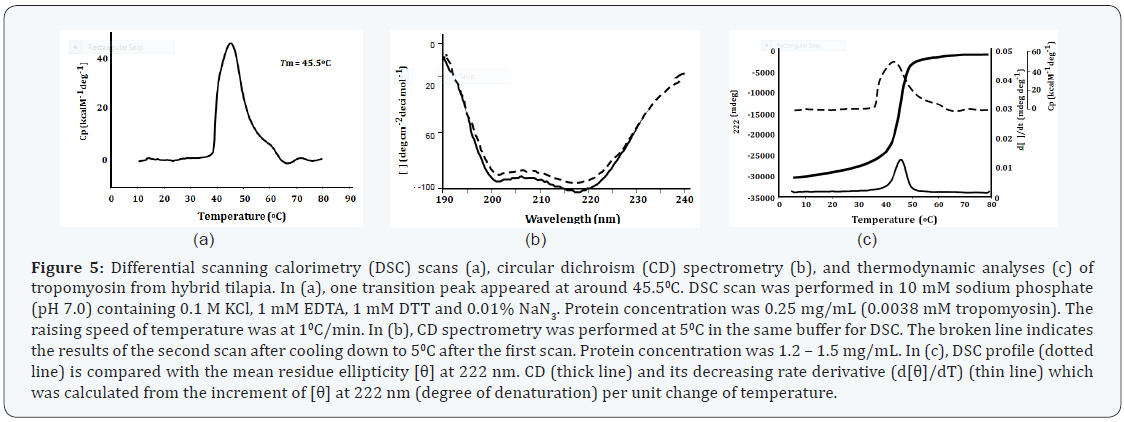
DSC analysis of tropomyosin
The result of DSC scan for hybrid tilapia muscle tropomyosin is presented in Figure 5a. The DSC patterns of seawater and freshwater tropomyosins were almost the same to that of hybrid tilapia tropomyosin. One prominent endothermic peak appeared at 46.1±0.40C, 46.3±0.30C and 45.5±0.50C for freshwater, seawater and their hybrid tilapia, respectively. The Tm values for thermal unfolding of fish tropomyosins and heat-induced conformational changes have been reported to be reversible for most fish species [37]. In the case of tilapia tropomyosins, both first and second DSC scans of the same sample gave essentially an identical endothermic curve (data not shown). These results suggest that tilapia tropomyosins are able to refold to the original structure when temperature was reduced after the first scan.
CD spectroscopy
The CD spectra obtained for hybrid tilapia tropomyosin are shown in Figure 5b. The patterns of seawater and freshwater tilapia tropomyosins were almost the same as that of hybrid tropomyosin (data not shown). When measured at 50C, tilapia tropomyosins exhibited a typical pattern of α-helix having two ellipticity minima at the wavelengths of 222 and 208nm (Figure 5b). All of these tropomyosin preparations showed CD spectra, typical of α-helix at lower temperature range. Assuming that 100% of α-helical content corresponds to molar ellipticity value [θ] of -36,000 deg cm2 at 222 nm [38], the average α-helical content of tilapia tropomyosin was calculated to be about 100% at 50C and 14% at 800C. The increase in the ellipticity minimum levels at 222 nm with increasing temperatures indicated the decrease of α-helical content in tropomyosin. Tilapia tropomyosin was in a helical state at 50C and gradually unfolded with the increase of temperature. At 800C, the ellipticity minimum at 222 nm became smallest, and the spectra did not change appreciably even with further increase in temperature. When treated at 800C and subsequently cooled down to 50C, the tropomyosin showed again a typical pattern of α-helix (as shown in a broken line in Figure 5b), the average yield of the helical content being more than 92% as revealed by the secondary structure prediction program GOR IV [39].
Thermodynamic profiles as revealed by DSC and CD analyses are illustrated in Figure 5c. The decreasing rate derivatives of the ellipticity minima at 222 nm of CD spectra together with the data by DSC analysis are superimposed in this figure. It was revealed that the thermodynamic change at around 460C is due to the destruction of α-helical structure of tropomyosin.
Discussion
It has been reported that tropomyosin is a highly conserved protein [40]. In comparison with other α-type tropomyosins, the sequence identify was in the range of 95 to 99%. The relationships between tilapia tropomyosin and other fish counterparts were examined by phylogenetic analyses (Figure 3). Bluefin tuna tropomyosin showed the closest relationship with that of tilapia, followed by that of white croaker.
As it was hypothesized that the tropomyosin from the hybrid tilapia shares the properties of those of the parent fish, the tropomyosins from the ordinary tilapia species and the hybrid were essentially the same. The results of deduced amino acid sequence, pattern of gel electrophoresis, and the thermal stability were shown to be identical, except the partial differences in the nucleotide sequences of the coding region (Figure 1).
The nucleotide sequences encoding the tilapia tropomyosins were different at three positions, namely, at the 99th (A/G), 183rd (T/C) and 846th (T/C) nucleotides (Figure 1), without affecting the deduced amino acid sequences. These differences were found between the tropomyosins from freshwater and seawater tilapia, but not between the freshwater and hybrid tilapia. The gene type of tropomyosin from hybrid tilapia (O. mossambica × O. niloticus) were the same as freshwater tilapia (O. niloticus ), suggesting that the gene encoding hybrid tropomyosin originated from the freshwater tilapia. 2D-PAGE analysis of purified tropomyosin provided only one spot, indicating that hybrid tilapia had no isoform of tropomyosin like those from the ordinary species.
Compared with the tilapia (O. mossambicus) tropomyosin (accession no. JX193712) in NCBI database, variations from the freshwater tilapia tropomyosin were found at seven nucleotides, namely, at 48th (A/G), 99th (A/G), 183rd 252 (T/C), 230thsup (T/A), 411th (T/C), 680th (T/C) and 846th (T/C). The variations at 48th (A/G), 230th (T/A), 411th (T/C) and 680th (T/C) were not found in the present tilapia species. In the present study, the above-mentioned variations provided different deduced amino acids, namely K77I and V227A. It is not clear why the tilapia tropomyosin in this study showed the amino acid sequence different from those in the database. One reason might be the presence of different strains of tilapia. It is likely that small changes of DNA sequences have been accumulated during the evolution of tilapia like single nucleotide polymorphisms (SNPs). The two amino acid variations are located in the d and g positions of the coil-coiled structure. The d and g positions are located inside the coil-coiled structure, and these variations have not so far been reported for tropomyosins from closely related species.
In the amino acid sequence of tropomyosin, C-terminal 8 residues (ALNDMTSI) and N-terminal 8 residues (MDAIKKKM) are conserved in the vertebrate species. Moraczewska and Hitchcock-DeGregori [41] indicated that the N- and C- terminal regions of neighbouring two tropomyosin molecules overlap by about nine amino acids. The highly conserved N- and C-terminal sequences could be deeply associated with the structural necessity for head-to-tail polymerization of the tropomyosin molecule, because the removal of either end greatly reduced the actin affinity [1,40].
DSC and CD analyses gave similar patterns showing only one major denaturation peak for the three tropomyosins. The transition temperatures of tropomyosins were 46.1±0.40C (freshwater tilapia), 46.3±0.30C (seawater tilapia) and 45.5±0.50C (hybrid), and those of CD were around 460C suggesting that the α-helical structure unfolded at almost the same temperature range for all the tropomyosin in spite of their possible differences in post-translational modification. In DSC analysis, only one endothermic peak appeared and Tm was around 460C in the first scan. The second scan also gave similar endothermic peaks (data not shown). Since 12 tropomyosin is supposed to take α-helical coiled coil conformation almost all over the molecule, the present tropomyosins showed a similar thermal decay as observed in DSC and CD analyses (Figure 5c). Tm values observed in DSC are considered to correspond to temperatures at which α-helices collapse.
Some heart diseases in human are believed to be caused by the mutations of tropomyosin. Dilated cardiomyopathy (DCM) including X-linked cardiomyopathy may be inherited through the maternal lineage mutations in many of the sarcomeric genes (β-myosin, α-tropomyosin, myosin binding protein-C, troponin-T) [42, 43]. In Drosophila, tropomyosin 1 is part of the maternal deposit during oogenesis. It is required to localize the oskar mRNA at the posterior pole of the oocyte [44]. In fish, maternal inheritance has been reported for zebrafish nucleoplasmin (npm2) during egg developmental competence [45]. Based on these models, the probability of maternal inheritance in tilapia tropomyosin is high.
Conclusion
The nucleotide sequence of the coding region from hybrid tilapia tropomyosin gene was shown to be the same as those from the maternal tilapia (O. niloticus) except for three positions. It is likely that the skeletal muscle tropomyosins of tilapia are basically a maternal inheritance type.
Acknowledgement
We would like to thank Ms. Yui-Han Liu and Mr. Jing-Lin Lee, for their help in the experimental work. This study was supported in part by a National Science Council of Taiwan, R.O.C. (NSC-100-2313-B-024-002).
References
- Hitchcock DeGregori SE, Varnell TA (1990) Tropomyosin has discrete actin-binding sites with seven fold and fourteen fold periodicities. J Mol Biol 214(4): 885-896.
- Smillie LB (1979) Structure and functions of tropomyosin from muscle and non-muscle sources. Trends Biochem Sci 4(7): 151-155.
- Lees-Miller JP, Helfman DM (1991) The molecular basis for tropomyosin isoform diversity. Bio Essays 13(9): 429-437.
- Crick FHC (1953) The packing of α-helices: simple coiled coils. Acta Cryst 6(8-9): 689-697.
- Fraser RDB, MacRae TP, Miller A (1965) X-ray diffraction patterns of α-fibrous proteins. J Mol Biol 14(2): 432-442.
- McLachlan AD, Stewart M (1976) The 14-fold periodicity in α-tropomyosin and the interaction with action. J Mol Biol 103(2): 271- 298.
- Bernstein SI, O’Donnell PT, Cripps RM (1993) Molecular genetic analysis of muscle development, structure, and function in Drosophila. Int Rev Cytol 143: 63-152.
- Johnson P, Smillie LB (1977) Polymerizability of rabbit skeletal tropomyosin: effects of enzymic and chemical modification. Biochemistry 16(10): 2264-2269.
- Ebashi S, Endo M (1968) Calcium and muscle contraction. Prog Biophys Mol Biol 18: 123-183.
- Ebashi S, Endo M, Ohtsuki I (1969) Control of muscle contraction. Quart Rev Biophys 2(4): 351-384.
- Seki N, Iwabuchi S (1978) On the subunit composition of fish tropomyosins. Bull Japan Soc Sci Fish 44: 1333-1340.
- Heeley DH, Hong C (1994) Isolation and characterization of tropomyosin from fish muscle. Comp Biochem Physiol 108B (1): 95- 106.
- Ochiai Y, Ozawa H, Huang MC, Watabe S (2010) Characterization of two tropomyosin isoforms from the fast skeletal muscle of bluefin tuna Thunnus thynnus orientalis. Arch Biochem Biophys 502(2): 96-103.
- Jackman D, Waddleton DM, Younghusband B, Heeley DH (1996) Further characterization of fast, slow and cardiac muscle tropomyosins from salmonid fish. Eur J Biochem 242: 363-371.
- Ochiai Y, Ahmed K, Ahsan MN, Funabara D, Nakaya M et al. (2001) cDNA cloning and deduced amino acid sequence of tropomyosin from fast skeletal muscle of white croaker Pennahia argentata. Fish Sci 67(3): 556-558.
- Ochiai Y, Huang MC, Fukushima H, Watabe S (2003) cDNA cloning and thermodynamic properties of tropomyosin from walleye pollack Theragra chalcogramma fast skeletal muscle. Fish Sci 69(5): 1033- 1041.
- Togashi M, Kakinuma M, Hirayama Y, Fukushima H, Watabe S, et al. (2000) cDNA cloning of myosin rod and the complete primary structure of myosin heavy chain of walleye pollack fast skeletal muscle. Fish. Sci. 66(2): 249-357.
- Huang MC, Ochiai Y, Watabe S (2004) Structural and thermodynamic characterization of tropomyosin from fast skeletal muscle of bluefin tuna Thunnus thynnus. Fish Sci 70(4): 667-674.
- Mak AS, Smillie LB, Stewart GR (1980) A comparison of the amino acid sequences of rabbit skeletal muscle α- and β- tropomyosin. J Biol Chem 255(8): 3647-3655.
- Heeley DH, Bieger T, Waddleton DM, Hong C, Jackman DM, et al. (1995) Characterization of fast, slow and cardiac muscle tropomyosins from salmonid fish. Eur J Biochem 232: 226-234.
- Sambrook J, Russell DW (2001) Plasmids and their usefulness in molecular cloning. In: Molecular Cloning: A Laboratory Manual, Volume 1, (3rd edn), Cold Spring Harbor Laboratory Press, New York, USA, pp. 31-138.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12): 2725-2729.
- Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic tree. Mol Biol Evol 4(4): 406-425.
- Ohara O, Dorit, RL, Gilbert W (1989) One-sided polymerase chain reaction: the amplification of cDNA. Proc Natl Acad Sci USA. 86(15): 5673-5677.
- Heeley DH, Bieger T, Waddleton DM, Hong C, Jackman DM, McGowan C (1995) Characterization of fast, slow and cardiac muscle tropomyosins from salmonid fish. Eur J Biochem 232(1): 226-234.
- Ikeda D, Toramoto T, Ochiai Y, Suetake H, Suzuki Y, et al. (2003) Identification of novel tropomyosin 1 genes of pufferfish (Fugu rubripes) on genomic sequences and tissue distribution of their transcripts. Mol Biol Rep 30(2): 83-90.
- Toramoto T, Ikeda D, Ochiai Y, Minoshima S, Shimizu N et al (2004) Multiple gene organization of puffer fish Fugu rubripes tropomyosin isoforms and tissue distribution of their transcripts. Gene 331: 41-51.
- Bailey K (1948) Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem. J 43(2): 271-275.
- Cummins P, Perry SV (1974) Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J 141(1): 43-49.
- Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177: 751-765.
- Laemmli UK (1970) Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
- Matsudaira P (1987) Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem 262: 10035-10038.
- Nakaya M, Watabe S, Ooi T (1995) Differences in the thermal stability of acclimation temperature-associated types of carp myosin and its rod on differential scanning calorimetry. Biochemistry 34(9): 3114-3120.
- Nakaya M, Kakinuma M, Watabe S, Ooi T (1997) Differential scanning calorimetry and CD spectrometry of acclimation temperatureassociated type of carp light meromyosin. Biochemistry 36(30): 9179- 9184.
- Watabe S, Imai J, Nakaya M, Hirayama Y, Okamoto Y, et al. (1995) Temperature acclimation induces light meromyosin isoforms with different primary structures in carp fastskeletal muscle. Biochem Biophys Res Commun 208(1): 118-125.
- Imai J, Hirayama Y, Kikuchi K, Kakinuma M, Watabe S (1997) cDNA cloning of myosin heavy chain isoform from carp fast skeletal muscle and their gene expression associated with temperature acclimation. J Exp Biol 200(1): 27-34.
- Huang MC, Ochiai Y (2005) Fish fast skeletal muscle tropomyosins show species-specific thermal stability. Comp Biochem Physiol B Biochem Mol Biol 141: 461-471.
- Yang JT, Wu CC, Martinez HM (1986) Calculation of protein conformation from circular dichroism. Methods Enzymol 130: 208-268.
- Garnier J, Gibrat JF, Robson B (1996) GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol 266: 540-553.
- Perry SV (2001) Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil 22(1): 5-49.
- Moraczewska J, Hitchcock-DeGregori SE (2000) Independent function for the N- and C-termini in the overlap region of tropomyosin. Biochemistry 39(23): 6891-6897.
- Zeviani M, Gellera C, Antozzi C, Rimoldi M, Morandi L, et al. (1991) Maternally inherited myopathy and cardiomyopathy: association with mutation in mitochondrial DNA tRNA (Leu)(UUR). Lancet 338: 143-147.
- García-Castro M, Coto E, Reguero JR, Berrazueta JR, Alvarez V, et al. (2009) Mutations in sarcomeric genes MYH7, MYBPC3, TNNT2, TNNI3, and TPM1 in patients with hypertrophic cardiomyopathy. Rev Esp Cardiol 62: 48-56.
- Erdelyi M, Michon AM, Guichet A, Glotzer JB, Ephrussi A (1995) Requirement for Drosophila cytoplasmic tropomyosin in oskar mRNA localization. Nature 377: 524-527.
- Bouleau A, Desvignes T, Traverso JM, Nguyen T, Chesne,l F (2014) Maternally inherited npm2 mRNA is crucial for egg developmental competence in zebrafish. Biol Reprod 9192: 43(1-9).






