Cerebral Hyperperfusion Syndrome: A Mini-Review
Oleg Verbitskiy1, Michael Farberov2 and Darya Sitovskaya3,4*
1City Mariinskiy Hospital, St. Petersburg, Russia
2Hadassah Hebrew University Medical Center, Jerusalem, Israel
3Polenov Neurosurgical Institute - Branch of Almazov National Medical Research Centre, St. Petersburg, Russia
4Department of Pathology with a course of forensic medicine named after D.D. Lochov, St. Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
Submission: July 08, 2024; Published: July 15, 2024
*Corresponding author: Darya Sitovskaya, Department of Pathology with a course of forensic medicine named after DD Lochov, St. Petersburg State Pediatric Medical University, Saint-Petersburg, Russia. E-mail: daliya_16@mail.ru
How to cite this article: Verbitskiy O, Farberov M, Sitovskaya D. Cerebral Hyperperfusion Syndrome: A Mini-Review. Open Access J Surg. 2024; 15(4): 555918. DOI: 10.19080/OAJS.2024.15.555918.
Abstract
The prevention of strokes is crucial for healthcare, as it can significantly reduce morbidity, disability, and mortality rates. One important aspect of stroke prevention is the use of reconstructive and revascularization procedures on the carotid arteries, which are However, a major complication of carotid endarterectomies (CEA) is cerebral hyper perfusion syndrome (CHS), which can lead to fatal consequences in some patients. The pathophysiology of this condition is not fully understood, and there is currently no established diagnostic. criteria or optimal hemodynamic management for preventing CHS after carotid revascularization. In this mini-review, we address the current challenges surrounding CHS in the context of a vascular centre.
Keywords: Cerebral Hyperperfusion Syndrome; Arterial hypertension; Inflammatory mediators; Intracerebral hemorrhage; Carotid artery surgery
Abbreviations: CEA: Carotid Endarterectomies; CHS: Cerebral Hyperperfusion Syndrome; ICA: Internal Carotid Artery
Pathogenesis of Cerebral Hyperperfusion Syndrome
Preventing strokes is crucial in practical healthcare due to the rising rates of morbidity, disability, and mortality associated with this condition. One important aspect of prevention is the use of reconstructive and revascularization procedures in the carotid artery territory [1]. The number of carotid endarterectomies (CEA) performed annually in specialized clinics has been steadily increasing. According to statistics, approximately 100,000-150,000 of these operations are performed each year in the United States [2,3].
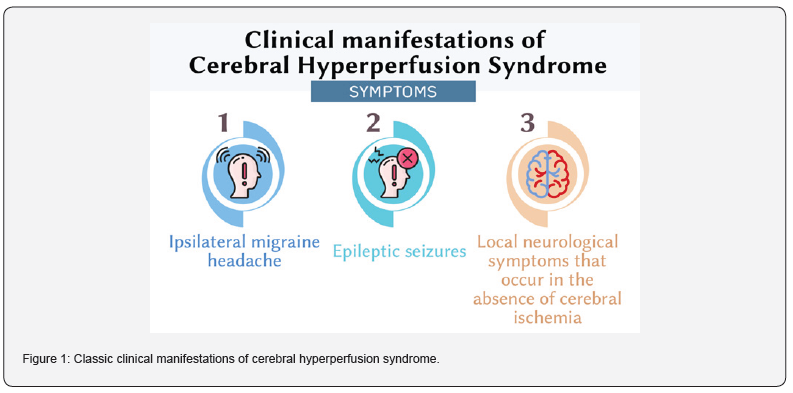
Cerebral hyperperfusion syndrome (CHS) is a potentially dangerous complication that can occur after cerebral revascularization and reconstruction of precerebral vessels. The first definition of this condition was given by Sundt in 1981. According to Sundt, CHS is a condition that results from an increase in cerebral blood flow after surgery, exceeding the metabolic needs of the brain [4]. The classic clinical manifestations of CHS include a triad of symptoms: ipsilateral headache, often in the orbital region, of a migraine nature, epileptic seizures, and local neurological symptoms that occur in the absence of cerebral ischemia (Figure 1) [5,6]. This definition is widely accepted by most authors.
According to current literature, carotid endarterectomy is considered the most effective treatment for symptomatic moderate to severe carotid artery stenosis [7]. However, it is important to note that CHS can occur in a significant percentage of patients undergoing this procedure, ranging from 0.4% to 7.7% [8-10]. This wide range can be attributed to differences in clinical inclusion criteria and sample size. For instance, studies have shown that headaches occur in 62% of patients, but most of these cases (78%) are classified as mild or moderate [11].
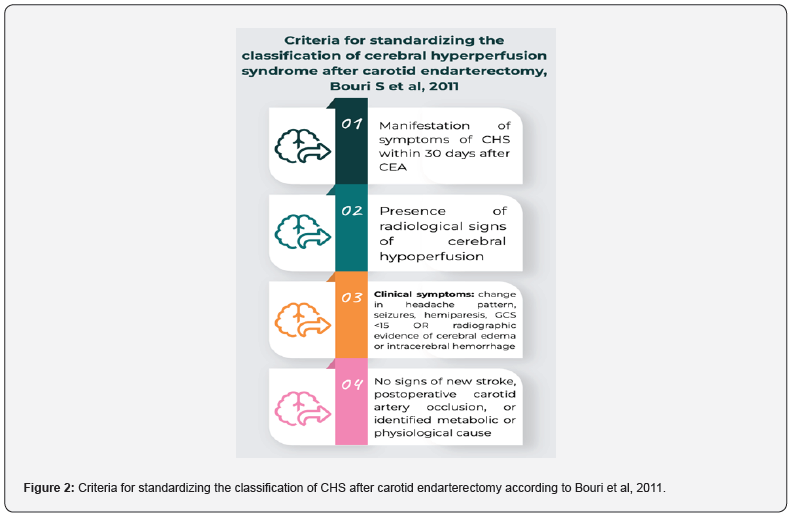
Although spontaneous carotid dissection was initially described as a complication of carotid revascularization, it has now also been reported in patients with acute stroke [12]. The criteria for diagnosing CHS in a patient include a combination of new clinical symptoms, radiological signs of hyperperfusion, and/or the presence of intracerebral hemorrhage within 30 days after manipulation of the carotid arteries or intracranial vessels [13,14]. In a 2011 meta-analysis, Bouri et al proposed four criteria to standardize the classification of CHS after carotid endarterectomy: (1) presentation within 30 days after CEA, (2) presence of radiographic evidence of cerebral hypoperfusion, (3) clinical symptoms such as changes in headache patterns, seizures, hemiparesis, the Glasgow Coma Scale (GCS) <15, or radiological evidence of cerebral edema or intracerebral hemorrhage, and (4) absence of evidence of new ischemic stroke, postoperative carotid occlusion, and identified metabolic or physiological causes (Figure 2) [14]. Edema associated with CHS is generally considered reversible, but the prognosis is worse when CHS is associated with intracerebral hemorrhage. This can result in partial disability for approximately 30% of patients and mortality for another 50% [15]. The main risk factors for developing CHS or intracranial hypertension after CEA include postoperative hypertension (systolic blood pressure >180 mmHg), a history of coronary artery disease, contralateral stenosis >70%, and a recent ipsilateral stroke within the past month [12,13,16]. Previous studies have also suggested that preventing CHS is best achieved through appropriate blood pressure control, timing of CEA in relation to transient ischemic attack or stroke, and careful selection of the anesthetic, considering its effect on the intracerebral vasculature [6,13,15].
Cerebral vasoconstriction is a normal physiological response under certain conditions that helps maintain normal cerebral blood flow [17,18]. However, in certain cases, such as patients with ipsilateral stroke, contralateral carotid artery stenosis ≥ 70%, arterial hypertension in the early postoperative period, and reduced cerebral vasoreactivity, this response can pose a high risk [13,19].
The main mechanism of CHS is the excessive flow of blood into the brain after revascularization or reconstruction of the internal carotid artery (ICA), which exceeds the brain’s metabolic needs. However, not all patients who experience hyperperfusion will develop this syndrome [20,21]. The primary risk factors for CHS are perioperative arterial hypertension and impaired autoregulation of cerebral blood flow. Under normal conditions, cerebral autoregulation mechanisms maintain a constant perfusion pressure by dilating or constricting arterioles. In cases of ICA stenosis or occlusion, compensatory arteriolar expansion occurs due to a decrease in perfusion pressure [1,22,23]. Clinical symptoms may arise when the volumetric blood flow velocity exceeds the preoperative level, typically by 100%. However, there have been reports of CHS with less significant increases in volumetric blood flow velocity in the literature. For example, Belyaev et al. found that CHS developed with a 48% increase in volumetric blood flow velocity compared to the initial level [24].
According to Krainik et al., clinical manifestations develop when there is a 30-50% increase in the volumetric velocity of cerebral blood flow compared to the initial level. In the absence of ischemia, blood flow reaches its maximum on the first day after surgery and returns to normal by 4-5 days. However, in cases of chronic hypoperfusion, this process can take up to 7 days [25]. Some authors suggest that during chronic hypoperfusion, cerebral metabolism decreases and the ability to constrict is lost for up to 4 weeks, resulting in reduced reactivity to carbon dioxide [14]. During ischemia, oxygen free radicals accumulate, leading to oxidative stress in the brain. This also triggers the production of pro-inflammatory cytokines (such as interleukins IL-1, IL-6, and tumor necrosis factor alpha) [26]. Inflammatory mediators are produced as a result of ischemia, causing endothelial dysfunction and increasing the permeability of the vascular wall. Additionally, nitric oxide is produced during ischemia, which acts as a vasodilator and further increases vascular permeability [20,27,28]. Arterial hypertension is another factor that can increase cerebral vascular permeability, leading to oxidative stress and the production of pro-inflammatory cytokines, even in the absence of ischemia [29]. Naylor defines hyperperfusion as reactive hyperemia after the start of blood flow [21]. Ultimately, the morphological substrate of cerebral hyperperfusion syndrome is cerebral edema and hemorrhage due to increased vascular permeability [25,30,31].
One important task in clinical work is the early recognition of subcortical hemorrhage, as cerebral edema in the initial stages is reversible. However, as the process progresses, particularly with hemorrhagic transformation of the lesion, the prognosis becomes less favorable. Up to 30% of patients may remain partially disabled, and the mortality rate can reach 50% [32,33]. Additionally, patients may experience transient mental disorders and hemorrhagic complications [1,34]. Those with a history of transient ischemic attack or stroke, who require urgent intervention or postoperative intravenous blood pressurelowering medications, or who are being treated for complete occlusion are at a higher risk for developing CHS. To prevent this complication in high-risk patients, some authors suggest using an angiotensin-converting enzyme inhibitor [35].
According to K. Ogasawara et al., intracerebral hemorrhages are a rare complication of chronic cerebral hypoperfusion after reconstructive surgery on the carotid arteries [36]. In their study, Galyfos et al. did not find statistically significant differences in the incidence of intracerebral hemorrhage after carotid endarterectomy and carotid artery stenting [16].
Conclusion
With the advancement of technology, manipulation of the carotid arteries has become more common. However, carotid revascularization can lead to a serious complication known as cerebral hyperperfusion syndrome. To reduce the risk of morbidity and mortality, it is important to remain vigilant and take preventive measures. The exact cause of CHS is not fully understood, but it is believed to be related to increased cerebral blood flow and impaired cerebral autoregulation, particularly in areas where the blood-brain barrier is disrupted. Additionally, baroreceptor dysfunction during carotid artery surgery may also contribute to the development of CHS.
Currently, there is no consensus on the target blood pressure to prevent cerebral hyperperfusion syndrome. It is possible that CHS shares a similar pathophysiological mechanism with hypertensive encephalopathies, which involve damage to the arteriolar endothelium, cerebral vasoconstriction, blood-brain barrier disruption, cerebral edema, and sometimes intracerebral hemorrhage, all of which can be fatal. Despite ongoing research, the pathogenesis of these syndromes is still not fully understood. It is likely that genetic and molecular factors, as well as individual characteristics of the patient, play a role in the development of CHS. Therefore, further research is needed to better understand and characterize these syndromes. Additionally, future studies should focus on developing diagnostic criteria and determining the best hemodynamic management after carotid revascularization to prevent cerebral hyperperfusion syndrome.
References
- Kokshin AV, Nemirovsky AM, Danilov VI (2018) Cerebral Hyperperfusion Syndrome in patients with stenotic and occlusive lesion internal carotid arteries after surgery. Literature review (In Russ.). Neurol Bullet 4: 44-51.
- Mukerji N, Manjunath Prasad KS, Vivar R, Mendelow AD (2015) Carotid endarterectomy-safe and effective in a neurosurgeon's hands: a 25-year single-surgeon experience. World Neurosurg 83(1): 74-79.
- Krylov VV, Luk'ianchikov VA (2014) Cerebral revascularization for the treatment of patients with acute ischemic stroke. Zh Nevrol Psikhiatr Im S Korsakov 114(12-2): 46-52.
- Sundt TM, Sharbrough FW, Piepgras DG (1981) Correlation of CBF and EEG changes during carotid endarterectomy with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin. Proc 56(9): 533-547.
- Adhiyaman V, Alexander S (2007) Cerebral hyperperfusion syndrome following carotid endarterectomy. 100(4): 239-244.
- Edwards AM, Birchler CR, Park S, Baker JM, Molnar RG (2021) Cerebral Hyperperfusion Syndrome Presenting As Status Epilepticus Following Carotid Endarterectomy. Cureus 13(12): e20551.
- Wangqin R, Krafft PR, Piper K, Kumar J, Xu K, et al. (2019) Management of De Novo Carotid Stenosis and Postintervention Restenosis-Carotid Endarterectomy Versus Carotid Artery Stenting-a Review of Literature. Transl Stroke Res 10(5): 460-474.
- Henderson RD, Phan TG, Piepgras DG, Wijdicks EF (2001) Mechanisms of intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 95(6): 964-969.
- Beard JD, Mountney J, Wilkinson JM, Payne A, Dicks J, et al. (2001) Prevention of postoperative wound haematomas and hyperperfusion following carotid endarterectomy. Eur J Vasc Endovasc Surg 21(6): 490-493.
- Hosoda K, Kawaguchi T, Shibata Y, Kamei M, Kidoguchi K, et al. (2001) Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke 32(7): 1567-1573.
- Tehindrazanarivelo AD, Lutz G, PetitJean C, Bousser MG (1992) Headache following carotid endarterectomy: a prospective study. Cephalalgia 12(6): 380-382.
- Vasilchenko NO, Rubleva OV, Verbitsky OP, Ordynets SV, Shabonov AA, et al. (2017) Syndrome of cerebral hyperperfusion after the carotid endarteretomy within the acute period of ischemic blood-stroke (In Russ.). Med Acad J 17(4): 17-21.
- Kirchoff-Torres KF, Bakradze E (2018) Cerebral Hyperperfusion Syndrome After Carotid Revascularization and Acute Ischemic Stroke. Curr Pain Headache Rep 22(4): 24.
- Bouri S, Thapar A, Shalhoub J, Jayasooriya G, Fernando A, et al. (2011) Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg 41(2): 229-237.
- Farooq MU, Goshgarian C, Min J, Gorelick PB (2016) Pathophysiology and management of reperfusion injury and hyperperfusion syndrome after carotid endarterectomy and carotid artery stenting. Exp Transl Stroke Med 8(1): 7.
- Galyfos G, Sianou A, Filis K (2017) Cerebral hyperperfusion syndrome and intracranial hemorrhage after carotid endarterectomy or carotid stenting: A meta-analysis. J Neurol Sci 381: 74-82.
- Delgado MG, Bogousslavsky J (2020) Cerebral Hyperperfusion Syndrome and Related Conditions. Eur Neurol 83(5): 453-457.
- Semenyutin VB, Nikiforova АА, Aliev VA, Panuntsev GК (2021) Informativity of blood flow distribution in the precerebral arteries for determining the hemodynamic significance of carotid stenosis. Regional Blood Circulation and Microcirculation 20(2): 45-51.
- Semeniutin VB, Aliev VA, Nikiforova AA, Svistov DV, Savello AV, et al. (2018) Role of cerebral blood flow autoregulation in internal carotid artery stenosis surgery. Grekov's Bulletin Surg 177(6): 81-86.
- Moulakakis KG, Mylonas SN, Sfyroeras GS, Vasilios A (2009) Hyperperfusion syndrome after carotid revascularization. J Vascular Surg 49(4): 1060-1068.
- Naylor AR, Evans J, Thompson MM, London NJM, Abbott R, et al. (2003) Seizures after carotid endarterectomy: hyperperfusion, dysautoregulation or hypertensive encephalopathy? Eur J Vascular and Endovasc Surg 26(1): 39-44.
- Awano T, Sakatani K, Yokose N, Tatsuya H, Norio F, et al. (2010) EC-IC bypass function in Moyamoya disease and non-Moyamoya ischemic stroke evaluated by intraoperative indocyanine green fluorescence angiography. Adv Exp Med Biol 662: 519-24.
- Dumont AS, Tjoumakaris SI, Jabbour PM, Fernando GL, Robert HR (2012) Cerebral hyperperfusion after neurovascular reconstruction surgery: what have we learned? World Neurosurg 78(5): 415-417.
- Beljaev A Yu, Usachev D Yu, Lukshin VA (2011) The syndrome of cerebral hyperperfusion after carotid endarterectomy. Burdenko's J Neurosurg 75(3): 31-38.
- Krainik VM, Kozlov SP (2011) Acute reperfusion lesions of the central nervous system during operations on the internal carotid arteries. Messenger of Anesthesiology and Resuscitation 8(2): 4957.
- Clark WM, Calcagno FA, Gabler WL, et al. (1994) Reduction of central nervous system reperfusion injury in rabbits using doxycycline treatment. Stroke 25(7): 1411-1415.
- Janigro D, West GA, Nguyen TS (1994) Regulation of blood-brain barrier endothelial cells by nitric oxide. Circu Res 75(3): 528-538.
- Lindegaard KF, Grolimund P, Aaslid R (1986) Evaluation of cerebral AVM's using transcranial Doppler ultrasound. J Neurosurg 65(3): 335-344.
- Poulet R, Gentile MT, Vecchione C, Maria D, Alessandra A, et al. (2006) Acute hypertension induces oxidative stress in brain tissues. J Cereb Blood Flow Metab26(2): 253-262.
- Nanba T, Ogasawara K, Nishimoto H, Shunrou F, Hiroki K, et al. (2012) Postoperative cerebral white matter damage associated with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy: a diffusion tensor magnetic resonance imaging study. Cerebrovasc Dis 34(5-6): 358-367.
- Nicosia A, Nikas D, Reinmers D (2011) Manifeastation, management and prevention of periprocedural complications of carotid artery stenting. J Med Res Sci 1(2): 12-28.
- Meyers PM, Higashida RT, Phatouros CC, Malek AM, Lempert TE, et al. (2004) Cerebral hyperperfusion syndrome after percutaneous transluminal stenting of the craniocervical arteries. Neurosurgery 47(2): 335-343.
- Piepgras DG, Morgan MK, Sundt TM, Yanagihara T, Mussman LM (1988) Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 68(4): 532-536.
- Fluri F, Engelter S, Lyrer P (2010) Extracranial-intracranial arterial bypass surgery for occlusive carotid artery disease. Cochrane Database Syst Rev 2010(2): CD005953.
- Hsu AC, Williams B, Ding L, Weaver FA, Han SM, et al. (2023) Risk Factors for Cerebral Hyperperfusion Syndrome following Carotid Revascularization. Ann Vasc Surg 97: 89-96.
- Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, et al. (2007) Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 107(6): 1130-1136.






























