Late Complications of Acute Pancreatitis: A Surgical Approach
Miguel Eduardo Rodriguez Rodriguez1, Jhon Navarro Gonzalez2, Vivaswan Dutt Mishra3, Coralvia Yaroslangna Villanueva Perez4, Luis Fernando Ochoa Meza5, Angeles Astrid Zamudio Fuentes6, Daniel Arias Vargas7, Aishwarya Yannamani8, Pushan Aggarwal8, Gioacchino De Sario Velasquez1, Uche Brigid Smith9, Paola Klaretsy Losada Muñoz1, Abisola Omokoyinsola Lawal9 and Maria Isabel Gomez10*
1Universidad de Oriente, Venezuela
2Universidad del Zulia, Venezuela
3Moti Lal Nehru Medical College, Allahabad, India
4Universidad Nacional Experimental Francisco de Miranda, Venezuela.
5Universidad Autónoma de Baja California, México
6Universidad de Monterrey, Mexico
7Universidad de Ciencias Médicas (UCIMED), Costa Rica
8Kasturba Medical College, Manipal, India
9Richmond Gabriel University, Saint Vincent and the Grenadines.
10Universidad del Valle de México, México
Submission: May 16, 2023; Published: May 30, 2023
*Corresponding author: Maria Isabel Gomez Coral, Universidad Del Valle de México, México, USA
How to cite this article: Miguel E R R, Jhon N G, Vivaswan Dutt M, Coralvia Y V P, Luis F O M, et al. Late Complications of Acute Pancreatitis: A 002 Surgical Approach. Open Access J Surg. 2023; 14(3): 555887. DOI: 10.19080/OAJS.2023.14.555887.
Abstract
Acute pancreatitis, a severe inflammatory pancreas disorder, often leads to significant morbidity and mortality due to its late complications, including fluid collections, pseudocysts, necrosis, obstruction, fistulation, and abdominal compartment syndrome. Despite its rising incidence and associated healthcare costs, the surgical management of these complications remains a clinical challenge due to the complex nature of the disease and patient comorbidities. Current literature provides an understanding of the pathophysiology, epidemiology, and standard management of these complications. However, there’s a need to focus on the surgical approach to address these complications more effectively. This article reviewed these late complications and discussed their clinical presentation and diagnostic methods, emphasizing surgical management and prognosis. The primary goal of the review was to provide clinicians with a comprehensive understanding of the surgical management of these complications, thereby improving patient outcomes. Our analytical methods involved a thorough review of current literature, including clinical studies, reviews, and case reports. The review revealed that surgical intervention plays a pivotal role in managing severe or complicated cases. However, the surgical technique choice depends on the complication’s type and severity, the patient’s overall condition, and the surgeon’s expertise. Further research is recommended to develop standardized protocols for the surgical management of these complications.
Keywords: Acute pancreatitis; Complications; Pseudocysts; Necrosis; Intestinal obstruction; Ileus; Fistulization; Abdominal compartment syndrome; Surgical approach; minimally invasive surgery.
Abbreviations: AP: Acute Pancreatitis; US: Ultrasound; CT: Computed Tomography; CECT: Contrast-enhanced Computed Tomography; ERCP: Endoscopic Retrograde Cholangiopancreatography; MRI: Magnetic Resonance Imaging; VARD: Video-Assisted Retroperitoneal Debridement
Introduction
Acute pancreatitis is an inflammatory condition of the pancreas that can cause local injury, systemic inflammatory response syndrome, and organ failure [1]. It is characterized by abdominal pain and elevated levels of pancreatic enzymes in the blood [2]. In the United States, more than 275,000 patients are hospitalized for AP annually, at an aggregate cost of >$2.6 billion per year. The incidence ranges from 5 to 30 cases per 100,000; evidence shows it has risen recently. The overall case fatality rate is roughly 5% and is expectedly higher for more severe diseases [1]. The most common causes of acute pancreatitis include gallstones, alcohol use, and hypertriglyceridemia. However, the rate of occurrence of each etiology of acute pancreatitis varies across geographic regions and socioeconomic strata. Other less common causes include idiopathic, post-procedural, ampullary stenosis, autoimmune pancreatitis, etc. [3]. The patient commonly describes moderate to severe abdominal pain in the epigastrium associated with nausea and anorexia; the pain characteristics depend on the etiology. A physical exam is often significant for fever, tachycardia, and in severe patients, hypotension. The abdominal exam typically reveals epigastric tenderness with possible guarding, rigidity, and decreased bowel sounds [3].
According to the Revised Atlanta Classification, the diagnosis of acute pancreatitis requires 2 of 3 of the following criteria: amylase and/or lipase levels greater than three times the normal upper limit, abdominal pain, and abdominal imaging is consistent with acute pancreatitis [4]. The management of acute pancreatitis includes early intravenous hydration, appropriate nutrition, pain management, antibiotics (in case of infected pancreatitis), and surgery (depending on the etiology) [4]. The most common complications of acute pancreatitis are fluid collections, pseudocysts, necrosis, abdominal obstruction, fistulization, and abdominal compartment syndrome. This narrative review aims to identify and manage these late complications, focusing on surgical strategies and considering the patient’s clinical context and comorbidities.
Fluid Collections
Acute peripancreatic fluid collections (APFCs) or Pancreatic Fluid Collections (PFCs) are common complications of pancreatitis, especially in the acute setting. It is estimated that 5-15% of pancreatitis episodes that have required in-patient hospitalizations have been noted to be associated with pseudocysts [5]. Acute fluid collections that develop in the backdrop of acute pancreatitis are usually accumulations of enzyme-rich fluid or semi-solids that lack well-defined boundaries or outlines and hold the potential to traverse through the retroperitoneal plane. Usually, such collections occur acutely (<48 hour period of onset), occur frequently (30-50% patients affected), and the majority of cases are associated with spontaneous resolution (nearly 50% cases) [6]. However, if these acute collections do not resolve on their own, they may evolve to become walled off or chronic in nature- pseudocysts, necrotic collections, even abscesses.
Pseudocysts are collections encapsulated by a non-epithelial network of granulation tissue that develop over 3-4 weeks. These collections become clinically apparent due to their presentation- persistent but mild abdominal pain after the resolution of an episode of pancreatitis associated with a palpable abdominal mass. Most acute pseudocysts will resolve spontaneously; however, if such collections fail to resolve acutely, they may worsen and lead to infections, bleeding, or rupture [7,8]. Pancreatic abscesses are collections of pus or infected fluid that arise in close proximity or contact with the pancreas in the backdrop of pancreatitis or trauma [9]. These collections arise from the sterile pancreatic fluid. However, they grow to become infected and possibly necrotic from the transmigration of bacteria [10,11]. This abscess may appear walled off and thicker walled on CECT and is associated with a broader range of complications [9]. Twenty percent of patients with acute pancreatitis can go on to develop pancreatic necrosis [8]. Pancreatic necrosis can also lead to gross peripancreatic fat necrosis [9]. These necrotic collections also contain semi-solid and solid debris that may get secondarily infected and spread to more complicated infections [12,13]. The revised Atlanta classification classifies Pancreatic Fluid collections as acute or chronic. The chronic collections can further be classified as Pseudocysts and walled-off pancreatic necrosis.
Pseudocysts
A pancreatic pseudocyst is a homogeneous fluid encapsulated by a wall that is well defined outside the pancreas with minimal or no necrosis [10]. The name “Pseudo” cyst refers to fluid collection surrounded by a non-epithelialized wall of fibrous and granulation tissue [11]. Pseudocyst is located as follows: one-third near the head of the gland and the other two-thirds in the tail. They usually occur four weeks after the initial onset of interstitial edematous pancreatitis in any age group [10]. Regardless of the cause, the incidence is low; 0.5 to 1 per 100,000 adults per year; in acute pancreatitis, incidence can be from 5 to 16 percent, and in chronic pancreatitis ranges from 20 to 40 percent. More common in males.
More commonly related to chronic pancreatitis; they occur when the damage or disruption of pancreatic ducts, either from inflammation or direct injury or frequently from biliary stones or alcohol; causes extravasation and collection of pancreatic fluid [11]. Alcohol-related pancreatitis is the major cause of pancreatic pseudocyst formation, especially in countries where alcohol consumption is higher, as it contributes to more than 70 percent of cases. Other causes include biliary stones, trauma, or idiopathic, but these are much less common. It often presents nonspecific symptoms in patients with chronic pancreatitis and less commonly in acute pancreatitis [11]. Present only with vague abdominal pain, anorexia, nausea, or vomiting. A history of pancreatitis, combined with classic imaging findings of a thick-walled fluid collection next to the pancreas, is pathognomonic of a pancreatic pseudocyst. Imaging studies will help to get the diagnosis. Transabdominal ultrasound is often the initial imaging study due to its portability, easy use, and cost; it has a 70 to 90 % sensitivity. The diagnostic modality of choice would be a contrast-enhanced CT scan of the abdomen, with a sensitivity of 82 to 100 % and a specificity of 98%. CT scan over ultrasound may have some advantages; it helps to visualize surrounding structures and allows to identify biliary stones or calcification. The only significant inability of the CT scan is the limitation in distinguishing between a pseudocyst and a neoplastic cystic lesion.
In treating pancreatic pseudocysts, characteristics of the pseudocysts and the presence or absence of symptoms must be considered to decide the conduct and follow-up therapy. Several recent studies have emphasized that asymptomatic and minimally symptomatic pancreatic pseudocysts identified through a CT scan or US may be managed conservatively without intervention management; it is considered that 40% of these cases are spontaneously resolved [14]. Patients who were more likely to have an intervention procedure were those with the following: 1) Complicated pseudocyst-like compression of prominent veins; gastric or duodenal obstruction; compression of a main bile duct; associated with pancreatic ascites or a pancreatic‑pleural fistula; infected pancreatic pseudocyst; or hemorrhage in pancreatic pseudocyst. 2) Symptomatic pseudocyst with satiety, nausea, vomiting, severe abdominal pain, and/or back pain or upper gastrointestinal bleeding. 3) Asymptomatic pseudocysts >6 cm without any regression for >6 weeks. 4) Patients with an extrahepatic pseudocyst [14,15]. There are multiple treatment techniques for pancreatic pseudocyst: percutaneous, surgical, and endoscopic drainage. Percutaneous drainage techniques, including simple percutaneous aspiration drainage and continuous percutaneous drainage, are both achieved through the help of a US or CT scan. This technique successfully resolves pseudocysts but has a high risk of infections [16].
Surgical pseudocyst drainage is accomplished by communicating between the pseudocyst cavity and the stomach or small bowel [14]. This approach to drainage is often reserved for those patients that cannot tolerate or have failed percutaneous or endoscopic drainage. The surgical stoma should be placed in the most dependent portion of the cystic cavity to maximize the chances of complete drainage. The stoma usually remains patent and functional for several months. SD is the first‑line therapy choice for symptomatic pancreatic pseudocyst; it demonstrates a permanent resolution of 91‑97% in cystogastrostomy and gastrojejunostomy, with mortality rates of 0‑13% and morbidity rates of 10‑30% [17]. Other indications for preferential surgical treatment are i) contraindication or failure of endoscopic or radiological methods; ii) complex main pancreatic duct stricture; iii) complex pathology such as an inflammatory mass in the pancreatic head; iv) main bile duct stricture caused by pseudocyst compression; v) venous occlusive disease; vi) multiple pseudocysts; vii) pseudocyst of the pancreatic tail; viii) hemorrhage not adequately controlled by angiographic embolization; and ix) suspicion of neoplastic cysts [17,18]. Endoscopic drainage of pseudocysts is the preferred therapeutic approach because it is less invasive than surgery, avoids the need for an external drain, and has a high long-term success rate [14,19]. Drainage is accomplished with either a transpapillary approach with ERCP or direct drainage across the stomach or duodenal wall. A transpapillary approach is used when the pseudocyst communicates with the main pancreatic duct, usually in the genue of the pancreatic duct. This approach is also successful for patients with pancreatic duct disruption. A transgastric or transduodenal approach is used when the pseudocyst is directly adjacent to the gastro-duodenal wall [18,19].
Pancreatic Necrosis
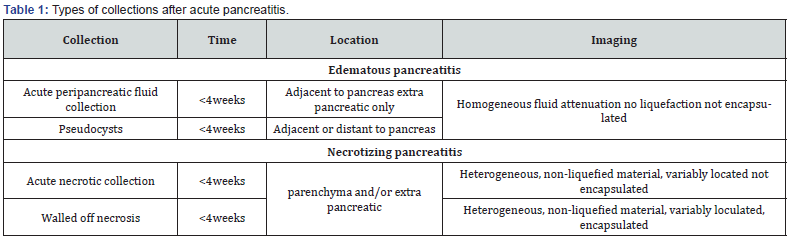
Pancreatic necrosis is a late complication that can occur after acute pancreatitis, resulting in the death of pancreatic tissue with loss of organ function. There are two types: sterile and infected necrosis. Sterile necrosis occurs without bacterial infection and is caused by severe inflammation and disrupted blood flow in the pancreas. Infected necrosis, on the other hand, involves bacterial colonization of the necrotic tissue [20]. Both types of necrosis present with symptoms such as severe abdominal pain, nausea, vomiting, and fever. Diagnosis is based on clinical symptoms, laboratory tests, and imaging findings. Imaging techniques like computed tomography (CT) scans can reveal non-enhanced areas in the pancreas, indicating necrosis. In infected necrosis, gas bubbles, fluid collections, or abscesses may be visible on imaging [21,22]. A comparison between the different types of collections seen after acute pancreatitis is depicted in Table 1.
Treatment for sterile pancreatic necrosis is usually conservative and supportive, focusing on pain control, intravenous fluids, nutritional support, and monitoring for complications. In some cases, drainage procedures may be necessary to manage fluid collections [22]. Infected pancreatic necrosis requires a combination of medical and surgical interventions [22,23]. Antibiotics are administered to target bacterial infection, and surgical debridement becomes necessary. Open surgical debridement involves removing necrotic tissue through a surgical incision. Minimally invasive procedures like video-assisted retroperitoneal debridement (VARD) or endoscopic necrosectomy are also used to remove necrotic tissue [24].
VARD is a minimally invasive procedure that aims to remove necrotic tissue using a retroperitoneal approach. VARD offers the advantages of decreased postoperative pain, shorter hospital stays, and faster recovery compared to open surgery. Endoscopic necrosectomy is another minimally invasive procedure performed through natural orifices, such as the mouth or rectum [25]. This technique allows direct visualization and targeted removal of necrotic material. Endoscopic necrosectomy is often performed in conjunction with other endoscopic interventions, such as the placement of stents or drainage catheters, to optimize outcomes. Lastly, open surgical debridement is a more invasive intervention typically performed through a midline or transverse incision in the abdomen [20,26]. There is dissection and removal of the nonviable pancreatic tissue, irrigation to the area to remove debris, and placement of drains to facilitate drainage of any residual fluid or infection. Open surgical debridement is usually reserved for cases with extensive necrosis, severe infection, or when minimally invasive options are not feasible [23,26]. The choice of surgical treatment depends on several factors, including the extent and severity of necrosis, infection, the patient's overall condition, and the expertise and resources available at the treatment facility [23,26]. The surgical approach is often determined through a multidisciplinary discussion involving gastroenterologists, radiologists, and surgeons to tailor the treatment to the patient's needs.
In summary, pancreatic necrosis is a relatively common late complication of acute pancreatitis. Sterile necrosis occurs without bacterial infection and is managed conservatively, while infected necrosis requires medical and surgical interventions. Treatment involves supportive care, drainage procedures, antibiotics, and surgical debridement options such as open surgery, VARD, or endoscopic necrosectomy. Therefore, prompt diagnosis and appropriate management are crucial in optimizing outcomes for patients with pancreatic necrosis (Table 1).
Intestinal Obstruction & Ileus
Intestinal obstruction is the blockage of the normal passage of luminal contents through the gastrointestinal tract that can be caused by extrinsic compression or an intraluminal process. On the contrary, ileus refers to a failure of normal intestinal motility in the absence of mechanical obstruction [27]. Obstruction complications in acute pancreatitis, such as intestinal obstruction or paralytic ileus, are rare [28]. Mechanical obstruction is most commonly seen on the splenic flexure and transverse colon, usually caused by severe inflammation of the body and tail of the pancreas causing extrinsic compression, retroperitoneal extravasation of pancreatic enzymes causing peri colitis or thrombosis of mesenteric arteries with infarction / ischemic necrosis of watershed areas. Although small bowel obstruction has been infrequently described during acute pancreatitis, it would undergo the same mechanism. Colonic paralytic ileus is a relatively more common and less severe complication than mechanical obstruction. Although the etiology of ileus is not entirely understood, it may rise from a visceral reflex in the superior mesenteric plexus secondary to retroperitoneal inflammation and/or transient colonic ischemia [28]. Moreover, severe acute pancreatitis with involvement of the duodenum can cause a duodenal obstruction that can be functional or both structural and functional in etiology. Incidence is approximately between 1 to 4 percent [28]. Intestinal obstruction secondary to acute pancreatitis usually resolves with conservative or supportive management, including pancreatitis treatment. However, surgical intervention is warranted when associated with retroperitoneal collections or necrotizing pancreatitis [28-30].
In conclusion, it is very important to be aware of obstruction complications of acute pancreatitis, which are rare but potentially deadly and should be managed aggressively. Management should be focused on underlying pancreatitis and reserved surgical management for unstable patients with refractory bowel obstruction [28,31].
Fistulization
Fistulas following acute pancreatitis refer to abnormal connections or passages between the pancreas and adjacent structures. These connections can involve the stomach, intestines, or bile ducts—the development of fistulas after acute pancreatitis can be attributed to the inflammatory process within the pancreas [32]. During acute pancreatitis, inflammation, and tissue necrosis can weaken the walls of the pancreas, forming a communication pathway or tract. This tract allows pancreatic secretions to escape into nearby structures, resulting in the formation of a fistula. The inflammatory response, enzymatic activity, and tissue destruction play significant roles in the pathophysiology of fistulas. The most common symptoms associated with pancreatic fistulas may include persistent abdominal pain, nausea, vomiting, diarrhea, weight loss, malabsorption, and symptoms related to the involvement of adjacent organs. However, the severity and specific symptoms can vary depending on the location and size of the fistula [32,33]. Patients may also develop an infection, abscess, sepsis, malnutrition, electrolyte imbalances, pancreatic insufficiency, pancreatic pseudocysts, or other complications related to the involvement of adjacent structures. Diagnosing pancreatic fistulas involves a combination of clinical assessment, imaging studies, and laboratory tests. Imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), or endoscopic retrograde cholangiopancreatography (ERCP) may be employed to visualize the fistula and assess its characteristics [33,34].
Some types of fistula that may be encountered after acute pancreatitis include pancreatic-pleural fistula (the passage of pancreatic enzymes, fluid, or necrotic debris into the pleural cavity, which may result in pleural effusion and respiratory symptoms), pancreatic-enteric (results in malabsorption, diarrhea, and other gastrointestinal symptoms), pancreatic-gastric (potentially causing gastric irritation, ulcers), pancreatic-biliary (leading to jaundice or cholangitis), pancreatic-cutaneous (persistent skin leakage, infection, and challenges in wound healing), pancreatic-vesical (causing recurrent urinary tract infections, hematuria or other urinary symptoms) [35].
The treatment of fistulas after acute pancreatitis depends on the condition's specific type, location, and severity [35,36]. Conservative management options may include supportive care, pain management, nutritional support, and fluid and electrolyte imbalance correction. In some cases, surgical intervention may be necessary to repair or divert the fistulous tract, remove necrotic tissue, or manage complications such as abscesses or pseudocysts [36].
Surgical strategies may vary depending on the fistula's specific type, location, and severity. In cases where the fistula tract is well-defined and accessible, surgical repair may be performed. The goal is to close the abnormal connection between the pancreas and the adjacent structure (e.g., stomach, intestine, bile ducts) [36,37]. This can be achieved through various techniques, including primary closure, suturing, or tissue flaps. On the other hand, in situations where direct repair is not feasible or the fistula is complex, diverting the flow of pancreatic secretions may be necessary. This involves creating a new pathway or rerouting the pancreatic fluids away from the fistula site. Standard diversion procedures include pancreatojejunostomy (creating a connection between the pancreas and the jejunum) or pancreaticoduodenal resection (removal of the diseased portion of the pancreas and duodenum). Surgical drainage may be required in cases with associated complications such as pseudocysts or abscesses [36,38]. This involves creating a controlled opening or drainage pathway to remove the accumulated fluid or infected material. In addition, procedures like percutaneous drainage, cystogastrostomy, or cystojejunostomy may be performed [39]. In severe cases with extensive tissue necrosis, uncontrolled sepsis, or recurrent fistulas, partial or complete pancreas removal (pancreatectomy) may be necessary. Pancreatectomy can involve distal pancreatectomy (removal of the tail and body of the pancreas) or total pancreatectomy (removal of the entire pancreas) [32,35]. This procedure is usually reserved for complicated cases or when other surgical options have been exhausted. Given the above, the choice of the best surgical strategy depends on factors such as the patient's overall condition, the location and complexity of the fistula, the presence of associated complications, and the surgeon's expertise.
Abdominal Compartment Syndrome
Compartment syndrome is defined as the increase in pressure within a certain cavity, in this case, the abdomen (ACS), thus producing organ damage or dysfunction. Usually, the abdominal pressure is below 10 mmHg, it is called abdominal hypertension when it is above 12 mmHg, according to the guidelines, and it is called abdominal compartment syndrome when the pressure rises above 20 mmHg. As a result, there is a sign of organ damage/ dysfunction [40]. The exact prevalence of ACS is challenging due to the varying definitions used in studies. However, estimates range from 1% to more than 20% in critically ill patients at risk. In addition, specific populations have an exceptionally high risk, including trauma patients, in which ACS occurs in up to 24% of severe trauma patients admitted to the ICU. Of burn patients, 40% develop ACS. After massive hemorrhage, 15% of patients develop ACS. After extensive abdominal surgery, 10% develop ACS [40,41].
Risk factors for developing ACS can be grouped into: Factors leading to intra-abdominal hypertension (extensive volume fluid resuscitation, massive transfusion, aggressive enteral feeding); factors reducing abdominal wall compliance (obesity, ascites, pancreatic inflammation, or necrosis); mechanical ventilation; increased intra-abdominal contents due to bleeding, edema, or inflammation. Abdominal compartment syndrome (ACS) occurs when intra-abdominal pressure rises to a level that impairs organ and tissue function. The normal intra-abdominal pressure is around 5–7 mmHg. An intra-abdominal pressure above 19 mmHg is considered pathological and leads to ACS. The underlying pathophysiology of ACS involves several mechanisms, which can be combined (cycle) or individually. These include: increased pressure is transmitted directly to the organs and blood vessels within the abdomen, impairing blood flow; reduced venous return and decreased cardiac output occur due to compression of the inferior vena cava and other veins; compromised respiratory function results from the decreased diaphragm and chest wall compliance and reduced lung volumes; impaired renal perfusion and decreased glomerular filtration rate lead to acute kidney injury; ischemia of intestinal mucosa causes increased intestinal permeability and bacterial translocation [42,43]. The clinical presentation of ACS has a constellation of symptoms that vary in intensity and location depending on where the problem is, or the organ involved (ex: pancreatitis). Patients typically exhibit difficulty breathing or shortness of breath, difficulty urinating, abdominal distention and pain, high blood pressure, decline in mental status, and oliguria. Diagnosing ACS after a presentation of pancreatitis involvesclinical symptoms as mentioned above, also by measuring intra-abdominal pressure (Intra-abdominal pressure above 20 mmHg is diagnostic of ACS) and tests to rule out other causes of symptoms like urinary obstruction, bowel obstruction, etc. [44,45]. The mainstay of treatment for ACS is surgical decompression by opening the abdominal wall. This can be done with any of the following procedures: Laparotomy, a full midline incision in the abdomen. Laparoscopy or Keyhole surgery uses small incisions and cameras to open the abdominal wall, and fasciotomy, an Incision of the abdominal wall fascia to, release pressure [40,46].
The choice of procedure depends on the patient's condition, the underlying cause of ACS, and surgeon preference. The prognosis depends on several factors, like how quickly the ACS is recognized and treated (early surgical decompression gives the best chance of recovery); the severity of organ dysfunction before and after decompression (the more organs that are affected, the worse the prognosis), the underlying cause of ACS (some causes have a better prognosis than others). With timely and appropriate treatment, the mortality rate of ACS can be as low as 20-30%. However, the mortality rises above 50% in patients with severe and refractory ACS. Overall, ACS is a life-threatening condition requiring a high index of suspicion, early diagnosis, and prompt surgical decompression to achieve the best outcomes [40,46-48].
Conclusion
Late complications of acute pancreatitis are a health concern due to the high rate of hospitalizations and high mortality per year in the United States. The surgical approach plays a significant role in managing late complications of acute pancreatitis, such as peripancreatic fluid collections, pseudocysts, pancreatic abscesses, pancreatic necrosis, intestinal obstruction, and abdominal compartment syndrome. However, the choice of surgical treatment depends on the specific complication, its severity, and the patient's clinical condition. Peripancreatic fluid collections, known as APFCs or PFCs, often arise as common complications of acute pancreatitis. While most acute collections resolve independently, persistent ones may evolve to become walled-off or chronic collections like pseudocysts or necrotic collections. Endoscopic drainage of pseudocysts is the preferred therapeutic approach because it is less invasive than surgery and has a high long-term success rate. Other treatment techniques involve percutaneous and surgical drainage. Surgical treatment is often reserved for those patients that cannot tolerate or have failed percutaneous or endoscopic drainage.
Pancreatic abscesses, which are collections of pus or infected fluid that arise nearby or in contact with the pancreas, often develop from a previously sterile pancreatic fluid that becomes infected due to the transmigration of bacteria. Treatment usually involves a combination of antibiotics and surgical drainage. On the other hand, pancreatic necrosis is caused by severe inflammation and compromised blood flow. It can occur in either sterile or infected forms, with the latter involving bacterial colonization. Sterile necrosis is managed conservatively, while infected necrosis requires antibiotics and surgical debridement. Surgical debridement can be performed through open surgery or minimally invasive techniques such as VARD or endoscopic necrosectomy. Open surgical debridement is usually reserved for cases with extensive necrosis, severe infection, or when minimally invasive options are not feasible. While rare, intestinal obstruction and paralytic ileus can occur as complications. Intestinal obstruction secondary to acute pancreatitis usually resolves with conservative or supportive management. The treatment should be focused on underlying pancreatitis and reserved surgical management for unstable patients with refractory bowel obstruction. Since the treatment of late complications of acute pancreatitis ranges from conservative management to minimally invasive endoscopic techniques, percutaneous drainage, and even open surgery, a multidisciplinary approach involving gastroenterologists, radiologists, and surgeons is often necessary to tailor treatment to the patient's individual needs.
References
- Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN (2018) American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 154(4): 1096-1101.
- Lankisch PG, Apte M, Banks PA (2015) Acute pancreatitis. Lancet 386(9988): 85-96.
- Chatila AT, Bilal M, Guturu P (2019) Evaluation and management of acute pancreatitis. World J Clin Cases 7(9): 1006-1020.
- Gapp J, Tariq A, Chandra S (2023) Acute Pancreatitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
- Poornachandra KS, Bhasin DK, Nagi B, Sinha SK, Rana SS, et al. (2011) Clinical, biochemical, and radiologic parameters at admission predicting formation of a pseudocyst in acute pancreatitis. J Clin Gastroenterol 45: 159-163.
- Johnson CD (1993) University Surgical Unit, Southampton General Hospital, UK. Timing of intervention in acute pancreatitis. Postgrad Med J 69: 509-515.
- Bradley EL, Clements JL Jr, Gonzales AC (1979) The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg 137: 135–141.
- Vitas GJ, Sarr MG (1992) Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery 111: 123–130.
- Baron TH, D E Morgan (1997). The Diagnosis and Management of Fluid Collections Associated with Pancreatitis. The Am J Med 102(6): 555-563.
- Banks PA (1996) Acute pancreatitis. Practice Parameters Resource Manual. Arlington, Virginia: American College of Gastroenterology 1: 91-107.
- Balthazar EJ, Freeny PC, vanSonnenberg E (1994) Imaging and intervention in acute pancreatitis. Radiology 193: 297-306.
- Bradley EL, Allen K (1991) A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg 161: 19-24.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JHC (1990) Acute pancreatitis: value of CT in establishing prognosis. Radiology 174: 331-336.
- Tan JH, Chin W, Shaikh AL, Zheng S (2021) Pancreatic pseudocyst: Dilemma of its recent management (Review). Exp Ther Med 21(2): 159.
- D'Egidio A, Schein M (1991) Pancreatic pseudocysts: A proposed classification and its management implications. Br J Surg 78: 981-984.
- Heider R, Meyer AA, Galanko JA, Behrns KE (1999) Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg 229: 781-789
- Núnez C, Jimenez Gonzalez A (2004) Surgical treatment of pancreatic pseudocyst. Rev Gastroenterol Mex 69 (Suppl 3): S119-S120
- Weckman L, Kylanpaa ML, Puolakkainen P, Halttunen J (2006) Endoscopic treatment of pancreatic pseudocysts. Surg Endosc 20: 603-607.
- Misra D, Sood T (2003) Pancreatic Pseudocyst. [Updated 2023 Feb 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
- Baron TH, DiMaio CJ, Wang AY, Morgan KA (2020) American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 158(1): 67-75. e1.
- Yasuda I, Takahashi K (2020) Endoscopic management of walled-off pancreatic necrosis. Dig Endosc 33(3): 335-341.
- Thomson JE, Van Dijk SM, Brand M, Van Santvoort HC, Besselink MG (2018) Managing Infected Pancreatic Necrosis. Chirurgia (Bucur) 113(3): 291-299.
- Ramia JM, de la Plaza R, Quiñones-Sampedro JE, Ramiro C, Veguillas P, et al. (2012) Walled-off pancreatic necrosis. Neth J Med 70(4): 168-71.
- Maurer LR, Fagenholz PJ (2023) Contemporary Surgical Management of Pancreatic Necrosis. JAMA Surg 158(1): 81-88.
- Wolbrink DRJ, Kolwijck E, Ten Oever J, Horvath KD, Bouwense SAW, et al. (2020) Management of infected pancreatic necrosis in the intensive care unit: a narrative review. Clin Microbiol Infect 26(1): 18-25.
- Bugiantella W, Rondelli F, Boni M, Stella P, Polistena A, et al. (2016) Necrotizing pancreatitis: A review of the interventions. Int J Surg 28 Suppl 1: S163-171.
- Kufa DW, Pollock RE, Weichselbaum RR, et al. (2003) Holland-Frei Cancer Medicine. 6th Hamilton (ON): BC.
- Etienne D, Caughey ME, Gaduputi V (2017) Small Bowel Obstruction Secondary to Acute Pancreatitis. Gastroenterology Res 10(1): 42-44.
- Sainani NI, Sahni VA, Jeffrey F Chick, Nikunj R Chauhan, Darwin L Conwell, et al (2014) Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 34: 1218-39.
- Hart PA (2017) Complications of chronic pancreatitis. Dig Dis Sci 62: 1745-1750.
- Tagore S, Denzil E, Caughey M, Gaduputi V (2017). Small Bowel Obstruction Secondary to Acute Pancreatitis. Gastroenterology Res 10(1): 42-44.
- Fielding GA, McLatchie GR, Wilson C, Imrie CW, Carter DC (1989) Acute pancreatitis and pancreatic fistula formation. Br J Surg 76(11): 1126-8.
- Mallick B, Nath P, Praharaj DL (2022) Gastrointestinal: Pancreatico-iliopsoas fistula in chronic pancreatitis. J Gastroenterol Hepatol 37(10): 1841.
- RISOLIA AJ (1950) Fästula pancreática [Pancreatic fistula]. Bol Univ B Aires 1950 26(190): 176-83. Undetermined Language.
- Renard Y, de Mestier L, Perez M, Avisse C, Lévy P, et al. (2018) Unraveling Pancreatic Segmentation. World J Surg 42(4): 1147-1153.
- Francisco E, Mendes M, Vale S, Ferreira J (2015) Pancreaticopleural fistula: an unusual complication of pancreatitis. BMJ Case Rep 2015: bcr2014208814.
- Alsumait AR, Jabbari M, Goresky CA (1978) Pancreaticocolonic fistula: a complication of pancreatitis. Can Med Assoc J119(7): 715-19.
- Brown A, Malden E, Kugelmas M, Kortz E (2014) Diagnosis of pancreatic duct-portal vein fistula; a case report and review of the literature. J Radiol Case Rep 8(3): 31-38.
- Skawran SM, Kambakamba P, Baessler B, von Spiczak J, Kupka M, et al. (2021) Can magnetic resonance imaging radiomics of the pancreas predict postoperative pancreatic fistula? Eur J Radiol 140: 109733.
- Andrew W Kirkpatrick, Derek J Roberts, Jan De Waele, Roman Jaeschke, Manu L N G Malbrain et al. (2013) Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 39 (7): 1190-206.
- Smit M, Koopman B, Dieperink W, Jan B F Hulscher, H Sijbrand Hofker, et al. (2020) Intra-abdominal hypertension and abdominal compartment syndrome in patients admitted to the ICU. Ann Intensive Care 10 (1): 130.
- ML Cheatham, MLNG Malbrain, A Kirkpatrick, M Sugrue, M Parr, et al. (2006) Results from the International Conference Experts on Intra-Abdominal Hypertension and Abdominal Compartment Syndrome. Intensive Care Med 32(11): 1722-1732.
- A Kirkpatrick, D J Roberts, J de Waele, R Jaeschke, MLNG Malbrain, B de Kuelenaer, et al. (2013) Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 39 (7): 1190-1206
- Maluso P, Olson J, Sarani B (2016) Abdominal Compartment Hypertension and Abdominal Compartment Syndrome. Crit Care Clin 32 (2): 213-22.
- A Bauman, F Jones, G Bishop, A Flabouris, M Parr, et al. (2002) Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 26 (1): 1428-1431.
- Crandall ML, Nicolson NG, Smith-Singares E, Merlotti GJ, Jalundhwala Y, et al. (2016) Abdominal compartment syndrome in trauma patients: New insights for predicting outcomes. J Emerg Trauma Shock 9 (2): 53-57.
- HD Wittmann, C Aprahamian, JM Bergstein. (1990) Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg, 14 (1): 218-226
- Cheatham ML, Safcsak K (2010) Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med 38(2): 402-407.






























