Indication for Robotic-Assisted Surgery Influences Perioperative Outcomes and Hospital Readmissions among Women Undergoing Gynecologic Procedures for Benign and Malignant Pathologies
Kelsey I Musselman1, Melissa K Frey1, LiJin Joo2, Nigel I Madden3, Jessica Lee4, Stephanie V Blank5 and Bhavana Pothuri2
1Weill Cornell Medicine , United States
2New York University Langone Health , United States
3Columbia-Presbyterian, United States
4UT Southwestern, United States
5Icahn School of Medicine at Mount Sinai, United States
Submission:June 12, 2020;Published:February 24, 2021
*Corresponding author: Bhavana Pothuri, MD, NYU Langone Health, 550 First Avenue, New York, New York 10016, United States, IRB exemption: New York University (NYU) School of Medicine Institutional Review Board (#09-0102, 12/03/2014)
How to cite this article: Kelsey I M, Melissa K F, LiJin J, Nigel I M, Jessica L, et al. Indication for Robotic-Assisted Surgery Influences Perioperative Outcomes and Hospital Readmissions among Women Undergoing Gynecologic Procedures for Benign and Malignant Pathologies. Open Access J Surg. 2021; 12(4): 555845 DOI:10.19080/OAJS.2021.12.555845.
Abstract
Objective: Differences in complication rates and outcomes are expected when comparing surgery for benign and malignant indications, however there are limited data addressing this in robotic-assisted gynecologic surgery. As this distinction and its ramifications can influence patient counseling, surgical planning and reimbursement as we transition to value-based payment models, we sought to evaluate perioperative outcomes for women undergoing gynecologic robotic-assisted surgery for benign versus malignant indications.
Methods: We reviewed the medical records of all patients undergoing robotic-assisted gynecologic surgery at a single institution by high-volume robotic surgeons from January 2013 - May 2016. Perioperative outcomes were evaluated using univariate and multiple regression analysis to compare complications for benign versus malignant surgical indications.
Results: Two thousand seven hundred and fifty-seven patients were included (benign 2316, malignant 441). Malignant cases were significantly older (58 vs. 42y, P<0.001) with more medical comorbidities and higher BMI. Compared to benign cases, malignant cases included a higher percentage of hysterectomies (82.3% v. 34.7%, P<0.001) with a greater number of cases including lymph node dissection (54.2% v. 0.8%, P<0.001) or staging biopsies (3.4% v. 0.4%, P<0.001). Malignant cases also had longer surgical time (3.7 v. 2.8 hours, P<0.001), higher rates of intraoperative complications (7.5% v. 4.6%, P=0.01), conversion to laparotomy (3.4% v. 0.9%, P<0.001), length of hospital stay (10.5h vs. 7.0h, P<0.001), emergency department (ED) visits within six weeks (8.2% vs. 5.4%, P=0.02), and hospital readmission within six weeks (5.7% vs. 2.0%, P<0.001). There was no difference in estimated blood loss or postoperative complications. Post-operative complications in benign vs malignant cases, respectively included: fever [75 (5%) 21 (5%)]; urinary tract infection [59 (4%), 24 (6%)]; wound infection [38 (2%), 8 (2%)]; abscess [14 (1%), 4 (1%)]; other infection [13 (0.8%), 10 (2%)]; port-site hernia 11 (0.7%), 4 (0.9%)]; small bowel obstruction [7 (0.4%), 3 (1%)]; arrhythmia [19 (1%), 13 (3%)]; pulmonary embolism [4 (0.3%), 3 (0.7%)] and re-operation [12 (0.8%), 5 (1%)]. On multiple regression analysis adjusting for age, BMI, medical comorbidities and perioperative complications, malignancy remained associated with longer operating time, lower rate of same-day hospital discharges, higher rate of conversion to laparotomy and more hospital readmissions within six weeks.
Conclusion: Robotic surgery performed for gynecologic malignancy is associated with higher rates of conversion to laparotomy and hospital readmission compared to robotic surgery performed for benign disease. Malignant cases had longer operating time and fewer same-day discharges. This information is very important for physician and patient surgical expectations and anticipated healthcare costs and should be accounted for when determining models of value-based reimbursement.
Keywords: Hospital readmission; Hysterectomy; Malignant; Operating time; Robotic surgery
Introduction
There has been a substantial increase in robotic-assisted surgery for benign and malignant gynecologic surgery since the daVinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) was granted Food and Drug Administration (FDA) approval for gynecologic surgery in 2005 [1,2]. By 2010, 9.5% of all hysterectomies for benign gynecologic disorders were performed robotically, and this number continues to grow [3]. In 2015, a survey of Society of Gynecologic Oncology members indicated that 97% of respondents performed robotic surgery, up from 27% in 2007 [4]. Robotic-assisted surgical technology offers advantages including three-dimensional high-definition stereoscopic vision, ergonomic positioning, wristed instruments and tremor cancelling software, which offer the surgeon additional manual dexterity and surgical precision [5-7]. Despite concern about potentially greater cost compared to open surgery and conventional laparoscopy, robotic surgery appears to be a safe minimally invasive approach for patients with comparable rates of intraoperative and postoperative complications compared to patients undergoing laparoscopy [8-10]. Additionally, robotic surgery may be superior to conventional laparoscopy with regard to lower estimated blood loss conversion rate, and hospital readmission rate [11-13].
The increasing prevalence and clinical advantages of conventional laparoscopic and robotic-assisted surgery have altered the standards of treatment in gynecologic oncology. In the Gynecologic Oncology Group LAP-2 study, laparoscopic surgical staging for uterine cancer was shown to be safe and effective in terms of short term outcomes with fewer complications and shorter hospital stay with no increased risk of cancer recurrence over time [14,15]. In recent years, a growing body of literature examining robotic-assisted gynecologic oncologic surgery has demonstrated increasing evidence regarding its utility, safety and efficacy in treatment of certain gynecologic cancers [9,13,16-19].
This growing use of robotic-assisted surgery in staging and treating gynecologic cancers necessitates awareness regarding higher surgical risks associated with malignancy, as differences in complication rates and perioperative outcomes are expected when comparing surgery for benign and malignant indications. Previous studies have shown higher rates of perioperative complications and post-surgical readmissions in patients with gynecologic malignancies compared to those with benign disease [20-22]. However, there are limited data concerning this difference in perioperative complications between benign and malignant disease in robotic-assisted gynecologic surgery. Perioperative complications have a dramatic influence on hospital costs, with a complicated case costing up to five times the amount of a similar operation without complications [23]. As we move to value-based payment models, this distinction and its implications are important for patient counseling, surgical planning and cost analysis. Therefore, we sought to evaluate perioperative outcomes for women undergoing gynecologic robotic-assisted surgery for benign versus malignant indications.
Materials and Methods
Approval was obtained from the New York University (NYU) School of Medicine Institutional Review Board (#09-0102, 12/03/2014); the study was exempt. A review was conducted of all patients undergoing robotic-assisted surgery at a single institution performed by a robotically-trained gynecologic surgeon or gynecologic oncologist between January 2013 and May 2016. The primary endpoint was to compare peri-operative and post-operative outcomes for patients undergoing surgery for benign and malignant indications.
Patient characteristic and demographic data were recorded, including age at time of surgery, body mass index (BMI), race, ethnicity and smoking status. Patient medical comorbidities such as hypertension, diabetes, coronary artery disease, asthma and chronic obstructive pulmonary disease (COPD) were documented, as well as history of malignancy and prior abdominal surgery. The type of surgical procedure performed for the cases in each of the two groups was accounted for. Intraoperative parameters including estimated blood loss (EBL), operative time and intra-operative complications (blood transfusions, anesthesia complications, conversion to laparotomy and structural damage) were recorded. Operative time was defined as the time from first surgical incision to skin closure.
The postoperative parameters examined included hours in the post-anesthesia care unit (PACU), time to hospital discharge (hours), hospital status and postoperative complications in the first six weeks after surgery. Hospital status was categorized as same-day discharge (observation care on the same calendar date as the surgery), 23-hour observation (observation care until the day after surgery without full inpatient admission) and full admission (inpatient care services requiring admission to the hospital gynecology service or intensive care unit). Postoperative complications included: fever, urinary tract infection, wound complication or infection, abscess, other infection, small bowel obstruction, ileus, fistula, postoperative transfusion, positionrelated nerve injury, arrhythmia, deep vein thrombosis, pulmonary embolism, intensive care unit (ICU) admission, reoperation and death. Emergency room and urgent care visits within six weeks after surgery occurring either at our institution or at an outside institution as reported in the medical record were documented, as were hospital readmissions within six weeks.
Statistical Analysis
The distribution of continuous variables was tested for normality via the Kolmogorov–Smirnov test. Univariate tests were applied based on whether the variable of interest was distributed normally (i.e. t -test, analysis of variance) or not normally (i.e. Mann–Whitney U test). Associations between categorical variables were evaluated by chi-square tests or Fisher’s exact tests as appropriate for category size. Logistic and linear regression analysis was also conducted to account for potential confounders. The acceptable α error level was set at P = 0.05 using 2-tailed tests. Data were analyzed using statistical program R (http:// www.r-project.org).
Results
Patient characteristics
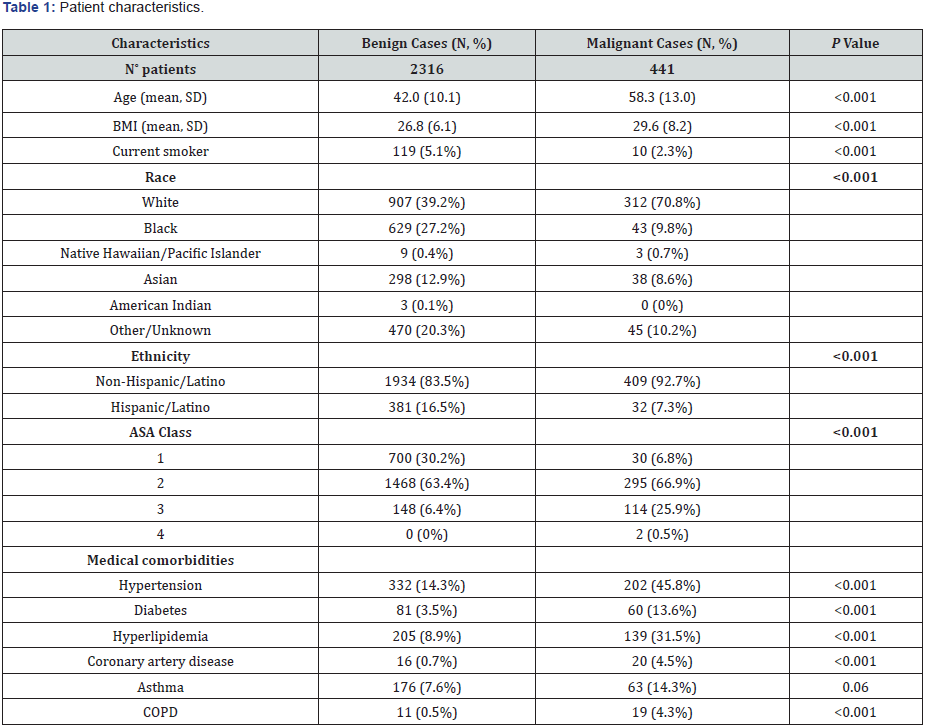
A total of 2,757 patients underwent robotic-assisted gynecologic surgery by high-volume gynecologic surgeons (8) and gynecologic oncologists (4) at our institution between January 2013 and May 2016 (Table 1). There were 2,316 roboticassisted surgeries performed for benign indications and 441 for malignant indications. Among these, 426 (18%) benign surgeries were performed by gynecologic oncologists with the remaining 1,890 (82%) performed by generalists. In malignant cases, 427 (97%) were performed by a gynecologic oncologist and 14 (3%) by generalists. Patient characteristics are reviewed in Table 1. Patients with a malignant indication for surgery were significantly older with higher BMI (P<0.001). Current smoking was more prevalent among benign cases (P<0.001). Benign cases had a higher number of non-whites and Hispanics/Latinos than malignant cases (P<0.001). Malignant cases had more American Society of Anesthesiologists (ASA) class 3 or greater (26.4% vs. 6.4%). Malignant cases had more medical comorbidities, including hypertension (45.8% vs. 14.3%), diabetes (13.6% vs. 3.5%), hyperlipidemia (31.5% vs. 8.9%), coronary artery disease (4.5% vs. 0.7%) and COPD (4.3% vs. 0.5%) than their benign counterparts (P<0.001).
Surgical procedures
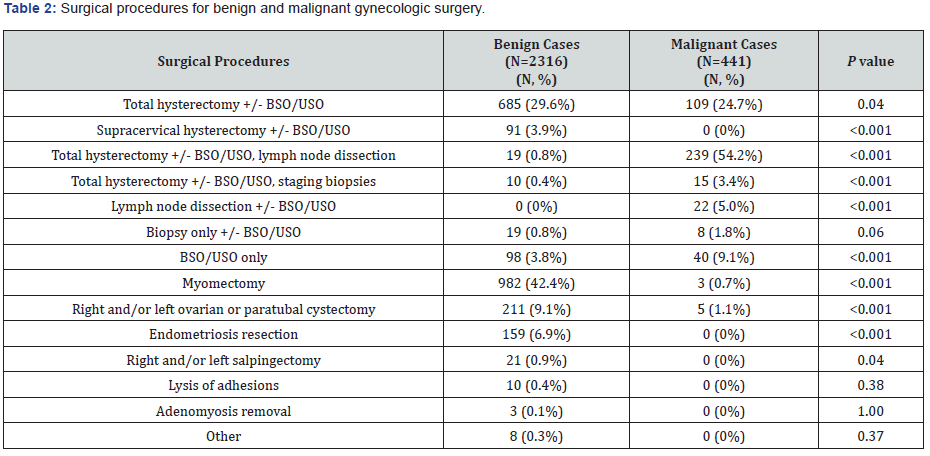
Types of surgical procedures differed between robotic surgeries performed for benign versus malignant indications (Table 2). Malignant cases had a higher percentage of patients undergoing hysterectomy (82.3% vs. 34.7%, P<0.001). Among those hysterectomies, the malignant group included a greater number of cases where lymph node dissection (54.2% v. 0.8%, P<0.001) or staging biopsies (3.4% v. 0.4%, P<0.001) were performed. For patients who did not undergo hysterectomy, the malignant group also had a higher number of cases who underwent lymph node dissection (5.0% v. 0%, P<0.001) or salpingo-oophorectomy only (9.1% v. 3.8%, P<0.001). Benign cases included a greater number of myomectomies (42.4% v. 0.7%, P<0.001), ovarian or paratubal cystectomies (9.1% v. 1.1%, P<0.001), endometriosis resection (6.9% v. 0%, P<0.001) and salpingectomy (0.9% v. 0%).
Intra-operative characteristics
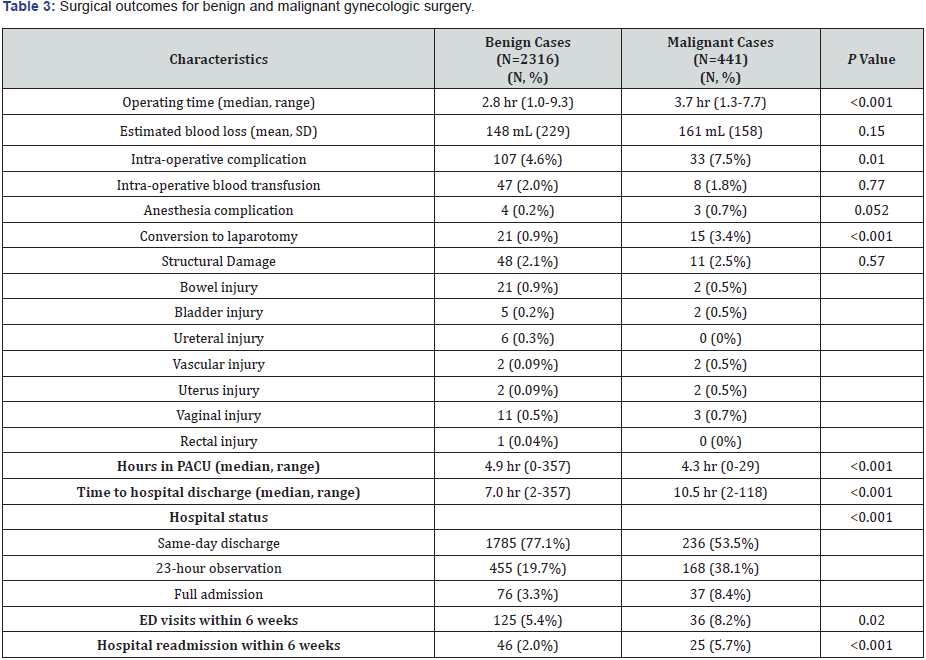
Median operative time was longer in the malignant cases than the benign cases (3.7 hours, range 1.3-7.7 vs 2.8 hours, range 1.0- 9.3, P<0.001) (Table 3). There was no difference in estimated blood loss between both groups (p=0.15). Intra-operative complications were higher in malignant cases (7.5%) than benign cases (4.6%) (P=0.01). There was no significant difference in the rate of intraoperative blood transfusions (P=0.77) or anesthesia complications (P=0.052). In malignant cases, 3.4% of patients required conversion to laparotomy compared to 0.9% of benign cases (P<0.001). There was no significant difference in the rate of intra-operative structural damage (p=0.57). Types of structural damage included bowel injury [benign 21 (0.9%), malignant 15 (3.4%)], bladder injury [5 (0.2%), 2 (0.5%)], ureteral injury [6 (0.3%), 0 (0%)], vascular injury [2 (0.09%), 2 (0.5%)], uterus injury [2 (0.09%), 2 (0.5%)], vaginal injury [11 (0.5%), 3 (0.7%)] and rectal injury (1 (0.04%), 0 (0%)].
P-value computed from T-test (for continuous variables) and Chi-squared test (for discrete variables).
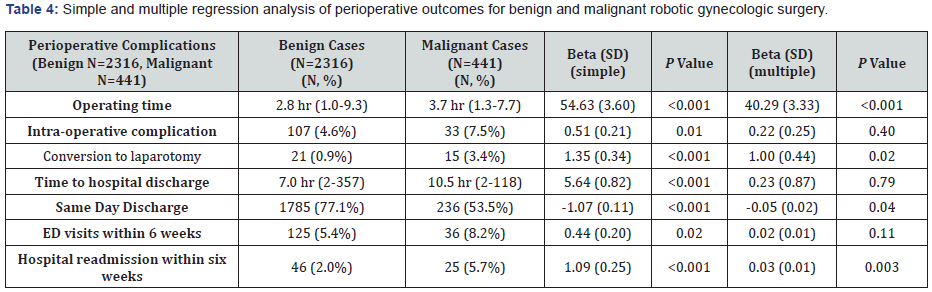
On multiple regression analysis controlling for age, BMI, race, ethnicity, medical comorbidities and perioperative outcomes, operating time and the rate of conversion to laparotomy remained significantly higher in malignant cases (P<0.001) (Table 4).
Multiple regression is controlled for age, race, ethnicity, BMI, smoking status, ASA class, medical comorbidities length of surgery, and perioperative outcomes and complications.
Post-operative characteristics
Malignant cases spent less time in the PACU (4.3 vs. 4.9 hours) but had a longer time to hospital discharge (10.5 vs. 7.0 hours) than their benign counterparts (P=0.001) (Table 3). Benign cases were more likely to be discharged the same day as their procedure, while malignant cases were more likely to undergo 23- hour observation or a full hospital admission (p<0.001).
Following discharge, malignant cases had more emergency department (ED) visits within six weeks (8.2% vs. 5.4%) than the benign cases (P=0.02). Hospital readmissions within six weeks occurred in 5.7% of malignant cases versus 2.8% of benign cases (P<0.001). Of the 2,757 patients who underwent robotic-assisted surgery at our institution, 2,023 had post-operative outpatient follow-up documented in our electronic medical record system. The 734 patients without post-discharge documentation were predominately in the benign group (731 benign vs. 3 malignant), and include those whose surgeons conduct their outpatient appointments independent from our hospital system and those lost to follow-up entirely. These 734 patients had complete preoperative and intra-operative data, and were included in the respective analyses as above. However, due to the incomplete post-operative complication data among these 734 patients, we decided to exclude them from analysis of post-operative complications within six weeks.
For those who had outpatient follow-up documented at our institution, there was no significant difference in the number of postoperative complications between the benign and malignant groups (P=0.13) (Table 5). The types and incidence of postoperative complications are recorded in Table 3. One patient with a gynecological malignancy expired as a result of complications following robotic-assisted surgery. This patient presented to the emergency department on post-operative day three with abdominal pain, weakness, emesis and altered mental status, and was found to have to have ascites of unknown origin on CT imaging. This finding prompted an exploratory laparotomy where a 5mm enterotomy was discovered, and the patient underwent bowel resection and anastomosis. Post-operatively the patient remained hypotensive despite vasopressors and went into ventricular tachycardia, requiring transfer to the surgical intensive care unit where the patient’s clinical status ultimately declined. On multiple regression analysis controlling for age, race, ethnicity, BMI, smoking status, ASA class, medical comorbidities length of surgery, and perioperative outcomes and complications, malignant cases show significantly fewer same-day discharges and higher readmission rates (Table 4).

Discussion
Our study found that robotic-assisted surgery performed for gynecologic malignancy is associated with longer operating time, higher rates of conversion to laparotomy, fewer same-day discharges and higher hospital readmission rate within six weeks compared to robotic-assisted surgery performed for benign disease. Alternatively, outcomes of malignant robotic gynecologic surgery were equivalent to benign robotic gynecologic surgery for estimated blood loss, anesthesia complications, intra-operative blood transfusions, structural damage and postoperative complications after discharge. Although prior studies have demonstrated these differences in perioperative surgical outcomes between benign and malignant gynecologic disease, there is limited data examining these differences for roboticassisted surgery [20-22].
Perioperative outcomes of benign and malignant roboticassisted gynecologic surgery have been extensively evaluated to confirm their safety and feasibility in gynecologic care. Previous studies on benign robotic-assisted gynecologic surgery report median lengths of stay ranging from 1-3.3 days, perioperative complication rates of 2-18.4% and conversion to laparotomy rates of 0-4% [24-26]. Comparatively, studies on malignant roboticassisted gynecologic surgery have reported median lengths of stay ranging from 5.3 hours-3.5 days and reported complication rates include 1-6.8% intra-operative complications, 1.4%-11% conversion to laparotomy, 5% blood transfusion, 2.3-21% postoperative complications and 1.4% readmissions [8,11,12,25,27- 30]. Our evaluation of benign and malignant robotic gynecologic surgery cases is comparable to the literature for intra-operative complications (4.6% benign v. 7.5% malignant), blood transfusion (2.0% v. 1.8%), conversion to laparotomy (0.9% v. 3.4%), postoperative complications (13.8% v. 16.0%) and readmissions (2.0% v. 5.7%). An important limitation of this study is that relied on the medical record from a single institution and therefore readmission rates may have been underestimated, as readmissions only to the reporting facility were accounted for.
Of note, there was no significant difference between the benign and malignant groups regarding post-operative complications (13.8% v. 16.0%, P=0.13), a finding that was unanticipated given that differences in post-operative complications might be expected between the two groups. This result excluded 734 patients (731 benign, 3 malignant) for whom we did not have data regarding their medical course following hospital discharge. We suspect that many of these patients did not experience postoperative complications after discharge, and that this may have affected our analysis. Lastly, as different surgical procedures are performed for benign and malignant gynecologic cases (Table 2), the likelihood of potential complications associated with the type of procedure may vary between the two groups.
As the type of surgery and extent of surgical dissection varies between the benign and malignant groups, one might indeed expect to see a difference in operative time, hospital stay, intraoperative complications and post-operative complications. The implications of the differences between the benign and malignant groups are important in accounting for variation in healthcare costs, especially in the context of value-based payment. Based on reports in the literature that the cost of operative time in robotic-assisted gynecologic surgical cases is $18-32 per minute, we estimate that a robotic surgery performed for a malignant indication costs an average of $972-1,728 greater than a benign case as a direct result of increased operating time [31-35]. Our malignant cases also had a longer mean time to hospital discharge by 3.5 hours than benign cases. Using a recent estimate which asserts that the cost of hospital stay after robotic gynecologic surgery is $6506 per day, we approximate an increase in cost of around $949 as a direct result of longer hospital stay [36]. Our findings were also notable for a higher rate of conversion to laparotomy in the malignant group. This is important to consider as the total cost of a conversion to laparotomy from robotic gynecologic surgery to 30 days post-surgery has been shown to be $7,829-15,249 greater compared to non-converted cases [34,37]. Furthermore, as our results show higher readmission rates among gynecologic oncology patients compared to benign patients, it is essential to consider that the cost of readmissions among this patient population is around $25,416 [38]. As there are very few cost analyses specifically examining how the above factors increase perioperative costs, further study is necessary to better estimate these cost differences between benign and malignant cases.
Advantages of our study include the pertinence of these results to gynecologic surgeons at other institutions who perform robotic-assisted surgery. To the extent of our knowledge, this is the first study that has examined the differences in perioperative outcomes between benign and malignant gynecologic surgical cases specifically for robotic-assisted surgery. A primary limitation of our analysis is the retrospective nature of this study. As mentioned previously, we could only capture data for patients who returned to our institution for follow-up after the surgical procedure. We likely did not account for all of the post-operative complications, ER and urgent care visits and hospital readmissions. Furthermore, this data is limited to a single institution, and may not be representative of all practices.
In conclusion, differences in perioperative outcomes are expected when comparing robotic-assisted gynecologic surgery for benign and malignant indications. In our study, robotic surgery performed for gynecologic malignancy is associated with longer operating time, higher rates of conversion to laparotomy, lower number of same-day discharges and more hospital readmissions compared to their benign counterparts. The results of this study should be considered when accounting for higher surgical risks associated with malignancy, as perioperative complications greatly influence patient counseling, surgical planning and healthcare costs. With our healthcare systems moving toward value-based payment models, knowledge of the distinction between benign and malignant robotic-assisted gynecologic surgery and its implications is imperative.
Conflict of Interest
The authors report no conflict of interest.
References
- Hockstein NGC, Faust R, Terris D (2007) A history of robots: from science fiction to surgical robotics. J Robotic Surg 1: 113-118.
- Visco AG AA (2008) Robotic gynecologic surgery. Obstet Gynecol 112: 1369-1384.
- Wright JD AC, Lewin SN, Burke WM, Lu YS, Neugut AI, et al. (2013) Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA 309(7): 689-698.
- Conrad LB RP, Burke W, Naumann RW, Ring KL, Munsell MF, et al. (2015) Role of Minimally Invasive Surgery in Gynecologic Oncology: An Updated Survey of Members of the Society of Gynecologic Oncology. Int J Gynecol Cancer 25(6): 1121-1127.
- Sinno AK FA (2014) Robotic-assisted surgery in gynecologic oncology. Fertil Steril 102(4): 922-932.
- Frick AC FT (2009) Robotics in gynecologic surgery. Minerva Ginecol 61(3): 187-199.
- Advincula AP WK (2009) Evolving role and current state of robotics in minimally invasive gynecologic surgery. J Minim Invasive Gynecol 16: 291-301.
- Corrado GCG, Pomati G, Mancini E, Sperduti I, Patrizi L, et al. (2015) Surgical and oncological outcome of robotic surgery compared to laparoscopic and abdominal surgery in the management of endometrial cancer. Eur J Surg Oncol 41(8): 1074-1081.
- Wright JD BW, Wilde ET (2012) Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. J Clin Oncol 30: 783-791.
- Pasic RP RJ, Fang H, Ross S, Moore M, Gunnarsson C (2010) Comparing robotic-assisted with conventional laparoscopic hysterectomy: impact on cost and clinical outcomes. J Minim Invasive Gynecol 17: 730-738.
- Xie W CD, Yang J, Shen K, Zhao L (2016) Robot-assisted surgery versus conventional laparoscopic surgery for endometrial cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 142(10): 2173-2183.
- Rivard C CK, Anderson M, Isaksson Vogel R, Teoh D (2015) Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol 22(2): 219-226.
- Gaia G HR, Santoro L, Ahmad S, Di Silverio E, Spinillo A (2010) Robotic-assisted hysterectomy for endometrial cancer compared with traditional laparoscopic and laparotomy approaches: a systematic review. Obstet Gynecol 116: 1422-1431.
- Walker JL PM, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. (2009) Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 27: 5331-5336.
- Walker JLPM, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. (2012) Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol 30: 695-700.
- Galaal KBA, Fisher AD, Al-Khaduri M, Kew F, Lopes AD (2012) Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev 12(9): CD006655.
- Backes FJ BL, Farrell MR (2012) Short- and long-term morbidity and outcomes after robotic surgery for comprehensive endometrial cancer staging. Gynecol Oncol 125: 546-551.
- Magrina JF ZV, Noble BN, Kho RM, Magtibay P (2011) Robotic approach for ovarian cancer: perioperative and survival results and comparison with laparoscopy and laparotomy. Gynecol Oncol 121: 100-105.
- Lowe MP JP, Kamelle SA, Kumar S, Chamberlain DH, Tillmanns TD (2009) A multiinstitutional experience with robotic-assisted hysterectomy with staging for endometrial cancer. Obstet Gynecol 114 :236-243.
- Wallace SK FM, Chen H, Cliby WA, Chalas E (2016) Outcomes and Postoperative Complications After Hysterectomies Performed for Benign Compared With Malignant Indications. Obstet Gynecol 128(3): 467-475.
- Lee MS VK, Growdon WB, Ecker JL, York-Best CM (2016) Predictors of 30-day readmission following hysterectomy for benign and malignant indications at a tertiary care academic medical center. Am J Obstet Gynecol 214(5): 607.
- Cory LA LN, Zhang X, Giuntoli R, Morgan M, Ko E (2016) Post-Surgical Readmissions Among Women Undergoing Benign and Malignant Gynecologic Surgery. Obstet Gynecol 127: Suppl 1:6S.
- Vonlanthen R SK, Breitenstein S (2011) The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 254: 907-913.
- Wechter ME MJ, Magrina JF, Cornella JL, Magtibay PM, Wilson JR, et al. (2014) Complications in Robotic-Assisted Gynecologic Surgery According to Case Type: A 6-Year Retrospective Cohort Study Using Clavien-Dindo Classification. Journal of Minimally Invasive Gynecology 21(5): 844-850.
- Tapper AM HM, Zeitlin R, Isojärvi J, Sintonen H, Ikonen TS (2014) A systematic review and cost analysis of robot-assisted hysterectomy in malignant and benign conditions. Eur J Obstet Gynecol Reprod Biol 177: 1-10.
- Nezhat FR SI (2014) Perioperative outcomes of robotic assisted laparoscopic surgery versus conventional laparoscopy surgery for advanced-stage endometriosis. JSLS 18(4).
- Guy MS SJ, Behbakht K, Wright JD, Guntupalli SR (2016) Comparative outcomes in older and younger women undergoing laparotomy or robotic surgical staging for endometrial cancer. Am J Obstet Gynecol 214(3): 350.e351-350.e310.
- Chan JK GA, Taylor K, Thompson CA, Blansit K, Yu X, et al. (2015) Robotic versus laparoscopic versus open surgery in morbidly obese endometrial cancer patients - a comparative analysis of total charges and complication rates. Gynecol Oncol 139(2): 300-305.
- Gala RB MR, Steinberg A, Murphy M, Lukban J, Jeppson P, et al. (2014) Systematic review of robotic surgery in gynecology: robotic techniques compared with laparoscopy and laparotomy. J Minim Invasive Gynecol 21(3): 353-361.
- Escobar PF LK, Magrina J, Martino MA, Barakat RR, Fader AN (2014) Feasibility and perioperative outcomes of robotic-assisted surgery in the management of recurrent ovarian cancer: A multi-institutional study. Gynecol Oncol 134: 253-256.
- Avondstondt AM WM, D'Adamo CR, Ehsanipoor RM (2017) Change in cost after 5 years of experience with robotic-assisted hysterectomy for the treatment of endometrial cancer. J Robotic Surg.
- El Hachem LAV, Mathews S, Friedman K, Poeran J, Shieh K, et al. (2016) Robotic Single-Site and Conventional Laparoscopic Surgery in Gynecology: Clinical Outcomes and Cost Analysis of a Matched Case-Control Study. J Minim Invasive Gynecol 23(5): 760-768.
- Bogliolo SFS, Cassani C, Musacchi V, Zanellini F, Dominoni M, et al. (2016) Single-site Versus Multiport Robotic Hysterectomy in Benign Gynecologic Diseases: A Retrospective Evaluation of Surgical Outcomes and Cost Analysis. J Minim Invasive Gynecol 23(4): 603-609.
- Bogani G MF, Dowdy SC, Cliby WA, Wilson TO, Gostout BS, et al. (2016) Incorporating robotic-assisted surgery for endometrial cancer staging: Analysis of morbidity and costs. Gynecol Oncol 141(2): 218-224.
- Dayaratna SGJ, Harrington C, Leiby BE, McNeil JM (2014) Hospital costs of total vaginal hysterectomy compared with other minimally invasive hysterectomy. Am J Obstet Gynecol 210(2): 120.e121-126.
- Herling SF PC, Møller AM, Thomsen T, Sørensen J (2016) Cost-analysis of robotic-assisted laparoscopic hysterectomy versus total abdominal hysterectomy for women with endometrial cancer and atypical complex hyperplasia. Acta Obstet Gynecol Scand 95(3): 299-308.
- Zakhari AC-SN, Spence AR, Gotlieb WH, Abenhaim HA (2015) Laparoscopic and robot-assisted hysterectomy for uterine cancer: a comparison of costs and complications. Am J Obstet Gynecol. 213(5): 665.e661-667.
- Wilbur MB, Angarita AM, Matsuno RK, Tanner EJ, Stone RL, et al. (2016) Unplanned 30-day hospital readmission as a quality measure in gynecologic oncology. Gynecol Oncol 143(3): 604-610.






























