The Clinical Outcomes Regarding to Tumor-Infıltrating Lymphocytes in the Breast Cancer Patients Treated with Neoadjuvant Chemotherapy
Ebru Şen1*, Mehmet Emin Güneş1, Cengizhan Özdemir1, Merve Karli1, Ayşegül Gemici2 and Serdar Altinay3
1Department of General Surgery, University of Health Sciences Bakırköy Dr. Sadi Konuk Training and Research Hospital, Turkey
2Department of Radiology, University of Health Sciences Bakırköy Dr. Sadi Konuk Training and Research Hospital, Turkey
3 Department of Pathology, University of Health Sciences Bakırköy Dr. Sadi Konuk Training and Research Hospital, Turkey
Submission:May 15, 2020; Published: July 06, 2020
*Corresponding author:Ebru Şen, University of Health Science, Bakırköy Training and Research Hospital, General Surgery Department Tevfik Sağlam cad. No: 11, Zuhuratbaba mah, Bakırköy/ Istanbul/Turkey
How to cite this article:Ebru Ş, Mehmet E G, Cengizhan Ö, Merve K, Ayşegül G, et al. The Clinical Outcomes Regarding to Tumor-Infıltrating Lymphocytes in the Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Open Access J Surg. 2020; 11(5): 555823. DOI:10.19080/OAJS.2020.11.555823.
Abstract
Objective: Host immune system is a new research area of breast cancer treatment. Tumor-infiltrating lymphocytes (TILs) as an immunologic marker may influence the patients clinical outcome. The aim of this study was to explore the prognostic and predictive roles of TILs in our breast cancer patients treated with neoadjuvant therapy and to determine its association with other clinicopathological parameters.
Materials and Methods: Between 2011-2016, 89 patients were included in this study. The patients were categorized according to molecular subtypes. All patients’ parafin sections were retrospectively evaluated and according to TILs value patients were distributed into two groups in each subtypes.
Results: HER2 positive patients had higher TILs value than others. No relationship was seen between TILs levels and presence of either locoregional recurrence or systemic metastasis. Both in survival outcomes and prediction of neoadjuvant therapy response no association was detected according to TILs value within all subtypes.
Conclusion: The relevance of TILs in small patient population is challenge. Further research is warrented.
Keywords:Tumor-infiltrating lymphocytes; Breast cancer; Response to neoadjuvant therapy
Introduction
In breast cancer great endeavors are going on to find new markers in determining the prognosis. In addition to well-known prognostic factors such as tumour size, grade, nodal status, molecular subtype, proliferation index, gene-expression based recurrence score, recently there is increasing evidence that tumor-infiltrating lymphocytes (TILs) are of important role as an immune biomarker in breast carcinoma [1-3]. TILs might be a reflection of host immune response which plays an important role in cancer control and patient’s clinical outcome. TILs are mononuclear immune cells that infiltrate tumour tissue and mainly comprised of cytotoxic (CD8+) T cells as well as macrophages, helper (CD4+) T cells, B cells, and NK cells [4,5]. There are two types of TILs: Stromal TILs (sTILs) are defined as the percentage of tumour stromal field in which lymphocytes are located without direct connection to tumour cells. In contrast, intratumoural TILs reflect immune cells within the tumour cells. Stromal TILs are commonly detected in H&E stained sections by light microscopy, and consist the majority of TILs in breast cancer [1]. Its assessment is the most reproducible parameter by pathologists. Moreover, lymphocytes-predominant breast cancer (LPBC) is defined as those cancer with TILs occupies at least 50 % to 60 % of the stroma [4].
The clinical importance and amount of TILs appear to vary significantly among different subtypes of breast cancer [2,6]. TILs are more common in triple-negative breast cancer (TNBC) and HER2-overexpressing breast cancer, with LPBC frequency of 15-25 % in these groups. Contrary, luminal tumour is the least immune infiltrated breast cancer subtype [3,6,7]. Large series suggest that tumour with higher TILs levels are associated with improvement of prognosis by longer survival rates and with reduction of locoregional recurrences [8,9]. This positive prognostic effect is especially striking on TNBC and HER2 positive patient populations [6,10]. In addition to prognostic value of TILs, trials also indicate the predictive role of TILs in response to neoadjuvant chemotherapy [11,12]. The aim of this study was to determine the association between TILs level and other clinicopathological parameters and to explore the prognostic and predictive roles of TILs in our breast cancer patients treated with neoadjuvant therapy.
Materials and Methods
The study was conducted to determine the association of TILs value with the clinical outcomes at neoadjuvant settings. Breast cancer patients treated after neoadjuvant chemotherapy at Bakırköy Sadi Konuk Training and Research Hospital from July 2011 to January 2016 were included in this study. All were followed up in our hospital. Patient’s demographic information, characteristics, outcomes and follow-up were obtained from hospital records. The study protocol was approved by the ethics committee of our institute (No:2017/09-20). Neoadjuvant therapy were taxane and anthracycline-based protocol, adding transtuzumab in the HER2 positive subtypes. Surgical treatment was decided as to treatment response. Radiotherapy and endocrine therapy were given in accordance with institutinal guidelines. Inoperable patients following neoadjuvant therapy were excluded of this study. The patients were categorized according to molecular subtypes as triple negative (TN), HER2 positive and luminal type. All patients in each subtypes were distributed into two groups according to TILs, with 10 % of cut-off value. In order to determine TILs value all patients’ paraffin sections stained hematoxilyn and eosin (H&E) from core biopsies were retrospectively assessed by an experienced pathologist (S.A.), blinded of clinical information. Briefly, all mononuclear cells (including lymphocytes and plasma cells) in the stromal compartment within the borders of invasive tumour were evaluated and reported as a percentage value. pCR was defined as ypT0/Tis ypN0.
We analysed associations of TILs with other clinicopathological factors such as menopausal status, tumour molecular type, ypTNM classification, histology, grade, lymphovascular and perineural invasion. In order to evaluate the predictive role of TILs, we investigated pathological responses to neoadjuvant therapy in high and low TILs groups of each subpopulation. To determine the prognostic role of TILs we recorded the patients’ locoregional recurrence and systemic metastasis in all groups and detected overall survival and disease free survivals. Statistical analysis was carried out using NCSS 11 package program. For categorical variables the frequency and percentage values were calculated and for continuous variables, average and standard derivation values were calculated. Standard distribution sort of variables was made with Kolmogorov-Smirnov test. Student t test was used in the comparison of two groups and single direction variance analysis was used in the comparison of more than two groups for variables exhibiting standard distribution. Disease-free survival (DFS) and overall survival (OS) were obtained using Kaplan-Meier survival analysis. The relations between categorical variables were examined with Chi square test, Fisher exact probability test and Fisher-Freeman-Halton test. P<0,05 was accepted as statistically significant.
Results
The study consisted of 89 breast cancer patients who underwent neoadjuvant chemotherapy. The mean follow-up duration of all patients was 33,8 (15-84) months. According to molecular subtypes, 39 patients had luminal tumour, and 25 patients each triple negative and HER2 positive.
TILs values in all groups and its relation with other clinicopathological factors
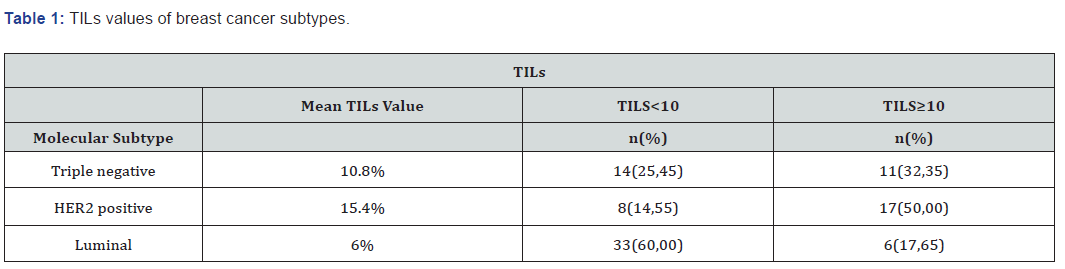
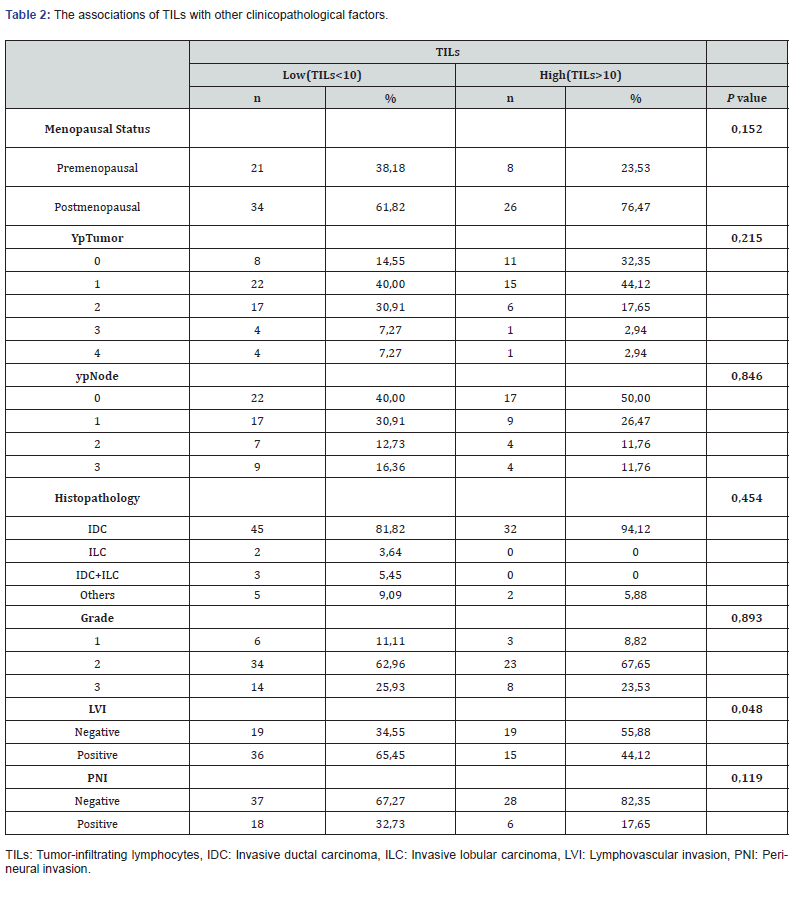
The mean TILs values were 10.8%, 15.4%, 6% in TN, HER2 positive and luminal groups, respectively. Among the patients of high TILs levels, HER 2 positive group was the most frequent molecular subtype (50 %), followed by triple negatives (32%) (Table 1). Sixty percent of patients with TILs less than 10 % had luminal tumour. There was statistically significant relationship between TILs value and both molecular type of tumour (p=0.0001) and presence of lymphovascular invasion (p=0.048). No association was detected between TILs and other variables such as menopausal status, ypTNM classification, histology, grade, and perineural invasion (Table 2).
TILs and prognosis
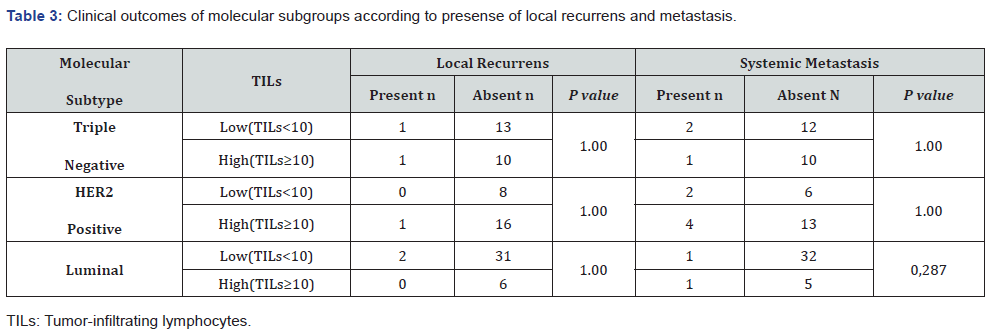
No association was seen in any subtypes between TILs levels and presence of either locoregional recurrence or systemic metastasis (Table 3). Neither OS nor DFS were significantly different between high and low TILs groups in both TN and HER2 positive groups. Because there was no death in luminal group, we could not detected survival analyses in this subtype.
Predictive role of TILs
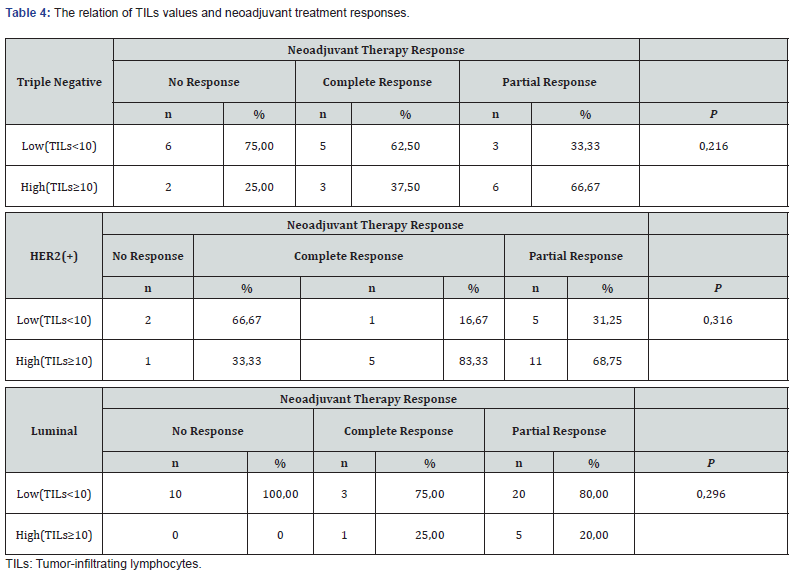
In the neoadjuvant setting, no interaction was found between TILs level and complete pathological response according to each subtypes (Table 4). But, there was non-significantly better pathological response rates in high TILs group of HER2 (+) population.
Discussion
The constant interaction between tumour and host immunity influences patient’s outcome. Innate and adaptive immune response in the tumour milieu require functional cytotoxic T-cells in order to achieve efficent tumour elimination [1]. The impact of TILs on the prognosis and treatment prediction suggests its importance on the reflection of individual immunity [13]. Various studies of TN and HER2 (+) breast cancer have pointed consistent results that the increased TILs value the patients have, the better OS and DFS in the adjuvant setting [6,14]. Moreover, the highest TILs also show the highest expression of T-cell checkpoint receptors which found on T cells [1]. Antitumour immunity is enhanced by monoclonal antibodies against checkpoint inhibitors. In addition to standard therapeutic options, immunotherapy in breast cancer is a new and promising implication especially in selected patient population. Therefore, TILs evaluation may be accepted as a biomarker for immunotherapeutics in patients with breast cancer at near future.
In the light of these rapidly evolving field, in 2014 international TILs working group has recommended TILs evaluation as a immune parameter along with standard histopathological practise to facilitate its use in research setting and clinical trials [4]. They reported TILs measurement as a percentage of stromal TILs on a continuous scale by visual assessment of H&E-stained sections of primary tumour specimens similar to any other quantitative histological biomarker. Several studies have evaluated lymphocyte subtypes by using immunohistochemical analysis, yet added value of immunohistochemistry on to TILs evaluation remains to be demonstrated [4,15]. Moreover, digital image analysis of TILs can provide more exact measurement and eliminate inter-pathologists differences in TILs assessment [13]. Visual measurement of TILs is prefered at many trials like our study [4,6]. Because this route is stated as rapid, easly available, less expensive and adequate tool in TILs assessment. There is no established threshold for high TILs. Various studies used different cut-off values [7,9,16,17]. Moreover, 50-60 % of TILs value for lymphocytes-predominant breast cancer had been arbitrarly chosen [17]. In this study, ROC Curve was applied for the estimation of pathological response of TILs values, but a significant breakpoint could not be found (unreported data). Therefore, we accepted 10 % of TILs level as a cut-off value similar to many studies [6,18].
In our study, we investigated the relation of TILs value with other clinicopathological parameters and its impact on patients with all molecular subtypes of breast carcinoma who were operable following neoadjuvant treatment. The positive relation of higher quantities of TILs and both negative steroid receptor status and presence of HER2 positivity is striking in the literature like in our study [6]. Also LPBC is commonly found in HER2 positive and triple negative subtypes [4,19]. Similarly, in our study HER2 positive group was the main molecular subtype (50%) that harbours the high percentage of TILs. But, interestingly unique patient with LPBC was seen in our series. In this study, patients with luminal type breast cancer had lower TILs value, with the median of 6%. Similar to litreture, no significant prognostic role of TILs was found in patients of our luminal group [1]. According to the trial by Huszno [7] higher grades of TILs were present more frequently in younger patients than older women (47% vs. 24%). Moreover, higher TILs levels was appeared to be associated with higher histological grades. On the other hand, TILs value also was significantly correlated with lymphovascular invasion in our study.
The positive association between TILs levels and favourable clinical outcomes was based on Sistrunk’s cohort trial at 1922 [20]. Eastern Cooperative Oncology Group’s phase III trials showed that each 10 % increase in TILs levels significantly predicted better DFS and OS in TNBC with a median follow-up of over 10 years [21]. A recent meta-analysis of twenty-five trials including more than twenty thousands patients confirmed the survival benefit of TILs in TN and HER2 positive breast cancer patients [18]. Moreover, according to secondary analysis of the NeoALTTO trial reported by Salgado [17] patients with high TILs levels at baseline had better outcomes for event free survival independent of whether they achieved pCR in the neoadjuvant phase. The association between higher levels of TILs and decreased distant recurrence rates in TN breast cancer was found in FinHER trial [22].
Contrary to obvious results as to prognostic impact of higher TILs levels on the adjuvant and neoadjuvant settings, we could not detected any significant relationship between TILs and survival analysis. Another issue related to TILs is its utility in prediction of adjuvant and neoadjuvant therapy response [6]. At the adjuvant setting, the association between higher levels of TILs and increased transtuzumab benefit in HER2 (+) subtype was detected in phase III trial including 1010 early stage BC patients [22]. Positive correlation between TILs levels and pCR rates was prominent in HER2 (+) tumours. According to the study of Carbognin [12] pCR rates were increased by nearly 30 % in the presence of TILs reported as LPBC. Another trial has also indicated that the level of TILs greater than 5% was associated with higher pCR rates [17]. Although we could not detected significant correlation between TILs value and pCR in our study, there were non-significantly higher pCR rates in HER2 (+) group. The minority of our patient population explains the limited power of the statistical analysis.
Conclusion
Host immunity controls cancer and influences patient’s outcome. How can a host enhance anti-tumor immune response and which biomarker provides better treatment approach are the main questions. Although which level of TILs should be used to determine optimal treatment strategy for breast cancer patients has not been certain yet, many trials indicate that TILs is of prognostic effect in long-term disease control and predictive effect of a better local response to treatment. But, further resaerchs are warrented before the utility of TILs as an immune biomarker for routine clinical practise of breast cancer patients.
References
- Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, et al. (2016) Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 13: 228-241.
- Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, et al. (2018) Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol 52:16-25.
- Li X, Oprea-Ilies GM, Krishnamurti U (2017) New Developments in Breast Cancer and Their Impact on Daily Practice in Pathology. Arch Pathol Lab Med 141: 490-498.
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, et al. (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26: 259-271.
- Lee KH, Kim EY, Park YL, Do SI, Chae SW, et al. (2017) Expression of epithelial-mesenchymal transition driver brachyury and status of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in predicting treatment responses to neoadjuvant chemotherapy of breast cancer. Tumour Biol 39(6).
- Solinas C, Carbognin L, De Silva P, Criscitiello C, Lambertini M (2017) Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast 20: 142-150.
- Huszno J, Nożyńska EZ, Lange D, Kołosza Z, Nowara E (2017) The association of tumor lymphocyte infiltration with clinicopathological factors and survival in breast cancer. Pol J Pathol 68: 26-32.
- Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, et al. (2017) Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol 18: 52-62.
- Solinas C, Ceppi M, Lambertini M, Scartozzi M, Buisseret L, et al. (2017) Tumor-infiltrating lymphocytes in patients with HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: A meta-analysis of randomized controlled trials. Cancer Treat Rev 57: 8-15.
- Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, et al. (2016) Tumor-Infiltrating Lymphocytes: A Predictive and Prognostic Biomarker in Neoadjuvant-Treated HER2-Positive Breast Cancer. Clin Cancer Res 22: 5747-5754.
- Ali HR, Dariush A, Thomas J, Provenzano E, Dunn J, et al. (2017) Lymphocyte density determined by computational pathology validated as a predictor of response to neoadjuvant chemotherapy in breast cancer: secondary analysis of the ARTemis trial. Ann Oncol May 19.
- Carbognin L, Pilotto S, Nortilli R, Brunelli M, Nottegar A, et al. (2016) Predictive and Prognostic Role of Tumor-Infiltrating Lymphocytes for Early Breast Cancer According to Disease Subtypes: Sensitivity Analysis of Randomized Trials in Adjuvant and Neoadjuvant Setting. Oncologist 21:283-291.
- Denkert C, Wienert S, Poterie A, Loibl S, Budczies J, et al. (2016) Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol 29: 1155-1164.
- Simon RM, Paik S, Hayes DF (2009) Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 101: 1446-1452.
- Buisseret L, Desmedt C, Garaud S, Fornili M, Wang X, et al. (2017) Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol Jun 16.
- Montagna E, Vingiani A, Maisonneuve P, Cancello G, Contaldo F, et al. (2017) Unfavorable prognostic role of tumor-infiltrating lymphocytes in hormone-receptor positive, HER2 negative metastatic breast cancer treated with metronomic chemotherapy. Breast 34: 83-88.
- Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, et al. (2015) Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol 1: 448-454.
- Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K (2016) The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS One 13: 11(4)
- Pujani M, Jain H, Chauhan V, Agarwal C, Singh, et al. (2020) Evaluation of Tumor infiltrating lymphocytes in breast carcinoma and their correlation with molecular subtypes, tumor grade and stage. Breast Dis 9.
- Sistrunk WE, Maccarty WC (1922) Life expactancy following radical amputation for carcinoma of the breast: a clinical and pathologic study of 218 cases. Ann Surg 75: 61-69.
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, et al. (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32: 2959-2966.
- Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, et al. (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25: 1544-1550.






























