Management of a Recurrent Malignant Pheochromocytoma: A Case Report and Review of Literature
Nadjet Azzi1*, Meziane El Houcine1, Becherki Yakoubi2, Radia Ait Chaalal3 and Tabouche Mounir3
1Surgical Department of BEO University Hospital, Algeria
2Neurosurgical Department of BEO University Hospital, Algeria
3Radiology Department of BEO University Hospital, Algeria
Submission:June 3, 2020; Published: June 15, 2020
*Corresponding author:Nadjet Azzi, Surgical Department of BEO University Hospital, Algiers, Algeria
How to cite this article:Nadjet A, Becherki Y, Radia Ait C. Operative Strategy for the Prevention of Common Bile Duct Injuries during Laparoscopic Cholecystectomy. Open Access J Surg. 2020; 11(4): 555820. DOI:10.19080/OAJS.2020.11.555820.
Summary
Pheochromocytoma is a rare tumor, of very variable clinical expression and probably underestimated in incidence. The last few years have been marked by advances in genetics with the identification and clinical and evolutionary characterization of new familial forms and the progressive generalization of screening. Significant progress has also been made both in terms of biological assays and imaging, facilitating the diagnostic process. Surgery currently remains the only curative treatment and the therapeutic possibilities remain limited in malignant or inoperable forms.
Keywords:Pheochromocytoma; Surgery; Malignant tumor
Introduction
Ten to 20% of pheochromocytomas are malignant and generally have a poor prognosis [1]. The malignancy of the tumor can be considered at the outset or during a recurrence, even late, which justifies clinical and biological surveillance at least every two years, of all patients with pheochromocytoma [2]. We report this case of malignant pheochromocytoma. The diagnosis was made immediately in one case and in a recurrence. Surgical treatment of pheochromocytoma is based on the complete excision of the tumor. It is the only curative treatment. This is often difficult and specific surgery due to the importance of the vascular supply of the tumor and its major risks of inducing blood pressure variations. Preoperative imaging allows elective approaches to be chosen.
Observation
A 55-year-old man has presented clinical symptoms suggestive of pheochromocytoma for 10 years. In 2007 before the exacerbation of the symptomatology the patient consulted. The exploration found a right adrenal mass with lesion of the peritoneal fat on the abdominal CT scan. The renal Doppler showed a inferior vena cava and permeable renal veins. The procedure (laparotomy) performed the same year consisted of a total removal of the adrenal gland. Histological examination confirmed the diagnosis of pheochromocytoma. The immediate postoperative course was favorable, but without biological confirmation of recovery. In 2012, 5 years later, the same symptomatology recurred. Ultrasound and abdominal CT scan concluded that the right adrenal gland expands.
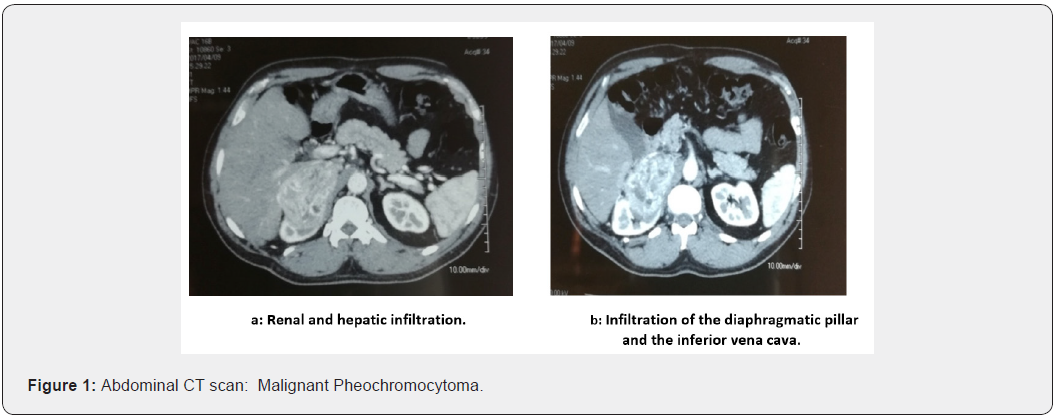
The patient was operated in 2012, and the locoregional tumor recurrence with infiltration of the surrounding adipose tissue was confirmed. The patient no longer consulted until 2017 for abdominal mass syndrome, such as pain and vomiting. An abdomino-pelvic CT scan (Figure 1) found a 66-64-53 mm right adrenal mass invading the kidney; segments V and VI of the liver and the right diaphragmatic pillar. The tumor infiltrates the lower vena cava over 4 centimeter; a Doppler ultrasound confirms the invasion of the left renal vein (Figure 1).
Surgery
Steps of surgery
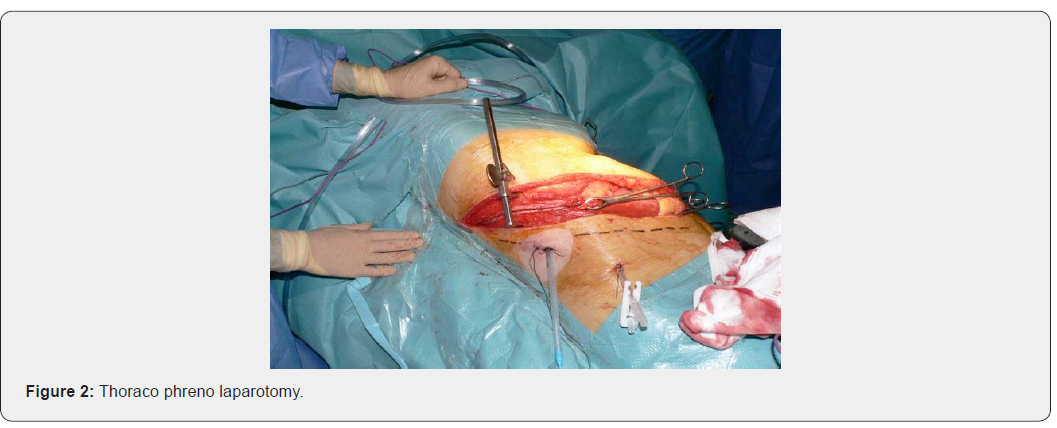
- Thoraco-Phreno-laparotomy (Figure. 2).
Installation
i. The patient is installed in an intermediate position between the dorsal and lateral decubitus.
ii. The thorax held by supports at 45° to the plane of the table, which is broken, the pelvis remaining as flat as possible to cause a slight rotation aimed at opening the intercostal spaces.
Incision
i. It follows the 8th or the 9th intercostal space or even the 7th, starting at the level of the posterior axillary line, it goes towards the umbilicus to the outer edge of the rectus where it can bend in para-rectal.
Procedure
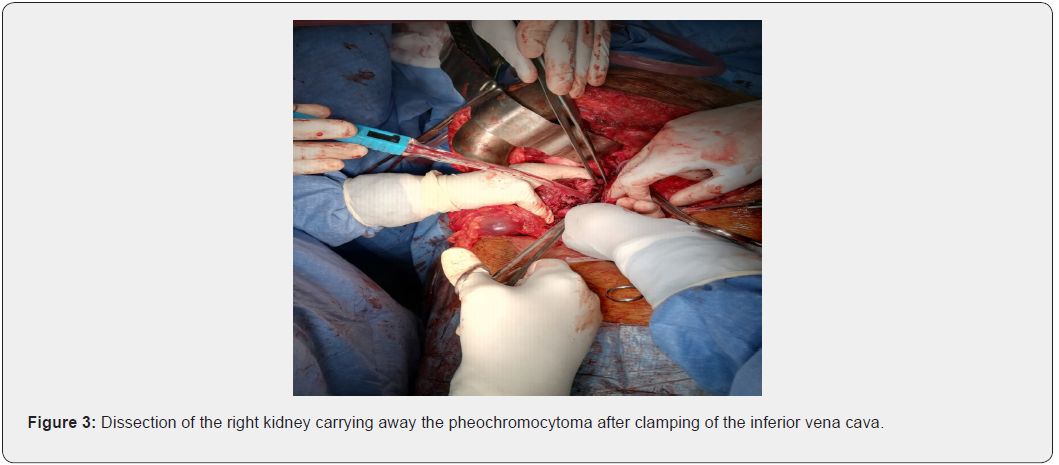
Vascular access, from the inferior vena cava (clamped) (Figure 3 & 4).
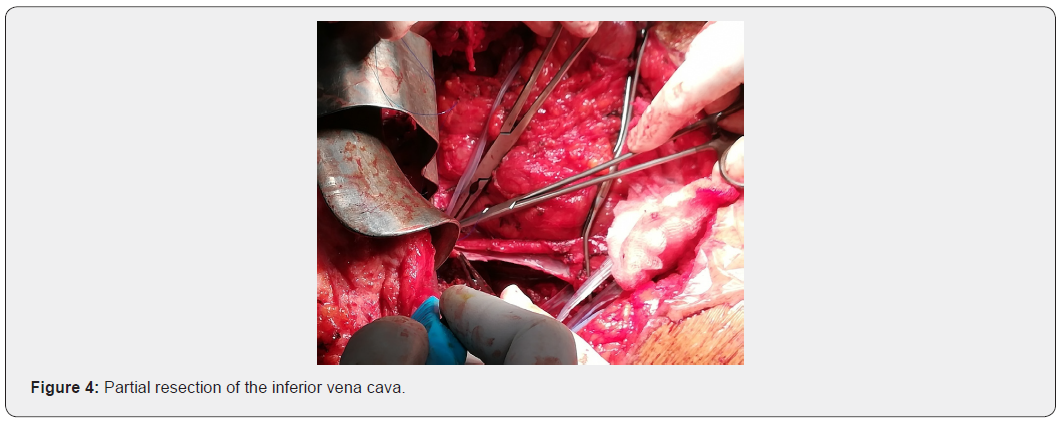
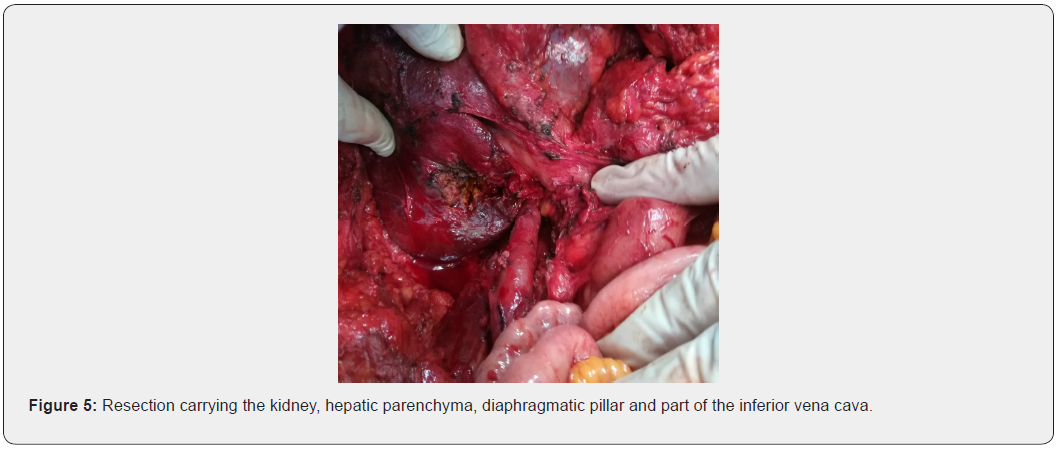
Resection of the tumor mass carrying the right kidney, the infiltrated hepatic parenchyma, part of the right diaphragmatic pillar and a pellet from the wall of the inferior vena cava (Figure 5).
Follow up
Favorable and patient discharge 8 days later
Anatomopathological study
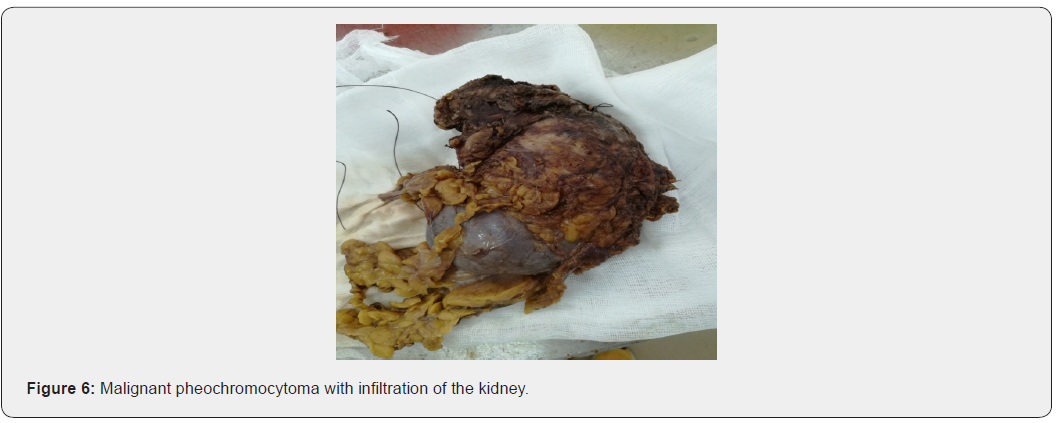
In favor of a recurrent metastatic malignant pheochromocytoma (Figure 6).
Indication of chemotherapy most often combining the CVD protocol, which includes cyclophosphamide (Procytox), vincristine (Oncovin) and dacarbazine (DTIC).
Discussion
The prevalence of metastatic pheochromocytomas is estimated at 10%, it varies from 5 to 26% according to some authors] [3-5]. The large size of the tumor, its ectopic site, an excessive excretion of dopamine (precursor of noradrenaline and adrenaline) and a tumor invading the capsule, or the neighboring organs recognized intra operatively are arguments in favor of the malignancy. In fact, only the presence of lymph node metastases or at a distance affirms the malignancy; histological examination does not provide any definite information. The malignancy is therefore affirmed preoperatively on the presence of lymphadenopathy on CT or MRI.
The distant metastases are most often pulmonary, hepatic and bones metastasis . Brain metastases are exceptional [6]. Liver metastases are formal proof of malignancy. While the loco-regional recurrence of the tumor, the invasion of the diaphragm pillar and the surrounding fat confirm the malignancy. Because metastases are microscopic at the time of diagnosis and malignancy is only declared after a delay of the initial surgery, generally after 5 years or even 10 years or more, clinical, biological and even morphological monitoring of all operated patients of a pheochromocytoma must be continued for a long time [2,7-9]. Moreover, in our observation, the malignancy was revealed late on 2 occasions and the diagnosis of recurrence is made thanks to the thoraco abdomino pelvic CT scan .
Survival at 5 years is generally less than 50% [10], but survival over 20 years without any treatment has been reported [11]. Due to the absence at present of an effective treatment of malignant tumors, several therapies with palliative aim allow tumor reduction and improvement of survival. From the diagnosis of malignancy, the therapeutic objective is to control blood pressure. Surgery is rarely curative, but tumor reduction or resection of metastases particularly reduces the cardiovascular risk [12,13]. Malignant pheochromocytoma surgery, even at the metastatic stage, increases survival [14-20]. Chemo-radiotherapy and MIBG are part of the therapeutic arsenal. The management of these patients requires multidisciplinary cooperation (surgeon, oncologist, endocrinologist, histopathologist, geneticist) [21-27].
Conclusion
Any pheochromocytoma should be considered as potentially malignant and monitored regularly over the long term. MIBG scintigraphy is widely used in the diagnosis and extension assessment of pheochromocytomas; the Pet-scan is also of considerable help in detecting recurrences. Progress is being made and others are expected in the treatment of malignant cases: surgery, embolization of visceral metastases, per cutaneous plasty of bone metastases, in situ radiotherapy with MIBG, antisecretory treatment with metyrosine or somatostatin analogues.
References
- Pannier-Moreau I, Massien-Simon C, Plouin PF (1999) Phé Encycl Méd Chir, Endocrinologie-Nutrition 10-015-B-50, 4p.
- Plouin PF, Gimenez-Roqueplo AP, La Batide Alanore A, Salenave S, Duclos JM (2000) Progrès récents dans le diagnostic, l’évaluation pronostique et le traitement des phéochromocytomes. Rev Méd Interne 21: 1075-1085.
- Edstrom Elder E, Hjelm Skog AL, Hoog A, Hamberger B (2003) The management of benign and malignant pheochromocytoma and abdominal paraganglioma. European Journal of Surgical Oncology 29: 278-283.
- Gimenez-Roqueplo AP, Favier J, Rustin P (2003) Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Research 63: 5615-5621.
- Goldstein RE, O’Neill JA Jr, Holcomb GW (1999) Clinical experience over 48 years with pheochromocytoma. Annals of Surgery 229: 755-766.
- William MM (2003) Editorial: In Search of Pheochromocytomas. J Clin Endocrinol Metabolism 88: 4080-4082.
- Lenders JW, Pacak K, Walther MM (2002) Biochemical diagnosis of pheochromocytoma: which test is best? Journal of the American Medical Association 287: 1427-1434.
- Morikawa T, Suzuki M, Unno M, Endo K, Katayose Y, et al. (2001) Malignant pheochromocytoma with hepatic metastasis diagnosed 10 years after a resection of the primary incidentaloma adrenal lesion: report of a case. Surgery Today 31: 80-84.
- Tanaka S, Ito T, Tomoda J, Higashi T, Yamada G, Tsuji T (1993) Malignant pheochromocytoma with hepatic metastasis diagnosed 20 years after resection of the primary adrenal lesion. Internal Medicine 32: 789-794.
- John H, Ziegler WH, Hauri D, Jaeger P (1999) Pheochromocytomas: can malignant potential be predict? Urology 53: 679-683.
- Yoshida S, Hatori M, Noshiro T, Kimura N, Kokubun S (2001) Twenty-six-years’ survival with multiple bone metastasis of malignant pheochromocytoma. Archives of Orthopedic Trauma and Surgery 121: 598–600.
- Mishra AK, Agarwal G, Kapoor A, Agarwal A, Bhatia E, et al. (2000) Catecholamine cardiomyopathy in bilateral malignant pheochromocytoma: successful reversal after surgery. International Journal of Cardiology 76: 89-90.
- Nonaka K, Makuuchi H, Naruse Y, Kobayashi T, Goto M (2000) Surgical excision of malignant pheochromocytoma in the left atrium. Japanese Journal of Thoracic Cardiovascular Surgery 48: 126-128.
- Ahn JT, Hibbard JU, Chapa JB (2003) Atypical Presentation of Pheochromocytoma as part of Multiple Endocrine Neoplasia IIa in Pregnancy. Obstrtrics and gynecology 102: 1202-1205.
- Conte-Devolx B, Niccoli-Sire P (2002) Groupe d’étude des tumeurs à calcitonine (GETC). Les néoplasies endocriniennes multiples de type 2. Presse Med 31: 1224-1230.
- Conte-Devolx B, Niccoli-Sire P (2003) Groupe des Tumeurs Endocrines (GTE). Les néoplasies endocriniennes multiples de type 2. Ann Endocrinol 64: 1389-1392.
- Igaz P, Patocs A, Racz K, Klein I, Varadi A (2002) Occurrence of pheochromocytoma in NEM 2a family with codon 609 mutation of the RET proto-oncogene. J Clin Endocrinol Metab 87: 2994.
- Ilias I, Yu J, Carrasquillo JA (2003) Superiority of 6-[18F]-Fluorodopamine Positron Emission Tomography Versus [131I]-Metaiodobenzylguanidine Scintigraphy in the Localization of Metastatic pheochromocytoma. J Clin Endocrinol Metab 88: 4083-4087.
- Lehnert H, Mundschenk J, Hahn K (2004) Malignant pheochromocytoma. Frontiers of Hormone Research 31: 155-162.
- Oishi S, Sasaki M, Sato T, Isogai M (1995) Coexistence of NEM2 a and papillary thyroïd carcinoma and a recurrent pheochromocytoma 23 years after surgery: report of a case and review of the Japanese literature. Jpn J Clin Oncol 25(4): 153-158.
- Pacak K, Fojo T, Goldstein DS (2001) Radiofrequency ablation: a novel approach for treatment of metastatic pheochromocytoma. Journal of the National Cancer Institute 93: 648-649.
- Punales M-K, Graf H, Gross JL, Maia AL (2003) RET codon 634 mutations in NEM type 2: variable clinical features ans clinical outcome. J Clin Endocrinol Metab 88: 2644-2649.
- Rose B, Mattahya KK, Price D (2003) High-Dose 131I-Metaiodobenzylguanidine Therapy for 12 Patients with Malignant Pheochromocytoma. Cancer 98: 239-248.
- Safford SD, Coleman RE, Gockerman JP (2003) Iodine-131 metaiodobenzylguanidine is an effective treatment for malignant pheochromocytoma and paraganglioma. Surgery 134: 956-962.
- Schlumberger M, Gicquel C, Lumbroso J (1992) Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. Journal of Endocrinological Investigation 15: 631-642.
- Takahashi K, Ashizawa N, Minami T (1999) Malignant pheochromocytoma with multiple hepatic metastases treated by chemotherapy and transcatheter arterial embolization. Internal Medicine 38: 349-354.
- Wiseman GA, Kvols LK (1995) Therapy of neuroendocrine tumors with radiolabeled MIBG and somatostatin analogues. Seminars in Nuclear Medicine 25: 272-278.






























