Important Medical Considerations when Planning Elective Surgery in Renal Transplant Recipients
Mohammed Mahdi Althaf1*, Mohamed Said Abdelsalam2 and Tamjeed Alam3
1Jack Pryor Renal Unit, Norfolk and Norwich University Hospital, United Kingdom
2Department of Internal Medicine, Alexandria University, Egypt
3Department of Surgery, William Harvey Hospital, United Kingdom
Submission:February 07, 2020; Published: March 05, 2020
*Corresponding author:Mohammed Mahdi Althaf, Consultant Nephrologist and Acute Physician, Jack Pryor Renal Unit, Norfolk and Norwich University Hospital - NHS Foundation Trust, Colney Lane, Norwich, Norfolk, NR4 7UY, United Kingdom
How to cite this article:Althaf MM, Abdelsalam MS, Alam T. Important Medical Considerations when Planning Elective Surgery in Renal Transplant Recipients. Open Access J Surg. 2020; 11(3): 555815. DOI:10.19080/OAJS.2020.11.555815.
Abstract
Renal transplantation remains the best modality of renal replacement therapy for those with end-stage renal disease. The number of renal transplants worldwide has been steadily increasing over the last decade. Survival of these patients has also significantly improved. These patients like the general population could require an elective surgical procedure at some point. This article highlights the salient factors that need to be considered when planning elective surgery to sure the best possible outcome for nontransplant surgery in the renal transplant recipient.
Keywords:Elective surgery; renal Transplant recipient; Medical considerations; Kidney transplant recipient.
Abbreviations: ESRD: End-Stage Renal Disease; RRT: Renal Replacement Therapy
Introduction
Renal transplantation is the preferred modality of renal replacement therapy (RRT) for patients with end-stage renal disease (ESRD). In comparison to dialysis, transplantation is cost-effective and confers better patient survival and quality of life [1-3]. According to 2008 data from the World Health Organisation, there are just over 100000 Solid organ transplants performed every year worldwide. Renal transplants make up 68 per cent of all solid organ transplants making it the most common solid organ transplant [4]. The number of kidney transplants has risen on a yearly basis in part due to the use of more marginal allografts and transplantation of older patients. Survival has also been promising with 5-year patient survival with a deceased donor transplant currently being 86.1% and 93.1% for living donor transplants [5]. If we consider the number to have remained static from 2008 to 2019 there are roughly 763000 new renal transplant recipients worldwide. With longer survival and an increasing pool of renal transplant recipients the chances of encountering one for elective surgery has increased. In a Canadian study that looked at the complexity of patients seen by different medical specialities based on nine different factors (number of comorbidities, presence of mental illness, number of types of physicians involved in each patient’s care, number of physicians involved in each patient’s care, the number of prescribed medications, the number of emergency department visits, the rate of death, rate of hospitalization and rate of placement in a long term care facility) the most complex patients were renal patients [6]. Therefore, this subgroup of patients requires careful consideration when planning elective surgery. This article will attempt to cover the most common medical factors that need to be considered prior to elective surgery.
Discussion
Planning elective nontransplant surgery for the renal allograft recipient is largely similar to non-transplant patients. However, there are important differences and salient factors need to be identified and addressed accordingly. It is strongly recommended that a renal transplant physician is involved at the very early stages when planning elective surgery. Factors to be considered are addressed under each subheading below:
Imaging
Imaging using modalities such as Magnetic resonance imaging (MRI) and Computed tomography (CT) are quite common prior to any major surgical intervention. The ideal modality for the clinical question at hand is best discussed and directed by the radiologist.
CT with and without contrast in renal transplant recipients
Renal transplant recipients can have varying levels of renal function. Patients with impaired baseline renal function defined as those with an estimated glomerular filtration rate less than 30 ml/min/ 1.73 m2 may be at risk of developing contrast-induced nephropathy following iodinated contrast administration. Generally, for patients undergoing contrast-enhanced CT scans it is important to have the renal function checked at least within one month for outpatient settings and within 48 hours for inpatient settings. The need for contrast in different clinical settings is summarised in Table 1.
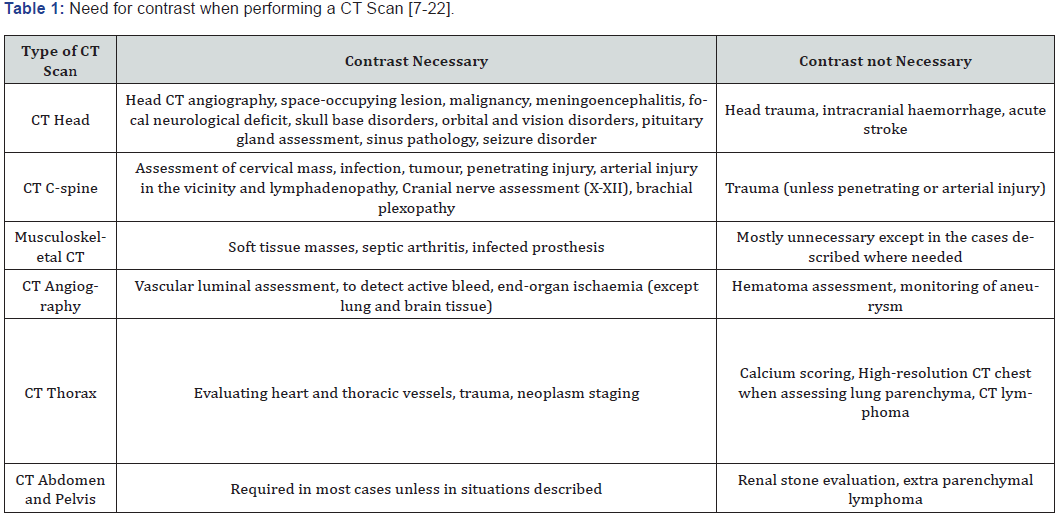
For studies where intravenous iodinated contrast is mandated the most effective prophylactic measure is that of volume expansion with an isotonic solution (normal saline or ringer’s lactate) [23-25]. There has been no reported benefit in using sodium bicarbonate or N-acetyl cysteine [26-28].
MRI with gadolinium-based contrast
It is important to remember that there is a risk of nephrogenic systemic fibrosis (NSF) in patients with impaired renal function who receive gadolinium-based contrast agents (GBCAs). GBCAs are classified as group I or group II. Group I agents have a higher risk of NSF compared to group II agents. NSF is characterised by thickening and hardening of the skin overlying the extremities and trunk with marked expansion and fibrosis of the dermis with CD34 positive fibrocytes. It can also progress to involve internal organs. Patient risk factors for the development of NSF include acute kidney injury, ESRD on either haemodialysis or peritoneal dialysis and chronic kidney disease with an eGFR of < 30ml/ min/1.73m2. There currently is no effective proven medical treatment for NSF and therefore is best prevented [29,30].
Blood transfusion
Transplant physicians prefer to avoid blood transfusions where possible in renal transplant recipients due to the increased risk of sensitisation. Sensitisation, in turn, could raise the risk of renal allograft rejection. However, in situations where blood transfusion is absolutely indicated it is best to offer filtered blood products or cytomegalovirus (CMV) seronegative blood. This is preferred over leukocyte reduced and/or leukocyte depleted products as viruses are can be transmitted by plasma. Blood products that are irradiated are probably not necessary [31,32].
Anticoagulation
There are no specific considerations for renal transplant recipients on warfarin, standard local protocols should be employed. It is, however, important to consider the eGFR and dose heparin appropriately.
Steroids and antirejection medications
After pairing of the donor and recipient and immunological risk is ascertained the next step in renal transplantation isimmunosuppression. Immunosuppression is done in two stages; induction followed by maintenance immunosuppression. The induction phase is where intense immunosuppressive agents are administered at the very onset of transplantation to minimise the burden of acute allograft rejection. Protocols are not standard across all transplant units as optimal induction immunosuppression therapy remains controversial [33,34]. However, it can broadly be classified into two strategies. The first one is based on the use of significant doses of standard immunosuppressive therapy (a calcineurin inhibitor, an antimetabolite and glucocorticoids). The other strategy employs antibodies targeted at T-cells in addition to standard immunosuppressive therapy.
The choice of agent is based on the recipient’s risk of developing acute rejection. Gebel et al. [35] stratified the prospective renal transplant patients into various categories according to immunological risk in renal transplantations. Based on this with further additions the principles of risk assessment are as follows:
i. High immunological risk: During transplant high titres of circulating antibodies targeting mismatched donor HLA also known as donor-specific antibodies are present. This can lead to hyperacute rejection. The presence of DSA precludes transplantation. However, there are reports of innovative pretransplant desensitisation regimens to reduce this risk.
ii. Intermediate immunological risk: The low titter of DSA at the time of transplantation and historic DSA is not detectable. It may be acceptable to consider intensified immunosuppression as well as immunological monitoring in the post-transplant period
iii. Standard immunological risk: Where there is no evidence of donor-directed sensitization to HLA.
It is worth noting that recipients of a renal allograft who currently have a functioning solid-organ transplant (such as heart, lung or liver) and are on maintenance immunosuppression are often spared from induction immunosuppression. However, this is not the standard in all centres, and some still prefer some form of induction agent [36]. Recipients of two haplotype identical living related kidneys who are caucasian have a significantly low risk of acute rejection and do not require induction therapy [37].
The most common maintenance immunosuppression consists of a triple regimen:
i. Calcineurin inhibitors (CNI) (tacrolimus, cyclosporine) or mammalian target of rapamycin (mTOR) inhibitors (Sirolimus, Everolimus).
ii. Antimetabolite (mycophenolate mofetil, azathioprine).
iii. Glucocorticoids (prednisolone).
It is worth noting that chronically immune-suppressed patients including those on low doses of maintenance glucocorticoids may frequency be noted intraoperatively to have ‘weak’ tissues. Thus, gentle handling of tissues is a commonly accepted surgical principle. It is particularly important in the technical execution of surgical methods in transplant patients. Wound healing is commonly slower in immunosuppressed transplant recipients. About specific maintenance immunosuppressive medication, it is worth noting that sirolimus was shown to have increased allograft wound complications such as peri graft fluid collections, superficial wound infections and incisional hernias. Thus, it is important to review maintenance immunosuppressive medications when planning elective surgery and notify the transplant physician early enough for review and consideration of an alternative agent to sirolimus for a short period pre and postoperatively.
For patients who cannot take their medications orally in the peri and post-operative period the following conversions of medication to intravenous forms should be done:
i. Tacrolimus – convert to intravenous and monitor trough levels (patients > 12 months post-transplant aim for levels between 5-8ng/ml).
ii. Cyclosporine – convert to intravenous and monitor trough levels (patients > 12 months post-transplant aim for levels between 50-100ng/ml).
iii. Mycophenolate Mofetil - convert to intravenous at oral equivalent dose
iv. Azathioprine – convert to intravenous at oral equivalent dose
v. Glucocorticoids – Maintenance glucocorticoid dose in stable renal transplant recipients is usually prednisolone 5mg once daily.
It is best to increase the dose for the stress of surgery. In one regimen generally, 100 mg of hydrocortisone is given eight hourly perioperatively and the dose is gradually reduced until the patient can be switched back to the oral maintenance dose.
Infections
It is reasonable to consider prophylactic antibiotics for renal transplant recipients undergoing surgery. Routine prophylaxis using a first-generation cephalosporin or amoxicillin is usually enough for minor procedures. Patients who received prophylactic antibiotics within a 2-hour window before the initial incision have significantly lower rates of surgical site infection as opposed to those who received them either too early or postoperatively [38]. Erythromycin and clarithromycin are two macrolides that should ideally be avoided in those being administered cyclosporin or tacrolimus. These antibiotics interfere with the CYP 3A4 enzymes. This, in turn, results in elevated levels of cyclosporin and tacrolimus which are nephrotoxic and can lead to an acute kidney injury. In contrast doxycycline, clindamycin and ciprofloxacin can be used as they do not significantly alter the levels of either cyclosporine or tacrolimus [39,40].
Analgesia
As we often do, non-steroidal anti-inflammatory drugs (NSAIDS) are best avoided in renal transplant recipients. For nociceptive pain, which is usually from tissue injury, paracetamol is preferred. For non-responders, this can be used in combination with tramadol at renal doses. Other preferred opioids include buprenorphine, methadone, fentanyl and hydromorphone.
Conclusion
Renal transplant recipients are a special population that require specific considerations due to the level of renal function, chronic immunosuppressed state, immunosuppressive therapy and its common drug interactions as well as the risk of allograft rejection.
References
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, et al. (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341(23): 1725-1730.
- Niakas D, Kontodimopoulos N (2009) Is renal transplantation the most cost-effective and preferable therapy for patients suffering from end-stage renal disease or not? Health policy 89(3): 329-331.
- Rebollo P, Ortega F, Baltar JM, Badía X, Alvarez‐Ude F, et al. (2000) Health related quality of life (HRQOL) of kidney transplanted patients: variables that influence it. Clin Transplant 14(3): 199-207.
- WHO (2008) GKT1 Activity and Practices. World Health Organisation.
- Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, et al. (2018) OPTN/SRTR 2016 Annual Data Report: Kidney. Am J Transplant 18 Suppl 1: 18-113.
- Tonelli M, Wiebe N, Manns BJ, Klarenbach SW, James MT, et al. (2018) Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open 1(7): e184852.
- Wintermark M, Sanelli PC, Albers GW, Bello J, Derdeyn C, et al. (2013) Imaging recommendations for acute stroke and transient ischemic attack patients: a joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol 34(11): E117-127.
- Mullins ME (2011) Emergent neuroimaging of intracranial infection/inflammation. Radiol Clin North Am 49(1): 47-62.
- Hijaz TA, Cento EA, Walker MT (2011) Imaging of head trauma. Radiologic clinics 49(1): 81-103.
- Phuttharak W, Sawanyawisuth K, Kawiwungsanon A, Tiamkao S (2011) The appropriate neuroimaging study in persons with epilepsy. Neurological sciences 32(5): 969-971.
- Groell R, Doerfler O, Schaffler GJ, Habermann W (2001) Contrast-enhanced helical CT of the head and neck: improved conspicuity of squamous cell carcinoma on delayed scans. American Journal of Roentgenology 176(6): 1571-1575.
- Keberle M, Tschammler A, Hahn D (2002) Single-bolus technique for spiral CT of laryngopharyngeal squamous cell carcinoma: comparison of different contrast material volumes, flow rates, and start delays. Radiology 224(1): 171-176.
- Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, et al. (2006) Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 354(22): 2317-2327.
- Desjardins B, Kazerooni EA (2004) ECG-gated cardiac CT. American Journal of Roentgenology 182(4): 993-1010.
- Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, et al. (2008) Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J 29(4): 531-556.
- Mark DB, Berman DS, Budoff MJ, Carr JJ, Gerber TC, et al. (2010) ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 55(23): 2663-2299.
- Davenport MS, Newhouse J (2018) Patient evaluation prior to oral or iodinated intravenous contrast for computed tomography.
- Yamashita Y, Komohara Y, Takahashi M, Uchida M, Hayabuchi N et al. (2000) Abdominal helical CT: evaluation of optimal doses of intravenous contrast material--a prospective randomized study. Radiology 216(3): 718-723.
- Keyzer C, Cullus P, Tack D, De Maertelaer V, Bohy P, et al. (2009) MDCT for suspected acute appendicitis in adults: impact of oral and IV contrast media at standard-dose and simulated low-dose techniques. American journal of roentgenology 193(5): 1272-1281.
- Fayad LM, Bluemke DA, Fishman EK (2005) Musculoskeletal imaging with computed tomography and magnetic resonance imaging: when is computed tomography the study of choice? Current problems in diagnostic radiology 34(6): 220-237.
- Panicek DM, Gatsonis C, Rosenthal DI, Seeger LL, Huvos AG, et al. (1997) CT and MR imaging in the local staging of primary malignant musculoskeletal neoplasms: Report of the Radiology Diagnostic Oncology Group. Radiology 202(1): 237-246.
- Fuentes-Orrego JM, Pinho D, Kulkarni NM, Agrawal M, Ghoshhajra BB (2014) New and evolving concepts in CT for abdominal vascular imaging. Radiographics 34(5): 1363-1384.
- Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, et al. (2003) A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract 93(1): c29-c34.
- Bader B, Berger E, Heede M, Silberbaur I, Duda S, et al. (2004) What is the best hydration regimen to prevent contrast media-induced nephrotoxicity? Clin Nephrol 62(1): 1-7.
- Taylor AJ, Hotchkiss D, Morse RW, McCabe J (1998) PREPARED: Preparation for Angiography in Renal Dysfunction: a randomized trial of inpatient vs outpatient hydration protocols for cardiac catheterization in mild-to-moderate renal dysfunction. Chest 114(6): 1570-1574.
- Golshahi J, Nasri H, Gharipour M (2014) Contrast-induced nephropathy; A literature review. Journal of nephropathology 3(2): 51-56.
- Zhang B, Liang L, Chen W, Liang C, Zhang S (2015) The efficacy of sodium bicarbonate in preventing contrast-induced nephropathy in patients with pre-existing renal insufficiency: a meta-analysis. BMJ open 5(3): e006989.
- Stenstrom DA, Muldoon LL, Armijo-Medina H, Watnick S, Doolittle ND, et al. (2008) N-acetylcysteine use to prevent contrast medium–induced nephropathy: premature phase III trials. J Vasc Interv Radiol 19(3): 309-318.
- Grobner T (2006) Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21(4): 1104-1108.
- Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB ,et al. (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 7(9): 2359-2362.
- Nichols WG, Price TH, Gooley T, Corey L, Boeckh M (2003) Transfusion-transmitted cytomegalovirus infection after receipt of leukoreduced blood products. Blood 101(10): 4195-4200.
- Triulzi DJ (2002) Specialized transfusion support for solid organ transplantation. Current opinion in hematology 9(6): 527-532.
- Kahan BD (2003) Individuality: the barrier to optimal immunosuppression. Nat Rev Immunol 3(10): 831-838.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9 Suppl 3: S1-S155.
- Gebel HM, Bray RA, Nickerson P (2003) Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant 3(12): 1488-1500.
- Cassuto JR, Levine MH, Reese PP, Bloom RD, Goral S (2012) et al. The influence of induction therapy for kidney transplantation after a non-renal transplant. Clinical journal of the American Society of Nephrology: CJASN 7(1): 158-166.
- John Vella M, Daniel C Brennan (2017) Kidney transplantation in adults: Induction immunosuppressive therapy. UpToDate.
- Rubin RH, Wolfson JS, Cosimi AB, Tolkoff-Rubin NE (1981) Infection in the renal transplant recipient. The Am J Med 70(2): 405-411.
- Van Gelder T (2002) Drug interactions with tacrolimus. Drug Saf 25(10): 707-712.
- Wadhwa NK, Schroeder TJ, Pesce AJ, Myre SA, Clardy CW (1987) Cyclosporine drug interactions: a review. Ther Drug Monit 9(4): 399-406.






























