Moyamoya Disease: Case Report and Review of Patient Operated at the Clinical Hospital in Uruguay
Gonzalo Bertullo1*, Gabriel Castelluccio2 and Humberto Prinzo3
1 Assistant Professor, Department of Neurosurgery, Clinical Hospital “ Dr. Manuel Quintela, University of the Republic, Montevideo, Uruguay
2 Department of Neurosurgery, Clinical Hospital “ Dr. Manuel Quintela, University of the Republic, Montevideo, Uruguay
3Professor, Department of Neurosurgery, Clinical Hospital “ Dr. Manuel Quintela, University of the Republic, Montevideo, Uruguay
Submission: November 20, 2019; Published: December 18, 2019
*Corresponding author: Gonzalo Bertullo, Department of Neurosurgery, Clinical Hospital “ Dr. Manuel Quintela”, Faculty of Medicine, University of the Republic. Montevideo-Uruguay, Rambla República de Chile 4287, Apto 501, Montevideo- Uruguay
How to cite this article:Gonzalo Bertullo, Gabriel Castelluccio, Humberto Prinzo. Moyamoya Disease: Case Report and Review of Patient Operated at the Clinical Hospital in Uruguay. Open Access J Surg. 2019; 11(2): 555807. DOI: 10.19080/OAJS.2019.11.555807.
Abstract
Moyamoya disease (MMD) is a progressive disease of the distal internal carotid arteries and their major branches that is characterized by occlusion of these vessels, which show intimal proliferation. A characteristic finding is the formation of a collateral network of blood vessels at the base of the brain with an unusual angiographic appearance described as a “puff of smoke.” Surgical revascularization is the main therapy for MMD as it prevents risk of a future stroke. Surgical options can be divided into indirect, direct, or combined approaches.The authors present the first case of a patient with MMD disease, operated in the Clinical Hospital in Uruguay by the combined technique with doppler and intraoperative angiography. Literature and the most accepted techniques were reviewed.
Keywords: Sister mary joseph nodule; Malignancy; Metastatic disease; Skin lesion; Chemotherapy; Abysmal prognosis
Introduction
MMD is now widely accepted as a disease process that not only affects patients of Asian descent, but it is also prevalent in North America and Europe. MMD is an occlusive, progressive cerebrovascular disease of both internal carotid arteries (ICA) or their branches, compensated by the development of a thin collateral vascular network. Its etiology is unknown, although different studies have associated its pathogenesis with genetic and environmental factors. A hereditary factor has been proposed, because the incidence of familial cases in Asian populations has been reported as high as 7% and because the incidence of MMD in patients with Down Syndrome is high as well. Other factors are: history of inflammation in the head and neck region, tuberculosis meningitis, arteriosclerosis, neurofibromatosis, irradiation, sickle cell anemia, connective tissue abnormalities [1,2]. The pathophysiology of MMD remains unclear. Pathologic specimens have shown that the outer diameters of the carotid artery are diminutive with increased intimal thickening. It usually evinces by transient ischemic attacks or cerebral infarcts. In countries where it is more frequent, such as Japan, it has been possible to make early diagnosis in patients thanks as a result of the developement of different Magnetic Resonance Imaging (MRI) techniques, for instance diffusion and FLAIR [1-3].
Revascularization surgery would reduce the incidence of new ischemic events and improve the long-term prognosis of patients suffering from this disease [2,3]. Surgical treatment is indicated when recurrent and progressive ischemic episodes appear. Different techniques have been carried out for more than three decades with the aim of revascularizing the brain, either directly or indirectly [2,4]. Although in the last decades different indirect revascularization techniques have been created for these patients, such as Encephaloduroarteriosynangiosis (EDAS), also known as Pial Synangiosis, Encephalomyosynangiosis (EMS) and Omental Transposition, only the first two are recognized nowadays. Direct revascularization is the most accepted of which we emphasize the superficial temporal artery anastomosis with the middle cerebral artery (ATS-MCA bypass).
Clinical Case
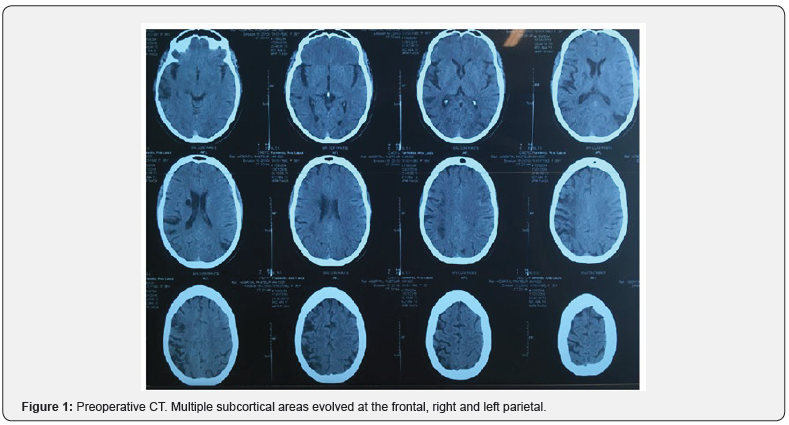
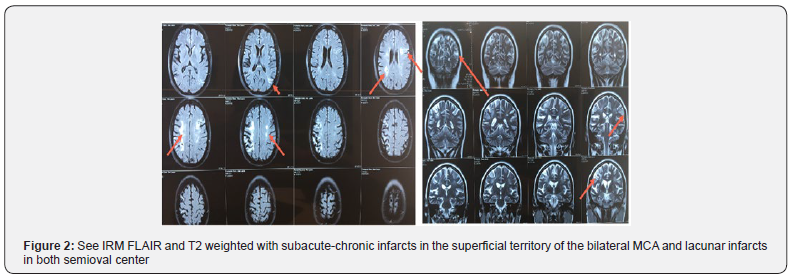
A 36-year-old female patient who is moderate smoker and with a history of hypertension difficult to control. In treatment with Enalapril, Methyldopa and Amlodipine. The initial symptoms were an abrupt motor deficit of the right side of the body, when she consulted for the first time. She was studied with a contrast-free cranial tomography (CT) showing that multiple subcortical areas had evolved at the frontal and right parietal. Additionally, there were dimensions in the white matter of both semi-oval centers, right periventricular image of evolved aspect (Figure 1). Subacute-chronic infarcts in the superficial territory of the left middle cerebral artery in addition to secular lesions and evolved lesions in right MCA territory and lacunar infarcts in both semioval centers were also present. There was significant reduction of the caliber of both extra and intracranial internal carotids to right predominance and in both MCA were observed from MRI of the cranial (Figure 2).
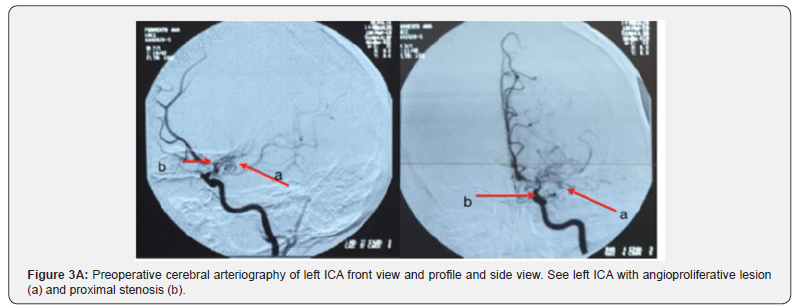
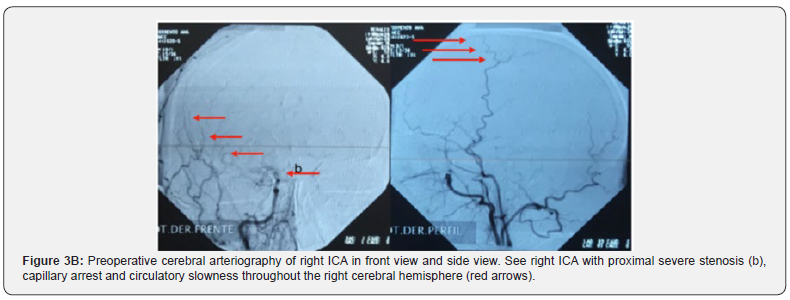
The Doppler study of the ICA and vertebral, the electrocardiogram, eyes fundus, and lipid profile were normal. Along with all studies mentioned above, the clinical case was evaluated jointly with colleagues in clinical round and it was decided to request neck and cerebral arteriography to rule out primary CNS vasculitis or other differential. Cerebral arteriography observations were angioproliferative type lesion with more severe stenosis on the right, capillary arrest and circulatory slowness predominantly throughout the right cerebral hemisphere, which is MMD in the context of having ruled out other causes (Figure 3). Two months later the patient consults again for diplopia and dysarthria without motor disorder on this opportunity. The physical exam showed mild paresis of the sixth cranial nerve. The patient was evaluated from the psychological point of view where no alterations were found.
Intraoperative
Coordination was fulfilled (Dr. Bertullo) with the objective of performing a direct and indirect revascularization with superficial temporal artery (Figure 4). Intraoperative Doppler and intraoperative angiography confirmed distal and proximal flow to the recipient artery.
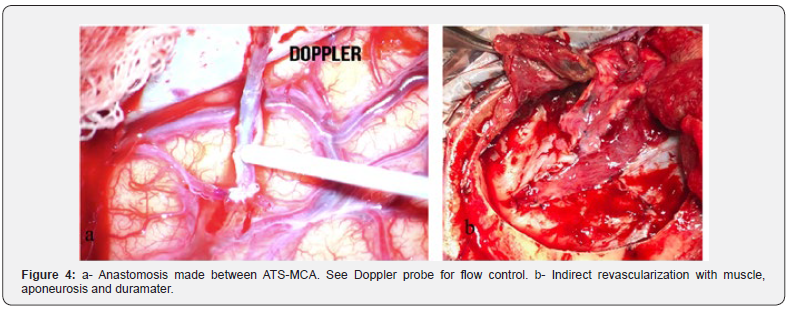
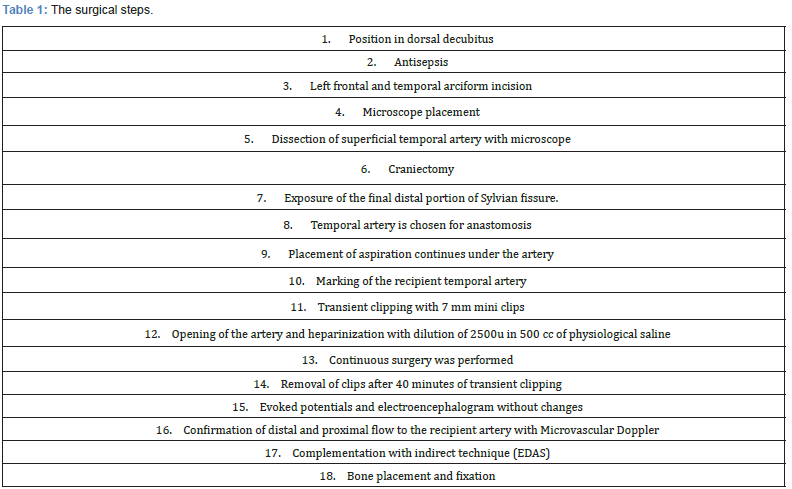
The surgical steps are detailed below (Table 1)
Postoperative
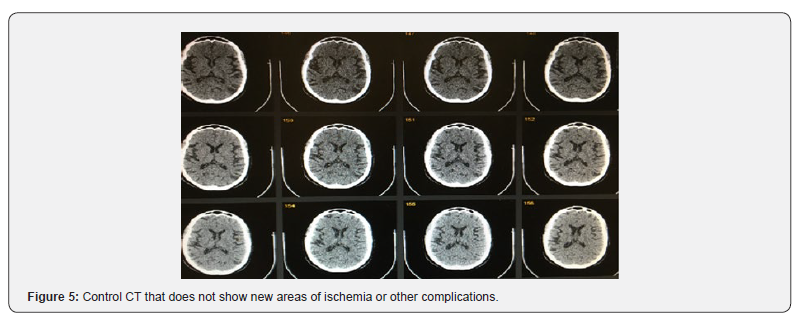
The patient was extubated without complications. Control CT did not show complications (Figure 5). It evolves favorably, lucidly, without headache, without focal neurological deficit. No symptoms were repeated 3 months after the current follow-up (Figure 6).
Discussion
MMD is a cerebrovascular disorder characterized by progressive occlusion of the supraclinoid internal carotid artery and its main branches within the circle of Willis [1-3]. This occlusion results in the formation of a fine and anomalous vascular network (Moyamoya vessels) at the base of the cervix.The Moyamoya vessels arise from the dilation of the perforating arteries that function as a collateral network to the stenosed sectors. These vascular alterations acquire an aspect of a cloud of smoke in angiographic studies (“Moyamoya” in Japanese) [4]. It is a progressive pathology. Early diagnosis allows to modify its evolution, thus avoiding progressive clinical deterioration, disability or even fatal consequences.
Epidemiology
The incidence of MMD is higher in countries in East Asia, such as Japan and Korea. In Japan, the annual prevalence and incidence rate has been estimated at 3.16 and 0.35 per 100,000 inhabitants respectively [5,6]. The female-male ratio is 1.8 to 1 and the age distribution at debut has two peaks: one at 5 years of age and one near 40 years of age. In recent years the improvement in diagnostic and therapeutic methods, as well as a better prognosis for these patients, have contributed to the increase in the incidence and prevalence of the disease [7,8]. A high incidence of family occurrence has been detected, representing approximately 15% of cases. Recent studies of family cases (studies limited to Japanese families) suggest a pattern of autosomal dominant polygenic inheritance, with a low penetrance and anticipation phenomenon.
Historical Review
The first case of MMD was described in Japan in the late 1950s [5]. The name of the disease was given by Suzuki and Takaku, who described them as a cloud of smoke. Later, in 1968, Kudo and Fukuta introduced it to the English language and described it as a spontaneous occlusion of the polygon of Willis [1-4]. Mahmut Gazi Yaşargil, Extracranial-intracranial cerebral vascular anastomosis surgery (ECr-ICr) seeks to restore blood flow to brain areas that are hemodynamically compromised [9]. This surgery was reported in dogs for the first time by Eck (1877) [9]. Later, with the advent of new suture materials and surgical instruments, it was perfected during the first half of the 20th century. Dr. Yasargil, pioneer of cerebral vascular microsurgery with the inclusion of the surgical microscope and the development of specialized instruments, reported for the first time in 1967 an ECr-ICr anastomosis from ATS to MCA to rid an obstructed segment of the internal carotid artery [8,9]. The refinement of the technique continued over the years with the participation of outstanding doctors, such as Dr. Kamiyama, in Japan, who during the past 30 years has perfected the surgical technique in an outstanding way, contributing practical advances, such as the use of methylrosaniline chloride (gentian violet, GV) for the adequate visualization of the surgical edges in the ostiums of the vessels to anastomosis, among other contributions of great importance for the advancement of this surgery [10]. Thus, the development of the technique of cerebral revascularization has been in constant progress until today.
Pathophysiology, Etiology and Clinical Presentation
The Moyamoya vessels arise from the dilation of perforating arteries that function as a collateral network to the stenosed sectors. The etiology that triggers these vascular modifications is not completely known. It is clear that the affected vessels do not present inflammatory or atherosclerotic changes [1,2,11- 13]. The anatomopathological findings show a clear decrease in the caliber of the terminal branches of the internal carotid artery as well as a fibrocellular thickening of the intima, irregular undulation of the internal elastic lamina and attenuation of the media [12-14]. Recent studies suggest that the caspase-3- dependent apoptosis pathway could be associated with these histopathological changes. The Moyamoya vessels present different histopathological changes, including parietal fibrin deposits, fragmentation of elastic lamina, attenuation of the media and formation of microaneurysms. The rupture or collapse of the arterial lumen of these vessels and the subsequent blood extravasation or thrombosis are closely associated with the appearance of haemorrhagic and ischemic stroke [15,16].
The clinical characteristics of MMD differ between children and adults. Most children develop transient ischemic attack (TIA) or cerebral infarction, while about half of adult patients suffer intracranial hemorrhage and the other half develop TIA and / or cerebral infarction. The symptoms and signs of MMD are attributed to alterations in cerebral blood flow as a consequence of the progressive stenosis of ICA and its main branches. Symptoms can be summarized in 2 major categories:
a) due to cerebral ischemia (infarction, TIA, epileptic seizures, etc.) and
b) due to the harmful consequences of the implementation of compensatory mechanisms against chronic ischemia (cerebral hemorrhage due to rupture of fragile dilated capillaries, headache due to dilatation of transdural collaterals, etc.) [14- 18].
Ischemia
Affects mainly frontal, parietal and temporal region. Frequent clinical forms of presentation are hemiparesis, dysarthria, aphasia and cognitive deterioration. They can also present with epileptic seizures, visual disturbances, syncope or behavioral alterations. Given the physiopathological mechanism of these ischemic events, they may be characteristically precipitated by hyperventilation in children (crying), intense exertion, anesthetic induction or dehydration. This is because in these patients cortical vessels that are already dilated to the maximum in response to chronic ischemia, are contracted by the reduction of partial pressure of carbon dioxide generating a greater local reduction of blood supply with subsequent ischemia focal [4,6,18].
Hemorrhage
Intracranial hemorrhage is more common in adults with MMD. This can be intraventricular, intraparenchymal (often basal ganglia) or subarachnoid. The hemorrhage is attributed to the rupture of fragile collateral vessels of the anomalous vascular network Moyamoya. The change in the usual vascular circulation pattern would also favor the development of cerebral aneurysms in areas of high parietal stress (top basilar artery, mainly posterior communicator). Dilatation and abnormal branching of the anterior choroidal artery and / or the posterior communicating artery are considered predictors of hemorrhagic vascular events [15,16].
Cephalea
Frequent symptom in patients with MMD. It is proposed that dilation of meningeal and leptomeningeal collateral vessels would stimulate dural nocioceptors, triggering the onset of headache. This is of migraineous characteristics and resistant to the usual therapeutics. It may even persist after successful revascularization surgery [8,14,15,17].
Chorea
Rare, described in children. Attributed to dilated collateral vessels in the basal ganglia region [18].
Cognitive deterioration
Although there are limited studies on the neuropsychological impact of MMD, the evidence shows relative preservation of intelligence and memory and commitment of executive functions (almost 50% of the population studied). These alterations are strongly associated with secondary damage to multiple vascular lesions of white matter as well as cortical infarcts [16,17].Although there are limited studies on the neuropsychological impact of MMD, the evidence shows relative preservation of intelligence and memory and commitment of executive functions (almost 50% of the population studied). These alterations are strongly associated with secondary damage to multiple vascular lesions of white matter as well as cortical infarcts [16,17].
Natural History of Disease
The natural history of MMD is variable. It can progress slowly, with episodic or fulminant vascular events with rapid neurological deterioration. Cerebral hematomas are the main cause of death in these patients. It progresses unavoidably in most patients, even in those who are asymptomatic. Medical treatment alone cannot stop the progression of the disease. It is estimated that up to two thirds of patients with MMD will experience symptomatic progression at 5 years. The estimated rate of progression of symptoms is close to 3% after surgery. The neurological status at the beginning of treatment and the age of the patient are the main predictors of the long-term therapeutic response [2,19,20].
Diagnosis
We must bear in mind the diagnosis of MMD in the etiopathogenesis of stroke in young people. We arrive at this diagnosis through a suggestive clinical picture and imaging studies with the characteristic findings of the disease. CT does not allow diagnosis of MMD. It may be normal or show to see small hypodense areas suggestive of hemorrhage or cerebral infarction in bordering cortical territories, basal ganglia and deep periventricular white matter [21]. Angiotomography (CTA) can provide a diagnostic approach, evidencing intracranial stenoses characteristic of the disease. It will be considered when MRI is not available [22]. MRI can be a diagnostic study of the disease. It will show:
a) acute infarctions (weighted diffusion) in atypical territories and honeycomb,
b) evolved infarcts,
c) decreased cortical blood flow due to vasculopathy characteristic of the disease that can be inferred in the FLAIR sequence by the sign of the creeper / ivy sign- linear sequences of high leptomeningeal signal following a sulcal pattern,
d) decreased flow gaps in ICA, MCA and ACA (severely stenosed arteries) together with prominent flow voids in the basal ganglia and thalamus region (very dilated collateral vessels).
The findings in point d) are characteristic of the vasculopathy of MMD and have diagnostic value [21-23].
Medical Treatment
It is a therapeutic option in patients with high risk for surgical procedures or patients with very mild cases of the disease [24]. Antiplatelets, although being contreversial (risk of intracranial hemorrhage from MMD), are commonly used in many centers to reduce the risk of embolism from in situ thrombosis in the stenosed arterial sectors [24,25]. The use of anticoagulants is not recommended as a routine practice. Calcium channel antagonists are widely used for modulator treatment of frequent headaches in these patients. They should be used with caution given the risk of hypotension and its negative consequences on cerebral hemodynamics (especially in these patients) [26,27].
Indication of surgical revascularization for MMD
The most important goal of surgical revascularization is to prevent cerebral infarction by improving cerebral blood flow (CBF) and restoring reserve capacity. The risk of surgical treatment is related in part due to the clinical condition and other underlying medical problems of the patient [28]. Patients who are in poor clinical condition as a result of ischemia or hemorrhage may not be ideal candidates for surgery unless significant improvement occurs. However, patients with significant mass effect and deteriorating neurologic condition from a hemorrhagic clot should be considered for emergent evacuation of the hemorrhage, with revascularization reserved for a later date [29,30]. Since pediatric MMD is characteristically more progressive than in adult patients, revascularization surgery is indicated in most children with MMD. As such, it is very important that early diagnosis and active intervention happen before irreversible brain damage occurs in order to achieve a favorable clinical outcome in children [29-31].
Surgical technique
Dissection and preparation of the superficial temporal artery. The semicircular incision is made as close as possible to the trajectory of the superficial temporal artery, for which scalpel No. 4 sheet 21 and dissection with teeth it is given to the surgeon. Then, bipolar electric scalpel is used to perform the corresponding haemostasis, and the syringe with serum is given to the assistant; the other assistant collaborates in the subsequent elaboration of the flap using farabeuff separators, aspiration and dissection forceps with teeth. At this time and throughout the surgery, we must keep the tips of the bipolar electric scalpel clean at all times and within reach of the surgeon’s loose gauze [28,31].
Once the skin has been incised in its superficial layers, dissection of the planes with bipolar forceps is continued with transverse movements that tend to move away from the vessels to avoid damaging them until dissection of the superficial temporal artery is completed. At this moment, operation begins under the vision of an electron microscope, which is previously prepared and conditioned for use. It must be covered by a sterile nylon sleeve and the nylon should be adjusted at the level of the lenses by means of elastic and sterile bands; and with a scalpel blade the corresponding nylon circles are cut to cover the surface of the lenses. The scalpel blade is discarded [32-39]. After dissection, a transient clip is placed at the most proximal point of the superficial temporal artery. The clip chosen by the surgeon is mounted on the corresponding clipper clip clamp, and clips are placed at the distal points of the front branches and the parietal branch, and are sectioned with curved scissors. Thus, achieving the release of the cutaneous flap artery, washed and irrigated with isotonic saline with heparin at a rate of 1,000 units of heparin in 100 ml of isotonic saline in a syringe 20 ml and a blue color abbocath. Then, another clip is placed at the distal end, making sure that the heparinized saline solution remains inside the superficial temporal artery. Once the donor artery and its branches are prepared, as a safety measure, it is placed next to the posterior border of the surgical incision covering it with wet gauze with physiological saline and keeping it at a safe distance from the area where the craniotomy will be performed [40,41]. The microscope is removed to make way for the craniotomy.
Craniotomy
The temporalis muscle is released in a single piece with a monopolar scalpel and retracted inferiorly with the help of hooks and elastic bands, fixing them to the Backhause tweezers. Then proceed to work at the time of the craniotomy, for which he it is delivered by monopolar electric scalpel to mark the periosteum and then Olver and Rugina bend to debone the bone [39,40].
Selection of the receiving artery
There are some characteristics to consider in selecting the recipient artery. The relationship in the diameters of the donor artery and the recipient artery should be very close to 1: 1, so it is necessary to adequately compare both vessels to locate the anastomosis at the ideal site according to the dimensions. Locate a vessel with healthy characteristics in the M3 segment of the middle cerebral artery, since in elderly patients it is common to find large plaques of atherosclerosis in the middle cerebral artery. The cortical vessels of patients with Moyamoya disease present two main color tones, pink and bright red. It is suggested to choose the pink ones as the receiving artery, since these have walls of better quality for the anastomosis [36-39].
The end of the artery is cut at an angle of 60° to the edge and a longitudinal incision is made on the shorter side. The tips are cut to change the shape of the ostium of the donor artery, making it rhomboidal and the edge is subtly impregnated with gentian violet to facilitate its visualization at the moment of anastomosing the vessels. At each vertex of the astium, a 10-0 prolene suture is inserted from the external wall towards the lumen, in a castro portal; these sutures will serve to make the fixation points at the vertex of the recipient artery [35,37-41].
Beneath the receiving artery slide a Kamiyama silicon millimeter field, which provides a smooth surface, easy to keep dry, with measurement preferences of 1 x 1 millimeters. As it is green, it absorbs the reflection of light and contrasts with the tonality of the arteries, brain, and suture threads; and gentian violet facilitates the visualization of each element. If necessary, below this field temporarily place to raise the artery some millimeters [36,37]. With the receiver artery list, the distance that exists between both vertices of the ostium of the donor artery is measured and with gentian violet a longitudinal line is drawn with this measurement on the wall of the recipient artery to mark the incision that will be carried out [35,37,39]. After completing each of these steps, the transient clips are placed proximally and distally in the segment of the recipient artery where the anastomosis will be performed. The recipient artery is incised with a microsurgical scalpel (ophthalmology) where it was previously framed with gentian violet and the incision is completed with straight scissors. This clipping segment is washed with dilution of serum with heparin preferably of the surgeon and the fixation points are completed in both vertices, starting from the lumen towards the wall so that both knots are outside the arterial lumen [40,41]. Continue with simple points until completing one side to continue with the other. It is important to take extreme precautions in the points near the vertices, since it can be easy to include the contralateral wall with the suture by mistake. The stitches should ideally be placed at a distance equivalent to the thickness of the wall of the superficial temporal artery and a distance of 300 to 500 μm between each point until completing the entire circumference. Usually, in addition to the two fixation points at the corners, 5 to 7 stitches are needed on each side of the donor artery to complete the anastomosis [38-41].
Since vascular anastomosis was performed, the transitory clips should be removed starting at the distal and then the proximal of the middle cerebral artery, then the distal one of the superficial temporal artery and, finally, the proximal of this same artery. The opening of the clips should be slow in order to locate the vanishing points that may arise. If this is the case, it will be necessary to place the clips again; perform a thorough lavage with serum with heparin and make the sutures that are necessary in order to occlude the bleeding. Once this step is completed, it is mandatory to confirm again the permeability of the superficial temporal artery with Doppler ultrasound. It is necessary to reshape the bone flap near the superficial temporal artery leaving a sufficiently broad space without bone to avoid collapsing the artery. The bone is fixed with vicril 2-0 in May needle holders and Boyle scissors to the helper to cut the threads, and the eco-doppler is performed once more. A drainage for negative pressure is placed by opening and fixing it to the skin with a nylon point 3-0 [28,30,38,41](Figure 6).

Complications
Complications There are several potential complications of STA-MCA bypass procedures. Ischemic changes are usually due to compression of the donor vessel by the scalp closure, graft occlusion or stenosis at the anastomosis site, or emboli [29]. Doppler and angiography studies generally identify the cause. As mentioned above, hypertension may result in leak at the anastomosis or hemorrhage. Hypertension may cause cerebral edema and dysautoregulation or, occasionally, hemorrhage, especially with the higher flow external carotid to MCA saphenous vein grafts [29,30]. Additionally, for STA- MCA bypasses, EDAs, and EMS, a non-water-tight dural closure is necessary to avoid any compression of the donor vessel, and this occasionally results in CSF leaks, pseudomeningoceles, and subdural hygromas. Finally, as with other craniotomies, systemic surgical complications include myocardial infarction, pneumonia, deep venous thrombosis, and pulmonary emboli. Complications from omental transposition include many of these adverse events, but also include the possibility of mass effect from the omentum itself [29,30,42]. Additionally, the abdominal laparotomy required to harvest the omentum may result in an ileus, bowel perforations, and peritonitis.
Outcome
Surgical treatment of MMD using various revascularization techniques has demonstrated to be safe and effective in reducing ischemic events, in both adults and children [25-28]. Post-revascularization angiography and MRI studies often reveal a reduction in Moyamoya vessels that closely parallels improvement in symptoms. Additionally, reports in the literature that revascularization techniques result in improvement of cerebral blood flow studies, in both adult and pediatric Moyamoya patients, are increasing in number [24,29,30,39,40- 42]. Our patient has a short time of evolution, she did not repeat symptoms or add others, so the current moment is satisfactory.
Conclusion
MMD is a pathology present throughout the world, including our country, for which a large number of surgical techniques have been promoted with the aim of developing neovascularization in the territories affected by the vascular occlusion that characterizes this disease. As much as it is a new technique, very difficult to employ and with a lot of risks, an excellent result was obtained, and reflected in the presentation of the case. This impels us to improve ourselves in terms of material resources, personnel training and investigation of the aforementioned pathology, in order to be able to face future cases with equal or better results in terms of quality and safety of patient care.
This paper has the intention to inquire, study and clarify one of the techniques of cerebral revascularization as a treatment to MMD, and to elaborate a reference material that provides specific information on cerebral revascularization as a treatment for MMD.
References
- Fujimura M, Sonobe S, Nishijima Y, Niizuma K, Sakata H, et al. (2014) Genetics and Biomarkers of Moyamoya Disease: significance of RNF213 as a Susceptibility Gene. J Stroke 16(6): 65-72.
- Fukui M (1997) Guidelines for the diagnosis and treatment of sponta- neous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg 99(Suppl 2): S238-S240.
- Narisawa A, Fujimura M, Tominaga T (2009) Efficacy of the revascu- larization surgery for adult-onset moyamoya disease with the progression of cerebrovascular lesions. Clin Neurol Neurosurg 111(2): 123-126.
- Suzuki J, Takaku A (1969) Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20(3): 288-299.
- Takeuchi K, Shimizu K (1957) Hypoplasia of the bilateral in- ternal carotid arteries. Brain Nerve 9: 37-43.
- Yamauchi T, Houkin K, Tada M (1997) Familial occur-rence of moyamoya disease. Clin Neurol Neurosurg 99(Suppl 2): S162-167.
- Starke RM, Crowley RW, Maltenfort M (2012) Moyamoya disorder in the United States. Neurosurgery 71(1): 93-99.
- Uchino K, Johnston SC, Becker KJ (2005) Moyamoya disease in Washington State and California. Neurology 65(6): 956-958.
- Vilela MD, Newell DW (2008) Superficial temporal artery to middle cerebral artery bypass: past, present, and future. Neurosurg Focus. 24(2): E2.
- Ishikawa T, Houkin K, Kamiyama H (1997) Effects of surgical revascularization on outcome of patients with pediatric moyamoya disease. Stroke 28(6): 1170-1173.
- Chen ST, Liu YH, Hsu CY, Hogan EL, Ryu SJ (1988) Moyamoya disease in Taiwan. Stroke 19: 53-59.
- Detwiler PW, de los Reyes RA (1996) Cerebrovascular disease of uncertain etiology and uncommon causes of stroke. In: Tindall GT, Cooper PR, Barrow DL (Eds.), The Practice of Neurosurgery, WIlliams & Wilkins, Baltimore, US State, pp. 1867-1898.
- Ellison PH, Largent JA, Popp AJ (1981) Moya-moya disease associated with renal artery stenosis. Arch Neurol 38(7): 467.
- Gewirtz RJ, Marks MP, Steinberg GK (1997) Clinical outcome of symptomatic patients with carotid artery occlusion and decreased hemodynamic reserve treated with STA-MCA bypass. J Neurosurg 86: 352A.
- Golby AJ, Marks MP, Thompson RC, Steinberg GK (1999) Direct and combined revascularization in pediatric moyamoya disease. Neurosurgery 45(1): 50-60.
- Halonen H, Halonen V, Donner M, Iivanainen M, Vuolio M, et al. (1973) Occlusive disease of intracranial main arteries with collateral networks in children. Neuropadiatrie 4(2): 187-206.
- Herman JM, Rekate HL, Spetzler RF (1995) Pediatric cerebrovascular disease. In: Carter LP, Spetzler RF, Hamilton MG: (eds) Neurovascular Surgery. New York, McGraw-Hill, pp. 211-229.
- Hinshaw EB,Thompson J, Hasso AN (1976) Adult arteriosclerotic moyamoya. Radiology 118: 633.
- Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis (2012) Health Labour Sciences Research Grant for Research on Measures for Intrac-table Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 52(5): 245-266.
- Horn P, Bueltmann E, Buch CV, Schmiedek P (2005) Arterio-embolic ischemic stroke in children with moyamoya disease. Childs Nerv Syst 21(2): 104-107.
- Kaku Y, Morioka M, Ohmori Y, Kawano T, Kai Y, et al. (2012) Outer-diameter narrowing of the internal carotid and middle cerebral arteries in moyamoya disease detected on 3D constructive interference in steady-state MR image: is arterial constrictive remodeling a major pathogenesis? Acta Neurochir (Wien) 154(12): 2151-2157.
- Yuan M, Liu ZQ, Wang ZQ, Li B, Xu LJ, et al. (2015) High-resolu- tion MR imaging of the arterial wall in moyamoya disease. Neurosci Lett 1 584: 77-82.
- Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, et al. (2010) High- resolution magnetic resonance wall imaging findings of moy- amoya disease. Stroke 2014;45:2457-2 Kim SJ, Heo KG, Shin HY, Bang OY, Kim GM, Chung CS, et al. Association of thyroid autoantibodies with moyamoya-type cerebrovascular disease: a prospective study. Stroke 41(1): 173-176.
- Im SH, Oh CW, Kwon OK, Kim JE, Han DH (2005) Moyamoya dis- ease associated with Graves disease: special considerations re- garding clinical significance and management. J Neurosurg 102(6): 1013-1017.
- Mineharu Y, Takagi Y, Takahashi JC, Hashikata H, Liu W, et al. (2013) Rapid progression of unilateral moyamoya dis- ease in a patient with a family history and an RNF213 risk vari- ant. Cerebrovasc Dis 36(2): 155-157.
- Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y (2007) Research Committee on Moyamoya Disease in Japan. Radiological find- ings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke 38(5): 1430-1435.
- Gross BA, Du R (2013) The natural history of moyamoya in a North American adult cohort. J Clin Neurosci 20(1): 44-48.
- Matsushima T, Inoue T, Suzuki SO,Fujii K, Fukui M (1992) Surgical treatment of moyamoya disease in pediatric patients-comparison between the results of indirect and direct revascularization procedures. Neurosurgery 31(3): 401-405.
- Wang KC, Phi JH, Lee JY, Kim SK, Cho BK (2012) Indirect revascularization surgery for moyamoya disease in children and its special considerations. Korean J Pediatr 55: 408-413.
- Kim SK, Cho BK, Phi JH, Lee JY, Chae JH, et al. (2010) Pedi- atric moyamoya disease: An analysis of 410 consecutive cases. Ann Neurol 68(1): 92-101.
- Karasawa J, Kikuchi H, Furuse S, Kawamura J, Sakaki T (1978) Treatment of moyamoya disease with STA-MCA anastomosis. J Neurosurg 49(5): 679-688.
- Kinugasa K, Mandai S, Kamata I, Sugiu K, Ohmoto T (1993) Surgical treatment of moyamoya disease: operative technique for encephalo-duro-arterio-myo-synangiosis, its follow-up, clinical results, and angiograms. Neurosurgery 32(4): 527-531.
- Irikura K, Miyasaka Y, Kurata A, Tanaka R, Yamada M, et al. (2000) The effect of encephalo-myo-synangiosis on abnormal collateral vessels in childhood moyamoya disease. Neurol Res 22(4): 341-346.
- Lee JY, Kim SK, Phi JH, Wang KC (2015) Posterior Cerebral Artery Insufficiency in Pediatric Moyamoya Disease. J Korean Neuro- surg Soc 57(6): 436-439.
- Bruzoni M, Steinberg GK, Dutta S (2015) Laparoscopic harvesting of omental pedicle flap for cerebral revascularization in children with moyamoya disease. J Pediatr Surg :19.
- Havlik RJ, Fried I, Chyatte D, Modlin IM (1992) Encephalo-omental synangiosis in the management of moyamoya disease. Surgery 111(2): 156-162.
- Zhao X, Wang C, Ji Y, Han C, Wang M (2015) Therapeutic effect of multiple burr hole operation combined with dural inversion and periosteal synangiosis for moyamoya disease. Br J Neuro-surg 29(6) :811-807.
- Kawaguchi T, Fujita S, Hosoda K, Shose Y, Hamano S, et al. (1996) Multiple burr-hole operation for adult moyamoya dis- ease. J Neurosurg 84(3): 468-476.
- Kazumata K, Ito M, Tokairin K, Ito Y, Houkin K, et al. (2014)The frequency of postoperative stroke in moyamoya disease following combined revascularization: a single-univer- sity series and systematic review. J Neurosurg 121(2): 432-440.
- Kawamoto H, Kiya K, Mizoue T, Ohbayashi N (2000) A modified burr-hole method ‘galeoduroencephalosynangiosis’ in a young child with moyamoya disease. A preliminary report and surgical technique. Pediatr Neurosurg 32(2): 272-275.
- Takeuchi S, Abe H, Ozawa T, Tanaka R (2000) Surgical treatment for moyamoya disease. Surgical effect of encephalo-galeo-synan- giosis on moyamoya disease. Surgery for Cerebral Strok 28: 98-103.
- Cho WS, Kim JE, Kim CH, Ban SP, Kang HS, et al. (2014) Long-term outcomes after combined revascularization surgery in adult moyamoya disease. Stroke 45(10): 3025-3031.






























