Upper Tract Transitional Cell Carcinoma (UTUC) With Sarcomatoid Change and Inferior Vena Cava Invasion: Imaging Findings of a Rare Entity
Mehak Raja1*, Sushila B Ladumor2 and Maneesh Khanna2
1Resident Radiologist, Clinical Imaging Department, Body Imaging, Hamad Medical Corporation, Hamad General Hospital, Qatar
2Consultant Radiologist, Clinical Imaging Department, Hamad Medical Corporation, HGH, Assistant Professor in Clinical Radiology, Weil Cornel Medical College, Qatar
Submission: October 23, 2017; Published: November 10, 2017
*Corresponding author: Mehak Raja, Resident Radiologist, Clinical Imaging Department, Body Imaging, Hamad Medical Corporation, Hamad General Hospital, Qatar, Email: mehak_uos@hotmail.com
How to cite this article: Mehak R, Sushila B L, Maneesh K. Upper Tract Transitional Cell Carcinoma (UTUC) With Sarcomatoid Change and Inferior Vena Cava Invasion: Imaging Findings of a Rare Entity. Open Access J Surg. 2017; 6(5): 555699. DOI: 10.19080/OAJS.2017.06.555699
Abstract
Upper tract urothelial carcinomas (UTUC) are generally uncommon and account for less than 3% of primary renal tumors [1]. UTUCs with IVC extension is rare, with only 27 cases reported in literature and a UTUC with sarcomatoid change is even rarer, with only 21 cases known in literature to date. We report on a case of a 70-year-old male patient of African descent, who presented with an incidentally detected right renal mass on ultrasound of the urinary tract performed for lower urinary tract symptoms secondary to benign prostatic hyperplasia. CT scan showed a right renal midpole mass associated with contiguous renal vein and inferior vena cava (IVC) tumour thrombosis and regional lymphadenopathy. MRI demonstrated an infiltrative mass invading the renal pelvis, extending along the right renal vein into the IVC with no definite vascular wall invasion.
With a preoperative diagnosis of RCC due to the presence of renal mass and contiguous tumour thrombus, the patient underwent right robotic nephrectomy, converted to open surgery with tumor thrombus clearance from the IVC. Histopathology revealed a high grade papillary urothelial carcinoma with sarcomatoid differentiation. Staging PET CT showed distant lymph node metastasis denoting stage IV disease. Following this the patient was started on three cycles of chemotherapy, comprised of gemcitabine and carboplatin.
Herein, we aim to exhibit the imaging findings in our rare case, which has not been elaborated in literature before.
<
Introduction
Urothelial carcinomas, also known as transitional cell carcinomas are the most common malignancy of the overall urinary tract. They commonly involve the bladder and urethra (90-95%) and uncommonly involve the renal pelvis, calyces and ureters (5-10%.) [2]. At diagnosis, 60% of UTUCs are invasive and concomitant bladder cancer is present in 33% of patients [3]. The most common presenting symptom is macro or microscopic hematuria [4]. CT urography is highly specific in high-risk patients and is generally preferred over Magnetic Resonance Urography (MRU).
Case Report
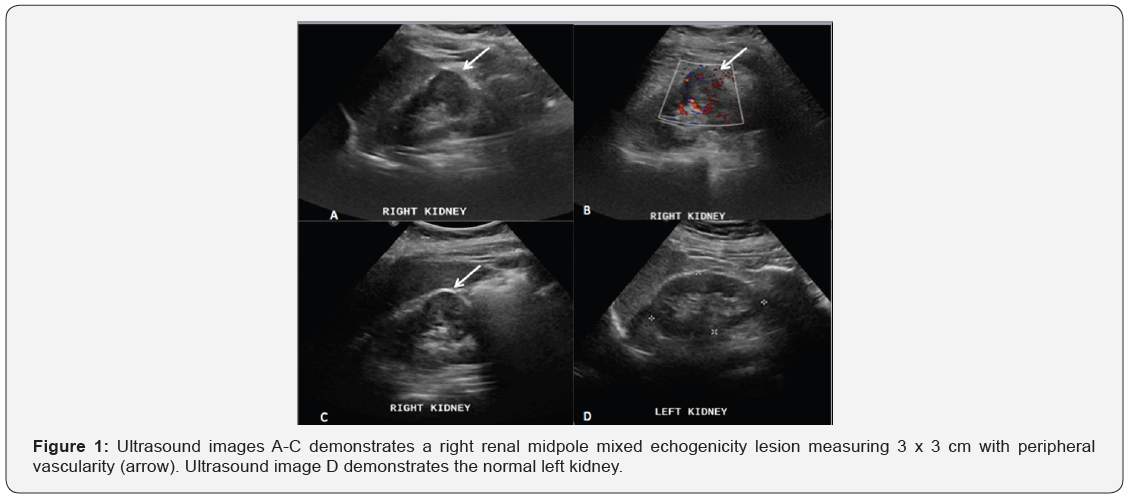
A 70 years old male, known to have DM, HTN and benign prostatic hyperplasia (BPH) on treatment, attended a regular follow up urology clinic for BPH. He had symptoms of urinary urgency pertaining to his BPH. Ultrasound of the urinary tract showed an incidental right renal solid mid-pole mass, demonstrating mixed echogenicity and peripheral vascularity, causing a focal contour bulge (Figure 1). A suspicion of primary renal malignancy was raised. Laboratory evaluation revealed iron deficiency anemia (Hgb: 8.6 gm/dL MVC 75.6 fL, MCH 22.3 pg), WBC: 6.9. Normal renal function (BUN: 4.9 mmol/L, Cr: 92 nmol/L) and normal liver function. Urinalysis was normal with no RBCs, 4 uL WBCs and high blood glucose of 5.5 mmol/L. Urine cytology was not performed.
A subsequent contrast enhanced CT scan was performed on a 64-slice CT scanner (LightSpeed; GE Healthcare, 436 mA, 100 kV, 1.5-mm section thickness, 0.5-second rotation time). The CT scan examination was protocoled for renal mass, which entailed three phases including a pre-contrast run, a post contrast venous phase (70s delay) and a urography phase (5 mins delay) from the top of the kidneys down to the pelvic cavity.
CT demonstrated a right renal hypodense lesion predominantly involving the antero-lateral aspect of the mid part of the right kidney measuring 6 x 3 cm. Peri-renal fascia appeared thickened with minimal surrounding fat stranding. Focal filling defects were noted in the right renal vein and IVC in continuity with the tumour denoting direct tumour extension/ thrombosis (Figure 2). Multiple enlarged retrocaval and aortic lymph nodes were additionally noted, suggesting metastatic involvement, cT3a N1 M0.
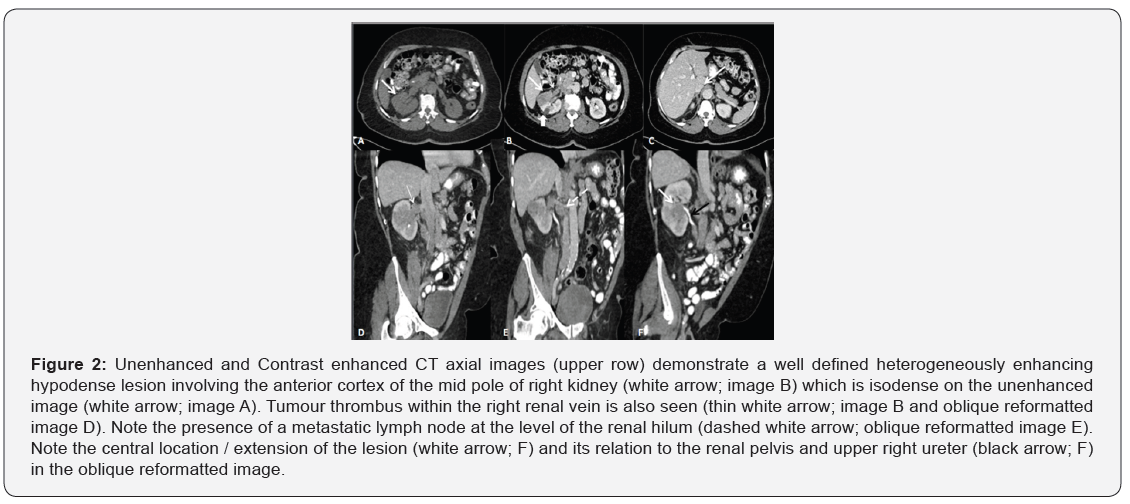
Left kidney, ureters and the urinary bladder were preserved.
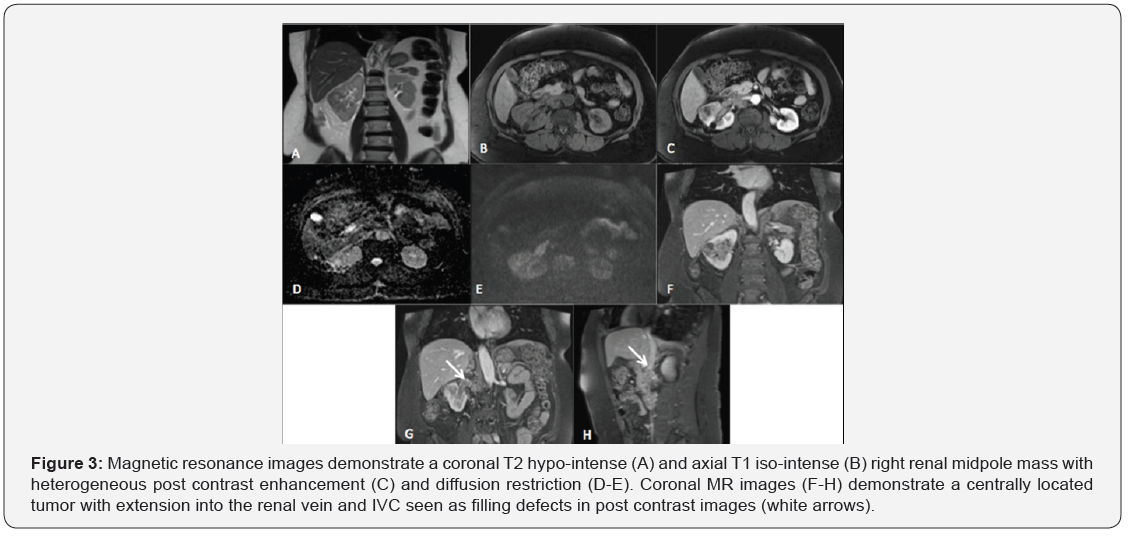
An MRI abdomen with intravenous contrast was performed for further characterization and assessment of the renal vein/ IVC wall invasion. On MR, an ill-defined heterogeneously enhancing infiltrative right renal mass was noted in the mid to lower pole of the right kidney. The mass was seen extending into the renal hilum and invading the renal pelvis. Tumoral thrombus was seen extending along the entire course of the right renal vein and projecting into the right side of the lumen of inferior vena cava without definite wall invasion (Figure 3). Considering direct extension of the tumour into the renal vein and IVC, a diagnosis of renal cell carcinoma was inferred. However, in view of the central location of the tumor involving the renal pelvis, upper tract transitional cell carcinoma was also listed in the differential.
With a most likely preoperative diagnosis of RCC, the patient went in for a right robotically assisted nephrectomy. At surgery, the right kidney demonstrated significant perinephric adhesions and soft tissue edema. It was mobilized and the renal vessels dissected. Upon dissection of the renal vein, extensive renal vein and IVC thrombus was seen reaching up to the hepatic veins. The procedure was converted to open laparotomy (Figure 4). Tumor thrombus retrieval from the IVC was successful. However, a dense mass posterior to the IVC was observed, that could only be partially excised. On macroscopic examination of the radical nephrectomy specimen cut section, a 5.5 x 4.4 x 4.5 cm tumor was seen in the renal pelvis growing towards and pushing the renal capsule but not infiltrating it. The tumor margins were 0.2 cm from the Gerota’s fascia sparing the perinephric fat. Soft tissue extracted from the intra-caval tumor thrombus appeared hemorrhagic and friable.
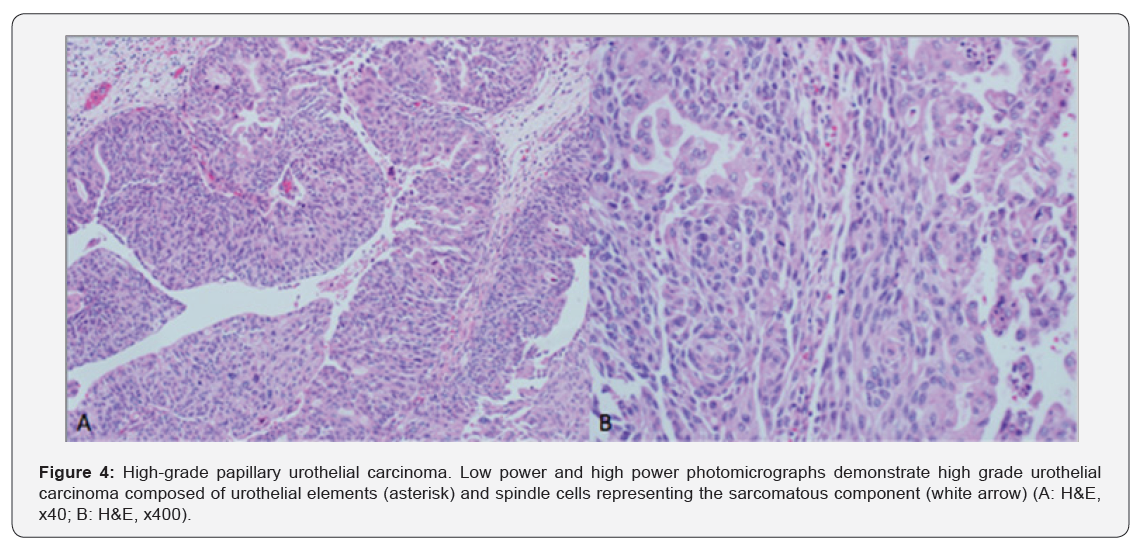
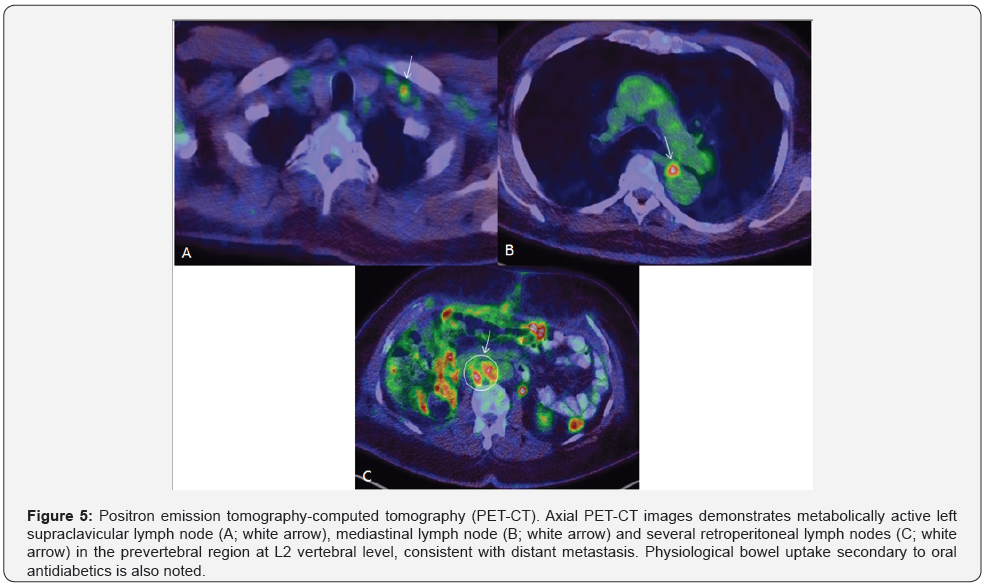
Histopathology demonstrated a uni-focal high-grade papillary urothelial carcinoma containing urothelial cells with large hyperchromatic nuclei, frequent atypical mitotic figures, frank anaplasia and spindle cells representing high-grade sarcomatoid features. The spindle cell component accounted for greater than 60 % of the tumor. Urothelial carcinoma in-situ was also noted. Extensive invasion of the renal parenchyma, renal sinus adipose tissue, renal vein, inferior venal cava and associated lymphovascular invasion by the tumor was seen. No lymph nodes were identified in the surgical specimen. A pathological stage of pT4 pNx was given. The patient had a long intraoperative course followed by SICU admission for gradual weaning from intubation. He was extubated the second day and had an uneventful post-operative recovery. A staging PET CT post-surgery revealed metabolically active metastatic regional retroperitoneal LNs and a solitary mediastinal LN with left supraclavicular LNs suspicious for distant metastasis. Categorically, the malignancy was labeled as a stage IV disease (Figure 5). Radiotherapy was not undertaken for reasons of advanced tumoral stage. The patient was started on carboplatin and gemcitabine for 3 cycles of chemotherapy.
Discussion
Upper tract urothelial carcinoma is a malignancy arising from the urothelium lining the renal calyces up to the distal ureter. UTUC of the renal pelvis has been frequently misdiagnosed as RCC on imaging. It was first described as a renal pelvic carcinoma in 1841 by a French pathologist named Rayer [5]. Diagnostic differentiation between a UTUC of the renal pelvis and its greatest mimic, a centrally located RCC is imperative in planning the surgical approach and management strategy. For instance, centrally located RCC is surgically treated with often minimally invasive nephrectomy, whereas UTUC requires nephroureterectomy and wider lymphadenectomy owing to its greater invasiveness. Follow-up strategies also differ. Patients with UTUC are started on an intensive surveillance plan because they have a lifelong increased risk of developing urothelial tumors in the contralateral upper urinary tract or bladder [6,7].
Of all cases of UTUC known in literature, 57% had been preoperatively misdiagnosed as RCC [8]. On cross-sectional imaging, as renal UTUCs infiltrate centrifugally into the renal parenchyma, they demonstrate features similar to centrally located renal cell carcinomas. In addition, the greater incidence of RCCs with renal vein or IVC extension (4-36%) compared to UTUCs with tumoral venous thrombus (5 to 7%) further adds to the difficulty in diagnostic differentiation between an RCC and renal UTUC with venous invasion [9,10].
UTUCs of the pelvis can be recognized with high accuracy on CT. A previous retrospective cross-sectional study, evaluated the diagnostic performance of individual CT features and their reliability in differentiating UTUCs of the renal pelvis from centrally located RCC. Six CT signs most helpful in the differentiation were, tumor center in collecting system, focal filling defect in renal pelvis, renal shape preservation, absence of cystic or necrotic change, homogeneous enhancement of tumor and extension towards ureteropelvic junction. These signs identified UTUC of the renal pelvis with a sensitivity of 68-82% and specificity between 79-89% [10]. Our case demonstrated five of the six CT signs with the differing sign being heterogeneous contrast enhancement.
The usefulness of MRI in UTUC detection and characterization is demonstrated by its greater soft tissue delineation ability over CT. Of particular interest in a series of MR sequences is the diffusion weighted functional imaging sequence. This sequence demonstrates good contrast between the tumor and the surrounding tissues, such that a positive DW-MRI is strongly indicative of UTUC, whereas a negative DW-MRI nearly excludes a diagnosis of UTUC in patients with negative urinary cytology [11]. Additionally, ADC value could be a biomarker of UTUC and reflect its aggressiveness, including histological grading and proliferative potential. In one study, ADC value was significantly lower in grade 3 cancers than in grade 1–2 cancers, potentially influencing the surgical management [12].
On histology, UTUCs in the renal pelvis are known to frequently display unusual morphological features unlike the UTUCs of the ureter. In a study of 108 high-grade urothelial carcinomas of the renal pelvis, up to 40% demonstrated unusual features. Some of the unusual morphological features were: micropapillary areas (4%), lymphoepithelioma-like carcinoma (2%), sarcomatoid carcinoma (6%), squamous differentiation and squamous cell carcinoma (14%), clear cells (4%), glandular differentiation (2%), rhabdoid, signet-ring or plasmacytoid cells (4%), pseudosarcomatous stromal changes (4%) and intratubular extension into the renal pelvis (3%) [13].
Sarcomatoid change in a UTUC has been seen in twentyone cases known in literature [1,13-17] to date. Piscioli et al reported the first case in a 62-year old male in 1984 [14]. However, only one known case in literature has shown IVC extension whilst exhibiting sarcomatoid changes [1] with our case being the second in line. We have demonstrated US, CT and MRI findings in our case, making ours the only case in literature with a comprehensive radiologic evaluation of sarcomatoid variant UTUC with IVC extension. Sarcomatoid carcinoma is a biphasic malignant neoplasm whose cells have morphologic and/or immunohistochemical properties of both epithelial tumours (“carcinoma”) and mesenchymal tumours (“sarcoma”) [15]. Microscopically, the carcinomatous component is mostly transitional cell carcinoma and the sarcomatous component is largely composed of spindle cells [16,17]. These cells show positivity of the spindle tumor cells for cytokeratin AE1/AE3 and vimentin on immunohistochemical staining [13].
Currently, open radical nephroureterectomy with bladder cuff excision is the standard for high-risk UTUC (i.e. hydronephrosis, tumour size > 1 cm, high-grade cytology, high-grade URS biopsy, multifocal disease, previous radical cystectomy for bladder cancer). Kidney-sparing surgery is considered for low-risk UTUC (sparing the morbidity associated with open radical surgery) and in all imperative cases with renal insufficiency or a solitary functional kidney. In patients with advanced metastatic disease, radical nephroureterectomy may play a palliative role; however, there are no proven benefits. Such patients are usually started on platinum based chemotherapy regimens similar to urothelial bladder cancer therapies [18].
Conclusion
In conclusion, the greater incidence of RCCs in centrally located renal pelvic tumors should not preclude the differential of a UTUC of the renal pelvis on imaging. Differentiation of these malignancies is challenging on imaging, but necessary because the management strategies and standard of treatment differs.
References
- Diaz RR, Kwon JK, Lee JY, Nahm JH, Cho KS, et al. (2014) Renal pelvic urothelial carcinoma with vena caval thrombus mimicking renal cell carcinoma. Korean J Urol 55: 624-627.
- Munoz JJ, Ellison LM (2000) Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 164(5): 1523- 1525.
- Cosentino M, Palou J, Gaya JM (2013) Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol 31(1): 141-145.
- Cowan NC (2012) CT urography for hematuria. Nat Rev Urol 9(4): 218-226.
- Rayer PFO (2000) Traités des Maladies des Reins, Des Alterations De La Secretion Urinaire. Tome 3. Paris: J.B. Baillière, years 1839-1841.
- Rathmell WK, Godley PA (2010) Recent updates in renal cell carcinoma. Curr Opin Oncol 22: 250-256.
- Sun M, Abdo A, Abdollah F (2010) Management of upper urinary tract urothelial carcinoma. Expert Rev Anticancer Ther 10:1955-1965.
- Li (2016) Transitional cell carcinoma with extension of the renal vein and IVC tumor thrombus: report of three cases and literature review. World Journal of Surgical Oncology 14: 309.
- Pouliot (2010) Contemporary management of renal tumors with venous tumor thrombus. J Urol 84: 833-841.
- Raza SA (2012) Centrally infiltrating renal masses on CT: differentiating intrarenal transitional cell carcinoma from centrally located renal cell carcinoma. AJR Am J Roentgenol. 198: 846-853.
- Yoshida S, Masuda H, Ishii C (2011) Usefulness of diffusion-weighted MRI in diagnosis of upper urinary tract cancer. AJR Am J Roentgenol 196: 110-116.
- S Yoshida (2014) Role of diffusion-weighted magnetic resonance imaging as an imaging biomarker of urothelial carcinoma. Int J Urol 21(12): 1190-200.
- Perez (2006) High-grade urothelial carcinoma of the renal pelvis: clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod Pathol 19(4): 494-503.
- Piscioli F, Bondi A, Scappini P (1984) True sarcomatoid carcinoma of the renal pelvis. Eur Urol 10: 350-355.
- D Bostwick, L Cheng (2008) Urologic Surgical Pathology, 2nd edn, Mosby Elsevier, Edinburgh, UK.
- MF Acikalin (2005) Sarcomatoid Carcinoma of the Renal Pelvis with Giant Cell Tumor-Like Features: Case Report with Immunohistochemical Findings. Int J Urol 12(2): 199-203.
- Lopez-Beltran A, Escudero AL, Cavazzana AO (1996) Sarcomatoid transitional cell carcinoma of the renal pelvis. A report of five cases with clinical, pathological, immunohistochemical and DNA ploidy analysis. Pathol Res Pract 192: 1218-1224.
- Roupret (2015) Guidelines on Urothelial Carcinomas of the Upper Urinary Tract. Eur Urol 68(5): 868-879.






























