Anaesthetic Management of Extramedullary Pheochromocytoma: Case Report & Review of Literature
Sandeep Kumar Kar*, Tanmoy Ganguly, Chaitali Sen Dasgupta and Anupam Goswami
Department of Cardiac Anaesthesiology, Institute of Postgraduate Medical Education & Research, India
Submission: April 23, 2017; Published: May 04, 2017
*Corresponding author: Sandeep Kumar Kar, Assistant Professor, Department of Cardiac Anaesthesiology Institute of Postgraduate Medical Education & Research, Kolkata, India, Email: sndpkar@yahoo.co.inspan>
How to cite this article: Sandeep K K, Tanmoy G, Chaitali S D, Anupam G.Anaesthetic Management of Extramedullary Pheochromocytoma: Case Report & Review of Literature. Open Access J Surg. 2017; 4(1): 555630. DOI: 10.19080/OAJS.2017.04.555630
Case Report
- Twelve years old girl.
- H/O repeated attacks of dyspnoea, palpitation, headache, diaphoresis and cold clammy skin.
- On examination her NIBP was 182/126 mm Hg.
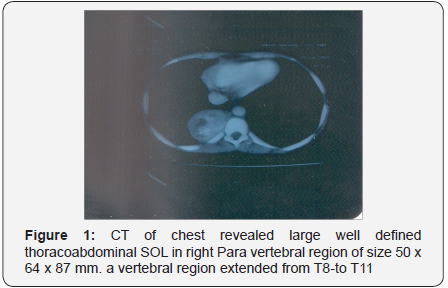
- CT of chest revealed large well defined thoracoabdominal SOL in right paravertebral region of size 50 x 64 x 87 mm.
- On CT abdomen the mass in right paravertebral region extended from T8-to T11
- Twenty four hour urinary VMA was 52.57 mg.
- So, it was diagnosed to be
Extramedullary Pheochromocytoma
Pheochromocytomas are catecholamine-secreting tumors of chromaffin tissue and are a rare cause of hypertension [1]. Less than 0.1% of the hypertensive population has a pheochromocytoma [2,3]. The hypertension caused by these tumors is usually curable. Surgery on a patient with an unrecognized pheochromocytoma can be fatal; similarly the administration of β-adrenergic-blocking drugs can have untoward side effects. These tumors can be associated with other potentially fatal but curable diseases.
Incidence: The high incidence of pheochromocytoma in families as a primary disease, in association with multiple endocrine neoplasia or other familial diseases such as Von Hippel Lindau syndrome and neurofibromatosis I, indicates the need for genetic counseling [4]. Approximately 16% of pheochromocytomas will be associated with other endocrine disorders, such as multiple endocrine syndrome 2, which is comprised of medullary thyroid carcinoma, pheochromocytoma, and parathyroid hyperplasia.
Pathophysiology: The incidence of pheochromocytoma as a benign tumor in one of the adrenal glands is 80%. Twenty percent are extra-adrenal, with half located below the diaphragm in areas such as along the aorta, near the urinary bladder, and in the organ of Zuckerkandl; the other half is located above the diaphragm in areas along the aorta, in the lungs or heart, or in the neck or carotid bodies. Ten percent occur in children. In non familial disease, the classical teaching is that 10% of patients have bilateral adrenal tumors and 10% have multiple extra-adrenal tumors; however, in familial disease, more than 80% are bilateral or in multiple sites. The incidence of a malignant pheochromocytoma is 10%. The occurrence of a pheochromocytoma is evenly distributed between the sexes and can occur at any age, although the peak incidence is between the fourth and sixth decades.
Catecholamine secretion is responsible for the signs and symptoms of a pheochromocytoma [5]. It is unusual that a tumor will grow large enough or be so invasive as to interfere with the function of the surrounding organs. The manifestations of a pheochromocytoma are primarily the result of the excessive secretion of norepinephrine, epinephrine, and dopamine [6]. The most common combination is predominantly norepinehrineand epinephrine (Figures 1-3). Some tumors secrete onlynorepinehrine, but <10% secrete only epinephrine. Dopamineand its metabolite are more likely to be significantly elevated inchildren with a pheochromocytoma.
The etiology for the increased production and secretion ofcatecholamines is not clear. It is conceivable that the negativefeedback mechanism of norepinephrine on tyrosine hydroxylaseis altered so that sensitivity to feedback is decreased, or perhapsmetabolism or release is so rapid that feedback does not transpirein the usual manner. Small tumors tend to secrete high levels ofcirculating catecholamines. With intracellular metabolism beingmore prevalent in large tumors, high levels of metabolites tendto be released, and free catecholamine secretion is reduced.
Approximately 50% of patients with a pheochromocytomahave sustained hypertension, 45% are normotensive withparoxysms of hypertension, and 5% are normotensive oreven hypotensive [4,6]. These differences, in part, relate tothe patterns of catecholamine secretion; bursts producehypertensive episodes. Patients with sustained hypertensionand some normotensive patients can have high or normallevels of norepinephrine. How sustained levels of highnorepinephrine concentrations result in persistent hypertensionis not understood. However, if elevated catecholamine secretionpersists, α- and β-receptors may become desensitized, or evendown regulated. Homodynamic mechanisms will no longerrespond to elevated levels of norepinephrine, and blood pressurewill normalize. Catecholamine levels and blood pressure do notusually correlate well, but a significant change in catecholamineconcentration will elicit a blood pressure response. Patients withthe rare, exclusively epinephrine producing tumors can presentwith normotension, or even hypotension, secondary to thevasodilating properties of epinephrine. Orthostatic hypotensionis another result of the ganglionic-blocking activity of excessiveamounts of catecholamines.
Clinical Presentation
The classic triad of symptoms in patients with apheochromocytoma consists of episodic headache,sweating, and tachycardia, all of which are largely due to thepharmacologic effects of the catecholamines secreted fromthese tumors [4,7]. Other signs and symptoms include pallor,orthostatic hypotension, visual blurring, papilledema, weightloss, polyuria, polydipsia, increased erythrocyte sedimentationrate, hyperglycemia, psychiatric disorders, and, rarely,secondary erythropoesis consistent with overproduction oferythropoietin. The abnormalities in carbohydrate metabolismare directly related to increases in catecholamine production;these changes resolve after adrenalectomy. There are two rarepresentations of a pheochromocytoma: Episodic hypotensionin patients with tumors that secrete only epinephrine and rapidcyclic fluctuations of hypertension and hypotension [7-9]. Thelatter group of patients can be treated by fluid repletion andα-adrenergic antagonists.
These patients can exhibit significant baselineelectrocardiographic (ECG) changes due to the toxic effectsexerted on the myocardium by the excessively high levels ofcatecholamines. Patients may also present with chest painand ECG changes suspicious for ischemia. Despite strikingrepolarization changes, many patients who proceed tocoronary angiography preoperatively are found to have noobstruction of their coronary arteries. Anecdotal reportssuggest ECG changes to be a manifestation of toxic myocarditis.In addition to the ECG changes mentioned, many patients witha pheochromocytoma are noted to have a long QTc intervalwhich may predispose to ventricular arrhythmias [10,11]. Afterremoval of the pheochromocytoma, the QTc intervals tend tonormalize. An elevated temperature more commonly reflectsa catecholamine mediated increase in the metabolic rate anddiminished heat dissipation secondary to vasoconstriction.Polyuria is an occasional finding, and rhabdomyolysis withresultant myoglobinuric renal failure may result from extremevasoconstriction and ensuing muscle ischemia.
Diagnosis
The diagnosis of pheochromocytoma is usually suggestedby the history in a symptomatic patient or the family historyin a patient with familial disease, and can usually be confirmedby measurements of urinary and plasma catecholamines andmetabolites and radiological tests.
Specific Tests for Diagnosing Pheochromocytoma
- The measurement of urinary catecholamines andmetabolites (preferably metanephrines rather than vinyl mandelic acid) is a useful screening test. Urinarymetanephrine excretion above 1.2 mg/d is highly suggestiveof a pheochromocytoma, and normal levels on three 24-hcollections of metanephrines virtually exclude the diagnosis(a,b). Many drugs (such as labetolol and buspirone) anddietary vanilla can interfere with these tests. Urinarymetanephrine and catecholamine secretion does not vary asa function of age or sex in normal subjects.
- Resting plasma catecholamines are elevated.
- Plasma chromogranin A (a storage vesicle proteinthat is co-stored and co-secreted with catecholamines) iselevated.
- Clonidine suppression and glucagon stimulation testsmay be appropriate, but should be performed in specializedcenters.
- Computer tomography scans, initially of the abdomen,are helpful to localize the tumors which are often large (c)
- Magnetic resonance imaging usually shows the lesionclearly (d)
- Scanning with [131I] metaiodobenzylguanidineproduces specific uptake in sites of sympathetic activity,with approximately 90% success; it is especially useful withextra-adrenal tumors (e)
- Data from: Sawka AM, Jaeschke R, Singh RJ (2003) Acomparison of biochemical tests for pheochromocytoma:Measurement of fractionated plasma metanephrinescompared with the combination of 24h urinarymetanephrines and catecholamines. J Clin Endocrinol Metab88: 553.
- Lenders JW, Pacak K, Walthar MM (2002) Biochemicaldiagnosis of pheochromocytoma: Which test is best? JAMA287: 1427.
- Mukherjee JJ, Peppercorn PD, Reznick RH (1996) CTimaging of pheochromocytoma: Effects of non-ionic contrastmedium on circulating catecholamine levels. Radiology 202:227.
- Bravo EL (1994) Evolving concepts in the pathophysilogy,diagnosis, and treatment of pheochromocytoma. EndocrinolRev 15: 356.
- Whalen RK, Althausen AF, Daniels GH (1992) Extraadrenalpheocromocytoma. J Urol 147: 1-10.
Anesthetic Considerations
Preparing the patient for surgical removal
Preparing the patient for surgical removal of apheochromocytoma entails the institution of α- and β-adrenergicblockade. The reduction in perioperative mortality rates, from ashigh as 45% to as low as 0% to 3% after the pheochromocytomais excised, has encouraged the administration of α-antagonistsfor preoperative therapy which is usually initiated once thediagnosis is established. To date, no one has investigated theoptimal duration of preoperative phenoxybenzamine therapy.Most patients require treatment for 10 to 14 days, as determinedby the time needed for stabilization of blood pressure andamelioration of symptoms. If the patient does not complainof nasal stuffiness, he or she is not ready for surgery. Becausepheochromocytomas spread slowly, little is lost by waiting untilthe patient’s preoperative condition has been optimized bymeans of medical therapy. The following criteria for an optimalpreoperative condition are recommended:
- No “in-hospital” blood pressure reading higher than160/90 mm Hg should be evident for 24 hours beforesurgery. I normally measure the blood pressure in eachpatient (as an outpatient) every minute for an hour in arecovery room setting during preoperative visits. This settingis most stressful to the medically naive and thus a good testof inhibition of responses to sympathetic stimulation. Ifno blood pressure reading higher than 160/90 mm Hg isrecorded, the patient is scheduled for surgery, assuming thefollowing three criteria are also met.
- Orthostatic hypotension, with readings above 80/45mm Hg, should be present.
- The electrocardiogram should be free of ST-Tabnormalities for at least a week; if abnormalities arepersistent, two-dimensional echocardiography should revealno evidence of global or regional dysfunction that cannot beattributed to a permanent deficit.
- The patient should have no more than one prematureventricular contraction every 5 minutes.
Phenoxybenzamine, a long-acting (24 to 48 hours),noncompetitive, presynaptic, α-adrenergic antagonist, is typicallyinitiated at least 7 days (usually for 2 to 4 weeks) before surgeryand added incrementally until the blood pressure is controlledand paroxysms disappear. The initial dose is usually 10 mg every12 hours. Most patients require between 80 and 200 mg per day.The combination of α-adrenergic receptor blockade and a liberalsalt intake will restore the contracted plasma volume towardsnormal [12]. Selective α-blockers, such as doxazosin, prazosinand terazosin, have also been used effectively, but their role inpreoperative management may be limited to the treatment ofindividual paroxysms. Nitroprusside, calcium channel blockers(where it was found that treatment extended postoperativelywith calcium channel blockers is also effective in treating theclinical manifestations of catecholamine-induced myocarditisand coronary vasospam), [13] and angiotensin-convertingenzyme inhibitors all can be used to reduce blood pressure inthese patients. Intraoperatively, nitroprusside is beneficial in thetreatment of hypertensive crises.
β-adrenergic blockade is initiated more than 3 days beforesurgery in patients with persistent tachycardia or reflextachycardia related to the initiation of α-blockade, or in thosehaving arrhythmias. It is important to initiate α-blockade beforeβ-blockade to avoid the situation of unopposed α-agonismwhereby the patient suffers from intense vasoconstriction fromthe α-adrenergic excess and is at risk for extreme hypertensionas well as a dramatic increase in myocardial workload. Low dosesoften suffice; a reasonable starting dose is 10 mg propranolol,three to four times per day, and is increased as needed to controlthe pulse rate. Labetolol, a β-adrenergic antagonist with someα-blocking activity, is effective as a second-line medication,but can increase the blood pressure if used alone, presumablybecause of its β-blocking effect being much more pronouncedthan its α-blockade. The pharmacologic adrenergic blockadehelps to blunt the intense surges in blood pressure that occurduring surgery and tumor manipulation.
Intravascular volume is also contracted in patients with apheochromocytoma. This is manifested by hemoconcentrationand orthostatic changes in blood pressure. α-adrenergicmediatedvasoconstriction, and possibly altered capillarypermeability, is thought to be responsible for these findings.Preoperatively, α-adrenergic blockade enables the patientto replete intravascular volume. The presence or absence oforthostasis and changes in hematocrit should be assessed at thetime of the preoperative visit. Despite the fact that hypotensioncommonly occurs after vascular isolation of the tumor, mostclinicians continue α-blockade up until the morning of thesurgery. Preoperative treatment with α-methyl-para-tyrosineresults in depletion of tumor catecholamine stores caused by thecompetitive inhibition of tyrosine hydroxylase, and decreasesboth blood pressure lability and intraoperative blood loss [13].This medication is currently reserved for patients with metastaticdisease or for situations in which surgery is contraindicated andlong-term medical therapy is needed.
Cardiovascular Monitoring and Management
Potentially life-threatening fluctuations in blood pressureindicate the need for direct arterial pressure monitoring, andlarge intraoperative fluid shifts underscore the importance ofexcellent intravenous access and urinary output monitoring.Young patients with healthy hearts may need only central venouspressure monitoring, whereas those with a history of cardiacdisease, including catecholamine-induced cardiomyopathy, maybenefit from transesophageal echocardiography or a pulmonaryartery catheter.
Endotracheal intubation should be attempted only after adeep level of anesthesia is attained. Intraoperative hypertensioncan be effectively treated with phentolamine (a short-actingα-adrenergic antagonist that may be given as an intravenousbolus of 2 to 5 mg or by continuous infusion), nitroprusside,or nicardipine titrated to effect. Other agents that can be used include nitroglycerin, fenoldopam [14], diltiazem, prostaglandinE1, and magnesium sulfate (as an infusion) [15].
Drugs to be avoided and problems in laparoscopicremoval
Anesthetic drugs and techniques that stimulate thesympathetic nervous system, such as ephedrine, ketamine,and hypercarbia; potentiate the arrhythmogenic effects ofcatecholamines (e.g., halothane); inhibit the parasympatheticnervous system (e.g., pancuronium) or release histamine (e.g.,morphine sulfate, atracurium) may precipitate hypertension andare best avoided.
During laparoscopic surgery, creation of thepneumoperitoneum may trigger the release of catecholaminesand huge fluctuation in hemodynamics that can be controlledwith a vasodilator. Interestingly, the hemodynamic changes aretypically very similar when pheochromocytomas are resectedlaparoscopically compared to when they are removed throughlaparotomy [16,17]. Blood loss and length of stay are less withthe laparoscopic than the open procedure [16]. Anesthesia isoften maintained with an opioid analgesic and a potent volatileagent. If a potent volatile anesthetic is used solely to maintainanesthesia, the drop in the blood pressure can be dangerousafter tumor resection.
After tumor ligation and resection, the drop in blood pressurecan be dangerously abrupt; however, this can be anticipatedthrough close communication with the surgical team. Manypatients require a vasoconstrictor (e.g., phenylephrine infusion)to support the blood pressure for a period of hours after thetumor is removed. If postoperative hypertension ensues, it mayindicate the presence of occult tumor or volume overload. Aftersuccessful surgery, catecholamine excretion returns to normal inabout two weeks.
- Premedicated with tab nitrazepam 5mg on H.S & M.O.S
- Preinduction IVI 500 ml R.L.
- Induction:- Fentanyl 5 μg/kg, propofol 1 mg / kg.
- Intubation:- Rocuronium 1.2 mg/kg after inj lignocaine1.5 mg/
- Preinduction B.P was 112/ 82 mmHg & post inductionB.P was 96/ 74mm Hg.
- Ventilation with T.V 10ml/kg & R.R 18/min
- Anaesthesia was maintained with vecuronium,Fentanyl, midazolam, isoflurane & O2/N2O mixture.
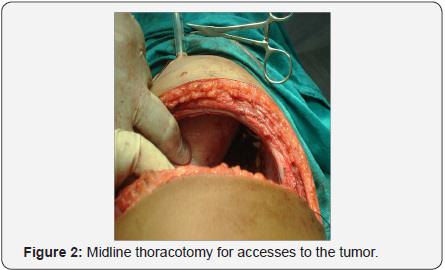
- The patient was placed in right up or left lateralposition and right sided thoracotomy was done.
- During manipulation of tumour, B.P ↑ 182/110 mm Hg& treated with sodium nitroprusside IVI.
- During retraction of lung, SpO2 ↓ to 91% & 100% O2was given to increase it to 99-100%.
- After the ligation of the vein, B.P ↓ to 60/40 mm Hg &was treated with volume infusion, dopamine, noradrenalineinfusion, phenylephrine bolus.
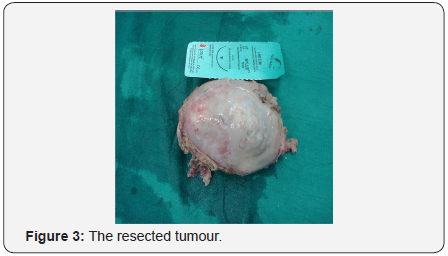
- The tumor was removed.
- The patient received 2000 ml RL, 500 ml hydroxyl ethylstarch and 1000 ml of 5% dextrose during 3 hrs surgery tokeep the CVP at around 10 mmHg.
- The 5% dextrose was infused after removal of thetumour to prevent hypoglycemia. Blood glucose wasmonitored every half an hour during surgery and was within120-180 mg/dl.
Extubation is done in deep plane of anesthesia with xylocard3 to 4 ml administered 2 minutes before extubation. Monitoringof the vitals and cardiovascular status postoperatively in theintensive care unit is mandatory.
Summary
A lack of controlled studies precludes definitivestatements about anesthetic management of patients withpheochromocytoma. It is known that the symptoms ofparoxysmal hypertension, sweating, and headache are highlysuggestive of the diagnosis. It appears that mortality can bereduced by preoperative α-adrenergic receptor blockadewith progressively increasing doses of a blocking agent for 10days to 2 months for treatment of symptoms, by treatment ofmyocarditis, and by restoration of intravascular volume.
In fact, the largest decrease in mortality of patients afterpheochromocytoma resection occurred with the introductionof preoperative α-adrenergic receptor blockade. We believethat knowledge on the part of the anesthetist about thepathophysiology of pheochromocytoma, preoperative patientpreparation and communication between surgeon andanesthetist are more important to patient outcome than is thechoice of the anesthetic or muscle-relaxing agent.
References
- Bravo EL, Gifford RW (1993) Pheochromocytoma. Endocrinol MetabClin North Am 22: 329.
- Ross NS, Aron DC (1990) Hormonal evaluation of the patient with anincidentally discovered adrenal mass. N Engl J Med 323: 1401.
- Pacak K, Linehan WM, Eisenhofer G (2001) Recent advances ingenetics, diagnosis, localization, and treatment of pheochromocytoma.Ann Intern Med 134: 315.
- Chew SL, Reznek RH, Sheaves R (1994) The diagnosis of bilateralpheochromocytomas in Von Hippel-Lindau disease by adrenal veinsamples. Q J Med 87: 49.
- Bravo EL (1994) Evolving concepts in the pathophysiology, diagnosis,and treatment of pheochromocytoma. Endocrinal Rev 15: 356.
- Bravo EL (1991) Pheochromocytoma: New concepts and future trends.Kidney Int 40: 544.
- Baxter MA, Hunter P, Thompson GR (1992) Pheochromocytomas as acause of hypotension. Clin Endocrinol (Oxf) 37: 304.
- Stein PP, Black HR (1991) A simplified diagnostic approach topheochromocytoma. A review of the literature and report of ourinstitutions experience. Medicine (Baltimore) 70: 46.
- Gangaly A, Grim CE, Weinberger MH (1984) Rapid cyclic fluctuationsof blood pressure associated with an adrenal pheochromocytoma.Hypertension 6: 281.
- Oishi S, Sasaki M, Ohno M (1988) Periodic fluctuations of bloodpressure and its management in patients with pheochromocytoma.Case report and review of the literature. JPN Heart J 29: 389.
- Liao WB, Liu CF, Chiang CW (2000) Cardiovascular manifestations ofpheochromocytoma. Am J Emerg Med 18: 622.
- Bogolioubev A, Keefe D, Groeger J (2001) Circulatory shock. Crit CareClin 17: 697.
- Bravo EL, Tagle R (2003) Pheochromocytoma: State-of-the-art andfuture prospects. Endocr Rev 24: 539.
- Cooper Z, Mihm F (1999) Blood pressure control with fenoldopamduring excision of a pheochromocytoma. Anesthesiology 41: 558.
- Pivalizza E (1995) Magnesium sulfate and epidural anesthesia inpheochromocytoma and severe coronary artery disease. Anesth Analg81: 414.
- Sprung J, O’Hara JF, Gill IS (2000) Anesthetic aspects of laparoscopicand open adrenalectomy for pheochromocytoma. Urology 55: 339-343.
- Gill IS (2001) The case for laparoscopic adrenalectomy. J Urol 166: 429.






























