Effective converting ratio when switching between Ona-botulinumtoxin-A and Abo-botulinumtoxin-A in cervical dystonia. A patient assessment cross over study
Ninel Nazarian*, and Erik Hvid Danielsen
Department of Neurology, Consultant Neurologist, Aarhus University Hospital, Nörrebrogade 44 DK-8000 Aarhus C, Denmark
Submission: May 01, 2019; Published: November 25, 2019
*Corresponding author: Ninel Nazarian, Department of Neurology, Consultant Neurologist, Aarhus University Hospital, Nörrebrogade 44 DK-8000 Aarhus C, Denmark
How to cite this article: Ninel Nazarian, Erik Hvid Danielsen. Effective Converting Ratio when Switching between on a-Botulinum toxin-a and Abo- Botulinumtoxin-a in Cervical Dystonia a Patient Assessment Cross over Study. Open Access J Neurol Neurosurg. 2019; 12(2): 555832. DOI: 10.19080/OAJNN.2019.12.555832.
Keywords: Abo-botulinumtoxin-A Ona-botulinumtoxin-A Cervical dystonia Effective conversion ratio VAS-score Follow-up Retrospective study
Introduction
In the 1950s it was hypothesized that Botulinum Neurotoxins (BoNT-A’s) could be used to reduce activity in muscle disorders [1]. Botulinum neurotoxin is made of a gram-positive anaerobic bacterial exotoxin-
clostridium botulinum [2]. When injected in the skeletal muscle, the botulinum toxin inhibits vesicular neurotransmitter (acetylcholine) release at the neuromuscular junction at the presynaptic membrane and a local chemo-denervation appears [1]. Thereby the toxin can reduce muscular contraction, which is the desire to reduce overactive muscle activity [3-5]. The medical therapies for dystonia are effective, and studies have shown that botulinum toxin is the most effective treatment for cervical dystonia [6]. The evidence from several Class I and Class II studies has led to a level A recommendation for use of BoNT-A for treating focal dystonia such as cervical dystonia, hemifacial spasm and blepharospasm [7]. Three main BoNT-A products are available on the market today: Ona-botulinumtoxin-A (Botox®), Abo-botulinumtoxin-A (Dysport®) and Inco-botulinumtoxin (Xeomin®). Due to different production methods, the biological nature of the three BoNT-A’s are different (e.g. the protein load, formulation, pH, dose and immunogenicity). Moreover, the BoNT-A’s are not interchangeable on an equivalent-dose basis [1, 7].
Study background
The goal of this study was to find the optimal conversion ratio for patients with cervical dystonia when switching treatment between Abo-botulinumtoxin-A and Ona-botulinumtoxin-Awhile obtaining a stable satisfying symptom improvement based on a subjective patient assessment. There is heterogeneity in treatment strategies and on how doses and the effect of BoNT-As are reported until now. This study represents a retrospective (follow-up), cross-over study (each patient acts as his/her own control). Effect of treatment was based on individual patient assessment. Individual based conversion rates were calculated using the optimal treatment dose for Abo-botulinumtoxin-A and Ona-botulinumtoxin-A. Due to the length of the present study with a follow-up time from 1991 to end 2014, it was possible to find stable toxin doses where each patient felt the best effect (min. 90%, VAS-score).
Methods
Study design
The clinical data was sampled from the patients’ medical records. Dystonic muscles and injection sites were identified by using electromyography (EMG). The neurologist determined the toxin doses based on each patient´s disease condition and the dystonic muscles activity (EMG). Each treatment (dosing) pr. clinical visit was individualized. At each consultation the neurologist reported the data (doses of toxin and the patient assessed effect and side effects) in the internal IT-system (Danish electronical patient journal) and also used standard sketches of the face/neck-regions to note which muscles have been injected
Botulinum toxin treatment
Ona-botulinumtoxin-A was diluted with saline to a concentration of 40U/ml. Abo-botulinumtoxin-A was dilutedwith saline to a concentration of 200 U/ml. For cervical dystonia the injection sites were between 2-8 sites. The injection doses in the specific muscles pr. patient and the total botulinum toxin doses pr. consultation were registered.
Effect of the procedure
The patients received treatment with BoNT-A´s every 3rd month at the neurological movement disorder clinic by an experienced neurologist. The subjective assessment of maximal treatment effect on dystonia symptoms between each treatment visits, were evaluated at each visit by the patient on a horizontal 100-mm Visual Analogue Scale (VAS) ; where the left end point indicated 0% treatment effect (no change in dystonia symptoms and no effect of the botulinum toxin) and the right end point indicated a total 100% treatment effect (indicate no dystonia symptoms and a maximal effect of botulinum toxin) between treatments)[8-11].
Data collection
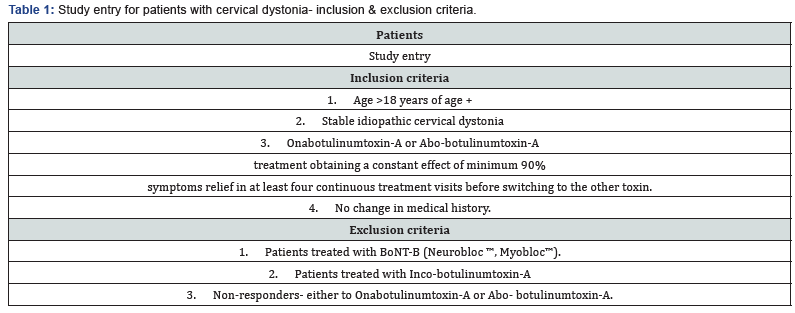

In this study, data was only obtained from patients with a stable idiopathic cervical dystonia, and no change in relevant medical treatment over the data recording period. (Patient study entry, Table 1). The mean individual dose of Onabotulinumtoxin- A was obtained from 4 continuous consultations from each patient with a symptom relief of >90%. The treatment was then changed to Abo-botulinumtoxin-A after filtration to maximal effect and the mean individual dose of this toxin was obtained in the same manner as Ona-botulinumtoxin-A. The conversion ratio of Ona- & Abo-botulinumtoxin-A could then be calculated for each individual by dividing the mean individual Ona-botulinumtoxin-A dose with the mean Abobotulinumtoxin- A dose. The final average conversion ratio (Ona- BoNT-A: Abo-BoNT-A) in the study was found by using all the individual conversion ratio data from the 48 patients (Figure 1).
Statistical analysis
For our statistical analysis of our data we used the software package SPSS Statistics. All averages are in mean± standard deviation.
Results
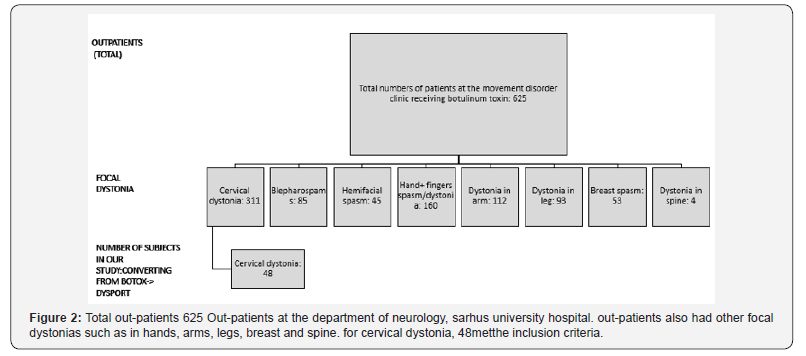
625 out-patients in total were registered at the movement disorder clinic and received treatments with botulinum toxins. Of these, 48 patients with cervical dystonia patients met the inclusion criteria (Figure 2).
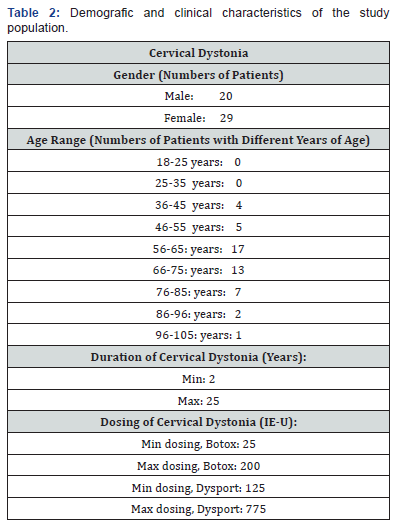
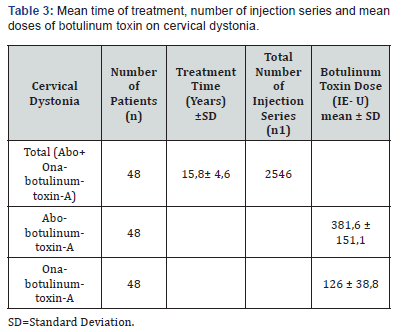
Cervical treatment was determined by the level of dystonia and ranged between 25- 200 units (Ona-botulinumtoxin-A) and 125- 775 units (Abo-botulinumtoxin-A) pr. clinical visit. More females had dystonia and most of the patients’ ages were between middle 50s to middle 70s (Table 2). All data wascollected in a period of 25 years from the movement disorder clinic. The VAS-outcome for each patient was minimum 90%. This retrospective data formed a cross-over design as each patient acted as his/her own control. Therefore, patients with confounders such as smoking, obesity etc. and comorbidities such as heart and lung diseases were not excluded, due to the cross over design. The average treatment time for patients with cervical dystonia receiving Ona-botulinumtoxin-A and Abobotulinumtoxin- A was 15,8±4,6 years. This average treatment time is the total period before and after switching botulinum toxin-A. 2546 injection series (total) were analyzed. Doses were 126 ± 38,8 IE-U for Ona-botulinumtoxin-A and 381,6 ± 151,1 IE-U for Abo-botulinumtoxin-A (Table 3). The mean conversion ratio for cervical dystonia was 1: 2.56. (Table 4).

Discussion
We are reporting 55 patients who converted treatment between Abo- and Ona-botulinumtoxin-A. All data wascollected in a period of 25 years from the movement disorder clinic. Patients have been stabilized with the Ona- or Abobotulinumtoxin- A treatment for at least a year before crossing over to treatment with the other toxin. Due to the long followup and moderate amount of data, we were able to find the average conversion ratio based on each patient´s subjective assessment. Former reports show no statistically significant difference on duration of the effect or adverse effects between Ona-botulinumtoxin-A and Abo-botulinumtoxin-A [12]. Data in these topics were excluded and not evaluated here. Treatment effect of Ona-botulinumtoxin-A and Abo-botulinumtoxin-A was in this study assessed by the treated patients. Due to the routine feedback from each of the treated patients over several years it was possible to achieve regular measurement of treatment effect from a subjective patient perspective without extra time for objective observations and evaluation in between toxin treatment sessions. The conversion ratio between the toxins (Ona-BoNT´s and Abo-BonT´s) using a patient-based assessment has in the present study shown to be equal to the study where they were using multicenter double-blind randomized trials [13]. Due to risk of different treatment techniques, toxin diffusion and especially individual variation in differentiating on relevant pain, a 90% margin was chosen as cut off for registration of desired effect.
Botulinum toxin effect & adverse effect
One unit of Ona-botulinumtoxin-A is not bioequivalent to one unit of Abo-botulinumtoxin-A. The ratio- Ona-botulinumtoxin-A: Abo-botulinumtoxin-A varies between 1:3 and 1:6 [14]. An earlier double-blinded study showed that the conversion ratio of Ona-botulinumtoxin-A and Abo-botulinumtoxin-A was 1:3 [14]. Abo-botulinumtoxin-A has been shown to have a doserelated significant longer duration of the effect rather than Onabotulinumtoxin- A, with a ratio 1:4 (Ona-botulinumtoxin-A: Abobotulinumtoxin- A, p-value= 0,02) [15]. Similar to former reports 12, this report have shown that the effect on dystonia, stabilizes after a period of treatments when changing between different toxin doses. In the present study the toxin doses were stabilized when the patients felt an effect of at least 90% measured on a VAS-score (0-100%) minimum at 4 continuous consultations.
Experimental results have shown that Abo-botulinumtoxin-A diffuses faster than Ona-botulinumtoxin-A which was explained by Abo-botulinumtoxin-A´s lighter molecular weight; Abobotulinumtoxin- A 3-400 kDa vs. Ona-botulinumtoxin-A 900kDa [16], and therefore the side effects in Abo-botulinumtoxin-A compared to Ona-botulinumtoxin-A was assumed due to a higher diffusion rate [17-19]. Other studies stated that botulinum toxin products dissociate under physiological conditions, and therefore the time of diffusion is not related to the toxin size [20]. In a study using Abo- and Ona-botulinumtoxin-A in the same patient with palmar hyperhidrosis, the patient was treated with Ona-botulinumtoxin-A in one palm, and Abo-botulinumtoxin-A in the other palm at the same session for 8 months. The patientswere their own control- like in the present study. The conclusion of that study was that the efficacy and safety of treating with Ona-botulinumtoxin-A and Abo-botulinumtoxin-A was by using the toxins in the conversion ratio of 1: 2.5 [21] which is similar to our conclusion. It has also been shown that conversion ratios of 1:3 or lower could be appropriate for the treatment of spasticity, cervical dystonia, hemifacial spasm, and blepharospasm 22. In the present study we found the conversion ratio of cervical dystonia to be 1:2, 56 which is similar to the former findings [21, 22].
The repeated administration and documentation of the usage of toxin doses (every 3rd month) at the movement disorder clinic reduced the information and selection bias. Due to the long follow-up period with a positive outcome and rare expected side effects, it was found to be effective to use a conversion ratio of 1: 2,56 (Ona-BoNT-A : Abo-BoNT-A) for botulinum toxin-A. The present study did not exclude any patients if they had confounders or comorbidities in behalf of that each patient was his/her own control in this cross-over design. The study design intended to prevent informational bias when evaluating the effect shortly after the toxin injections. To prevent selection and treatment variation bias the use of data was from patients who have been stabilized first with either Ona- or Abo-botulinumtoxin-A treatment for at least a year before crossing over to treatment with the other BoNT-A.
Conclusion
Considering the positive treatment effect, and the long followup period, we concluded that it is safe and clinically useful to use a conversion ratio at 1: 2,56 based on a patient assessment when changing treatment in between Ona- and Abo-botulinumtoxin-A for cervical dystonia. We consider the conversion ratios in our study applicable in other settings as well thus the effect of the botulinum toxin treatments was evaluated on a VAS-score (each patient is his/her own control) in this long follow-up study on dystonic out-patients.
Conflict of Interest
I certify that there is no actual or potential conflict of interest in relation to this article.
The study has been approved by the local scientific ethical committee of Jutland.
References
- Robles MJ, Esperanza A, Arnau Barres I Garrigós MT, Miralles R (2019) Frailty Falls and osteoporosis: Learning in elderly patients using a theatrical performance in the classroom. J Nutr Health Aging 23(9): 870-875.
- Ferreira ML, March L (2019) Vertebral fragility fractures How to treat them? Best Pract Res Clin Rheumatol 33(2): 227-235.
- Sugiyama T, Kim YT, Oda H (2014) Osteoporosis therapy: a novel insight from natural homeostatic system in the skeleton. Osteoporos Int 26(2): 443-447.
- Tamura H, Miyamoto T, Tamaki A, Gen Nawa, Hiroyuki Konya (2019) Osteoporosis complication is a risk factor for frailty in females with type 2 diabetes mellitus. J Phys Ther Sci 31(8): 621-624.
- Wicklein S, Gosch M (2019) Osteoporosis and multimorbidity. Z Gerontol Geriatr 52(5): 433-439.
- 6. Tassemeier T, Haversath M, Brandenburger D, Schutzbach M, Serong S, et al. (2019) Atraumatic fractures of the spine: Current strategies for diagnosis and treatment. Orthopade 48(10): 879-896.
- Waugh EJ, Lam MA, McGowan J, Papaioannou A, Cheung AM, et al. (2009) Risk factors for low bone mass in healthy 40-60-year-old women: a systematic review of the literature. Osteoporos Int 1: 1-21.
- Bliuc D, Alarkawi D, Nguyen TV, Eisman JA, Center JR (2014) Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the dubbo osteoporosis epidemiology study. J Bone Miner Res 30(4): 637- 646.
- Cieto Zapeda G, Duran Martinez N, Tena Sanabria ME (2019) Atypical femoral fracture, case report and literature review. Acta Ortop Mex 33(1): 39-41.
- Sugiyama T, Kim YT, Oda H (2014) Osteoporosis therapy: a novel insight from natural homeostatic system in the skeleton. Osteoporos Int 26(2): 443-447.
- Wang PW, Li YZ, Zhuang HF, Yu HM, Cai SQ, et al. (2019) Anti-Osteoporosis medications associated with decreased mortality after hip fracture. Orthop Surg 11 (5): 777-783.
- Hassler N, Gamsjaeger S, Hofstetter B, Brozek W, Klaushofer K, et al. (2014) Effect of long-term alendronate treatment on postmenopausal osteoporosis bone material properties. Osteoporos Int 26(1): 339-352.
- Cosman F (2014) Anabolic and antiresorptive therapy for osteoporosis: combination and sequential approaches. Curr Osteoporos Rep 12(4): 385-395.
- Wang Y, Ding H, Wang X, Shiqing Feng (2019) Associated factors for osteoporosis and fracture in Chinese elderly. Med Sci Monti 25: 5580- 5588.
- Lakkireddy M, Mudavath SV, Karra ML, Arrora AJ (2019) Hipovitaminosis D in patients with osteoporotic hip fractures. J Clin Orthop Trauma 10(4): 768-773.
- Stumpf U, Hesse E, Bocker W et al. (2019) Differential diagnoses of osteoporosis Z Gerontol Geriatr. 52(5): 414-420.
- Rincon Gomez M, Hernandez Quiles C, Garcia Gutierrez M, Galindo Ocaña J, Parra Alcaraz R, et al. (2019) Hip fracture co-management in the elderly in a tertiary referral hospital: a cohort’s study. Rev Clin Esp 3(19): 30154-30157.
- Mughal N, Inderjeeth AJ, Inderjeeth CA (2019) Osteoporosis in patients with dementia is associated with high morbidity and mortality: Findings from a single orthogeriatric unit. Aust J Gen Pract 48(1-2): 53-58.
- Kuliński W (2011) The importance of physical medicine in the prevention of disability in people age elderly. ActaBalneol 53(3): 201-202.
- Kuliński W (2012) Physical therapy in Medical Rehabilitation. Urban Partne, Elservier, Poland 351-411.






























