GAMALATE B6▪ Review of a GABAergic Product
Julio J Secades*
Scientific Department, Ferrer Group SA, Spain
Submission: November 22, 2017; Published: December 22, 2017
*Corresponding author: Julio J Secades, Senior Medical Advisor, Scientific Department, Ferrer Group SA, Spain, Email: jsecades@ferrer.com
How to cite this article: Julio J S. GAMALATE B6. Review of a GABAergic Product. Open Access J Neurol Neurosurg. 2017; 6(5): 555696. DOI: 10.19080/OAJNN.2017.06.555696.
Toxicology
Toxicology Gamalate B6 syrup. Determination of the LD50 by oral route in albino rat [1]
To determine the LD50 of Gamalate B6 syrup, the experimentation animal chosen was the albino rat (Wistar), weighing between 180 and 200g. A group of 10 animals received the normal diet established in our laboratory. The administration of the active components of the specialty Gamalate B6 syrup was made through a gastric tube in a dilution at 12% in distilled water. With a dose of 6g/Kg of the active components, only one death occurred in 24h. This has been the highest dose administered, corresponding to 100cc/kg of the specialty Gamalate B6. Therefore, the LD50 of Gamalate B6 syrup by oral route in albino rat can be considered indeterminable.
Gamalate B6 coated tablets. Determination of the LD50 by oral route in albino mice [1]
To determine the LD50 of Gamalate B, coated tablets, the experimentation animal chosen was the albino mouse, weighing between 20 and 25g. A group of 5 animals received the normal diet established in our laboratory. The administration of the active components of the specialty Gamalate B6 coated tablets was made through a gastric tube in a dilution in distilled water. Giving a dose of 5g/kg of the active components, no mortality or symptoms of toxicity were observed immediately or 24, 48 and 72h after the administration. This has been the highest dose administered, corresponding to more than 22 coated tablets per kg. This dose did not provoke any death. Therefore, the LD50 of Gamalate B6 coated tablets by oral route in albino mice can be considered indeterminable.
Pharmacology
Despite the great number of psychotherapeutic drugs developed in the last years, the problem of treating the neurologic and psychiatric symptoms, such as hyper excitability or overstrain, associated to mental deficiencies remains still unsolved, and the same applies to altered personality conditions in infancy, associated to changes in the affective development due to environmental or educational circumstances.
The present therapeutic arsenal does not provide the necessary psychotherapeutic effects, since the sedative- tranquilizer drugs, even achieving a symptomatic sedative effect, have little effectiveness to control the whole organic symptomatology, such as anorexia, gastralgia, cephalalgia, diarrhea, that is very frequent in this kind of patients.On the other hand, these drugs show the serious disadvantage of being difficult to handle and are associated with serious adverse events, thus being contraindicated in states of decrease intellectual performance or in children, mainly because they provoke mental sluggishness.
Properties of the Components
Magnesium glutamate hydrobromide (MGB), synthetized at the Research Department of Ferrer and patented in several countries, has proved to have a sedative effect of the central and neurovegetative nervous systems; it is entirely harmless and devoid of side effects. MGH has a curative effect in personality changes through a cerebral trophic action, improving and increasing the lucidity of the mental abilities. MGB acts as a glutamate receptor agonist, halting glutamic action. MGB has a sedative effect with anxiolytic action.
In order to improve the cerebral trophic action, MGB was associated with Vitamin B6 and with γ-aminobutyric acid (GABA) and γ-amino-β-hydroxybutyric acid (GABOB), two components which are known to take part in a great proportion in the composition of the nervous tissue and constitute, together with Glutamate, 80% of the non-protein aminic nitrogen, playing an important role in the physiology of nervous system as mediators in synaptic transfers processes. Gamalate B6 has a sedative effect on the hyperexcitability of the nervous system and brings amino acids that are very important to activate the mental function [1]. Its main indication are patients with intellectual deficiencies and, particularly in infancy, in difficult children with troubles of behavior, intellectual inhibitions, low school performance, and with somatic manifestations such anorexia, gastralgia, cephalalgia, etc., which fail to react to specific treatments in which the tranquilizers and similar drugs are contraindicated, not only by their side effects but also owing to the mental sluggishness they provoke.
Characteristics of the Active Principles
Γ-Aminobutyric Acid (GABA)
GABA is the major inhibitory neurotransmitter in the Central Nervous System (CNS), whereas Glutamate is the major excitatory neurotransmitter [2]. GABA plays a role in regulating neuronal excitability throughout the nervous system, and is involved in a variety of biological functions such as locomotor activity, learning, reproduction, circadian rhythms as well as cognition, emotions and sleep. The GABA system is widely used in the treatment of anxiety disorders, insomnia, epilepsy, restlessness, and aggressive behaviors, and it is the target for many intravenous and inhalational anesthetics and drugs of abuse, such as alcohol. GABA system is sensitive to a considerable number of pharmacological agents (ethanol, benzodiazepines, barbiturates, and neurosteroids). GABAergic mechanisms have been demonstrated in various peripheral tissues and organs. GABA is a neurotransmitter which is fundamental in neuron homeostasis [2].
The balance between excitation and inhibition is an essential feature which must be maintained in order to avoid pathological consequences (excitation-inhibition imbalance hypothesis). The inhibition-excitation balance controls adult brain plasticity and is at the core of the pathogenesis of neurodevelopmental disorders [3]. Inhibitory interneurons in general are crucial for normal cognitive processes [4-6]. Cortical glutamate and GABA neurotransmission critically involved in cognitive deficits [7]. Increase GABA transmission in the prefrontal regions may be the neural basis of the improvement of social competence [8].

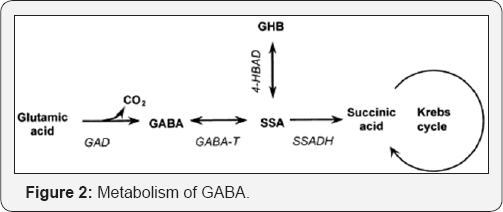
The dynamic balance between Glutamate and GABA is regulated by the enzymes glutamate decarboxylase (GAD) and GABA transaminase [2]. GABA is synthesized from the decarboxylation of glutamic acid GAD (Figure 1). GAD needs a pyridoxal phosphate coenzyme which is obtained by phosphorylation of vitamin B6. Thus Vitamin B6 deficiency can lead to reduced GABA synthesis. GABA transaminase enzyme catalyses the conversion of 4-aminobutanoic acid and 2-oxoglutarate into succinic semialdehyde and glutamate. Succinic semialdehyde is then oxidized into succinic acid by succinic semialdehyde dehydrogenase (SSADH) and as such enters the Krebs cycle as a usable source of energy (Figure 2). SSADH deficiency [9] is a rare disorder responsible of mental deficits, seizures, and various behavior problems.
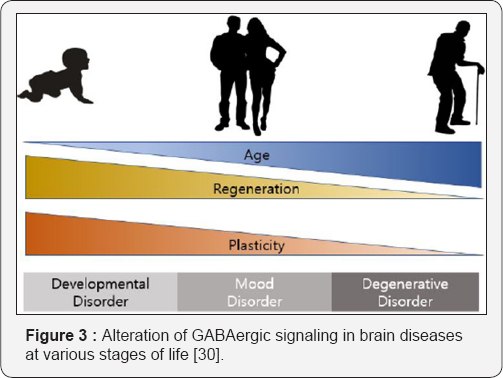
GABA is now used to treat all processes in which a mental activator is indicated [2]. Interestingly, recent research has demonstrated the involvement of GABA system in the pathophysiology of autism spectrum disorders [10-13], schizophrenia [14-18], anxiety [19-22], stress [23-25], acute posttraumatic stress disorder [26,27] and the cognitive function in older adults [28] and in Down Syndrome [29]. Thus, it can be detected an alteration of GABAergic [30] signaling in brain diseases at various stages of life (Figure 3) Γ-Amino-B- Hydroxybutyric Acid (GABOB).
GABOB can be considered as a derivative of GABA, since it differentiates from GABA only in the hydroxyl group in position 3 (Figure 4). Hayashi demonstrated that GABOB is one of the most important inhibitory factors of the brain as it is 5 to 19 times as active as GABA [31]. When used as an anticonvulsant, this compound has a very intense action at CNS level. The LD50 in mice is 13g/Kg by endovenous or subcutaneous route (unpublished data). This dose corresponds, approximately, to 1000 times the usual therapeutic dose. GABOB has been used mainly in the treatment of idiopathic or symptomatic epilepsies and in convulsive crises in children, as in the treatment of hypertension, behavioral disorders and mental deficiencies [32-36].

Magnesium Glutamate Hydrobromide (MGB)
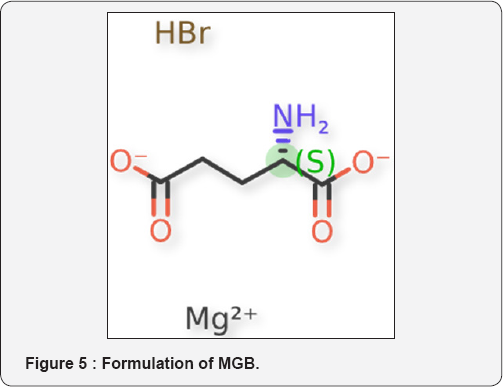
This product was developed by the Research Department of Ferrer. MGB is a double salt of glutamic acid, that is, magnesium joins the 2 carboxylic groups of glutamic acid in ionic or cyclic form, and bromine, joins the amino group (Figure 5). Pharmacologically, MGB can be classified as a mild-action sedative drug, with anxiolytic activity. The explanation for this fact must be searched for in its chemical molecule. The union with Mg and bromine in the form of chelate gives a compound with a rigid structure which will only act as a partial agonist of L-glutamate, thus decreasing the stimulation and achieving a mild sedative effect. This is the difference that MGB presents versus other tranquilizing drugs which produce a direct inhibition of the stimulation, such as benzodiazepines and barbiturates, but with a high rate of undesirable side effects, which have not been described so far for MGB [37]. MGB potentiates the action of the sedative drugs and protects the experimentation animals from the effect produced by the stimulating drugs [1]. Among the tests carried out to check up the depressive action on the CNS, we can quote:
Test of the rotary cylinder: It shows that MGB exerts a depressive action on the voluntary motility and static reflexes of the mouse. The action is proportional to the dose [1].
Test of the vibratory case: It shows the action on the spontaneous activity of the mouse [1].
Potentiation of the barbituric action: It is shown that MGB extends the average duration of barbituric sleep. There is a clear dose-dependent effect [1].
To check up its antagonism versus stimulants of the CNS, cardiazol and strychnine have been chosen as type drugs. MGB changes the aspect of the convulsive phenomenon produced by cardiazol. The clonic form becomes shorter and the tonic one is less intense. The residual hyperexcitability disappears [1]. MGB protects the animals against the convulsions produced by strychnine and, furthermore, it is seen that the hyperexcitability induced by strychnine disappears completely [1]. The innocuousness of MGB is confirmed in the LD50 orally in mice is 6750mg/Kg, and 190mg/Kg intravenously. In another connection, on equal therapeutic dose with potassium bromide, the bromine blood levels obtained with MGB were maintained a 60% below that obtained with the above mentioned inorganic bromide [1]. All this explains the perfect tolerance subsequent to the MGB therapy and makes it indicated in all usual conditions of hyperexcitability of psychosomatic origin.
Vitamin B6
This is one of the vitamin factors arousing the greatest interest by the importance of its functions. Vitamin B6 refers to a group of chemically similar compounds which can be interconverted in biological systems. Vitamin B6 is part of the vitamin B group of essential nutrients. Its active form, pyridoxal 5'-phosphate (Figure 6), serves as a coenzyme in some 100 enzyme reactions in amino acid, glucose, and lipid metabolism [38]. Vitamin B6 accelerates conversion of glutamic acid to GABA, as a coenzyme of GAD.
Gamalate B6[39]: The approved indications for Gamalate B6 are:
A. In adults:
a. Personality changes, aggressiveness, sleep disorders, agitation or anxiety, whatever the cause: school, family, work-related.
b. Concentration difficulties.
c. Nervous depression.
d. Emotional instability.
B. In children:
a. Poor performance at school.
b. Difficulties with concentration and learning.
c. Maladjustment to social, family and school environment.
Mechanism of action [39]
Direct supply of GABA increases GABA levels, facilitating inhibitory neurotransmission (anxiolytic action)(Figure 7). Vitamin B6 helps accelerate conversion of glumatic acid to GABA. GABOB, blocks the GABA A and GABA B receptors, halting breakdown of GABA. Halting glutamic action due to the presence of MGB, which has a sedative effect with anxiolytic action.

Clinical experience
In 1966 Espadaler et al. [40] published their experience with Gamalate B6 in neurologic practice. The study participants were: 30 children (2-12 years old) with syndromes characterized by an association of characteriologic disorders, motor disturbances, mental deficiency and epilepsy. 20 adults (14-65 years old) with sequelae of neurological diseases, dementia or vertigo. All the patients were treated for 1 year and the outcome was assessed by means of laboratory test, EEG and psychological explorations.
The authors concluded that Gamalate B6 showed a favorable action manifested by an improvement of the external behavior of the subjects as well as by improved environmental contact, without safety concerns.
Folch Camarasa et al. [41] carried out a study on 66 patients (8-18 years old) with different kinds of oligophrenic insufficiencies treated with Gamalate B6 during 3 months. The effect of the drug was assessed by a battery of psychological explorations.
After 3 months of treatment, Gamalate B6 improved the intellectual performance of those psychologic insufficiencies (attention, concentration, association, memory,...) and exercises a positive influence in the formulation and development of automatic intelligence mechanisms, without safety concerns.
In 1972, Knobel [42] reported his experience with Gamalate B6 in some infantile mental deficiencies. It was a double-blind placebo controlled study in 60 children (8-12 years old) with nonspecific organic mental deficiency treated for at least 3 months. He found statistically significant differences between the groups with a clear improvement in the 61.11% in Gamalate B6 group vs. 8.33% in placebo group, without safety concerns. The effects were more relevant in cases with EEG alterations.
In 1973, Vendrell et al. [43] published a study of evolution of speech disturbances in children, on combining the logo therapeutic treatment with Gamalate B6. The study was made with two random matched groups (control vs. treated), involving 28 children with speech disorders (dysphasic, dyslexic or mentally defective). The duration of the treatment was 8 months and the evaluation method was the verbal IQ from the Wechsler infantile intelligence scale. The results show a favorable influence of Gamalate B6 on the performance of the speech abilities, improving the possibilities of interrelation with the environment, without safety concerns.
In 1982, Espadaler [44] published his experience in the treatment of minimum cerebral dysfunctions with Gamalate B6. It was an Open study on 30 children (5-12 years old) with cerebral dysfunctions consequent to minimal connatal encephalopathies, treated for 2 years with Gamalate B6. The effects were assessed by means of neuropsychological and EEG evaluations. The author found positive effects on attention and learning abilities, making easier their educational processes, without safety concerns.
In 1983, FolchSoler [45] published an open study with 100 children (0-17 years old) with different behavioral disorders, treated at least for 2 months with Gamalate B6. The author found some improvement in 46% of the cases and reported as side effects Excitation (3 cases) and sedation (1 case).
In 1990, Zamora & Perez [46] published their study with Gamalate B6as treatment of anxiety. It was an open study in 60 patients (14-75 years old) with anxiety, treated for 60 days. The authors found significant improvements at 30 and 60 days (p<.01) on anxiety symptoms (Figure 8). No side effects were detected during the study.
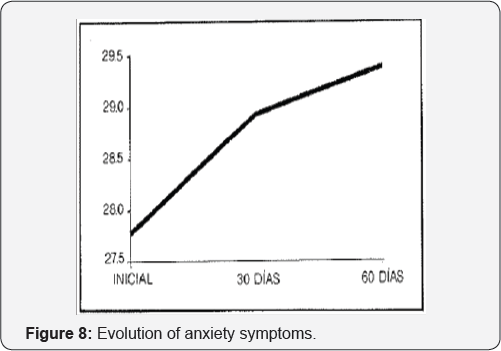
The authors concluded that Gamalate B6 presents a restraining activity of vital anxiety, improves the psychomotor activity of the patient and increases the degree of effectiveness.
Also in 1990, Nunez & Lopez-Pousa [47] published a study to assess the effects of Gamalate B6 in patients with tension headaches. It was an open study with 36 patients (42-71 years old) with tension headache of different origin treated for 30 days with Gamalate B6. After the 30 days of treatment, the drug was considered effective in the 75% of the patients (Table 1).
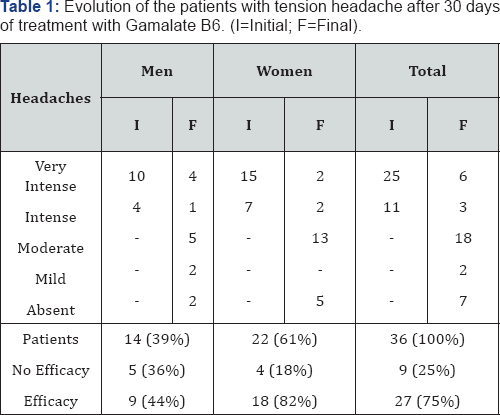
As side effects, greater calmness and somnolence were reported by the 27% and 22% of patients respectively, but these effects were not considered as negative by the patients. Insomnia, gastralgia and nightmares were reported for 1 patient each.
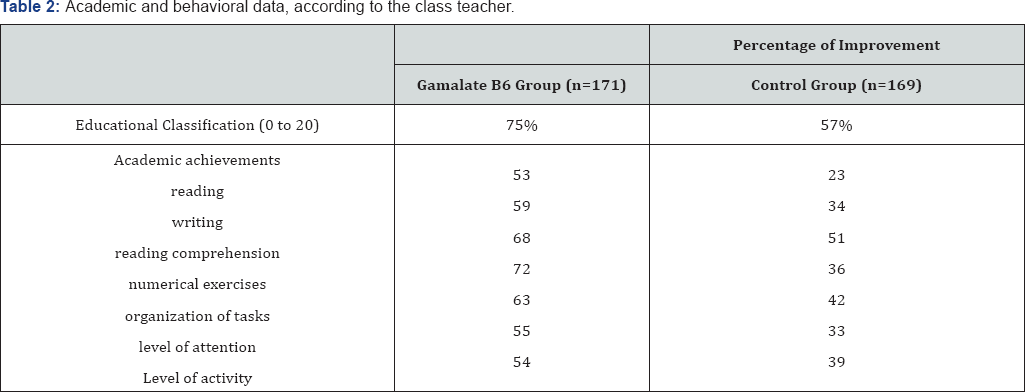
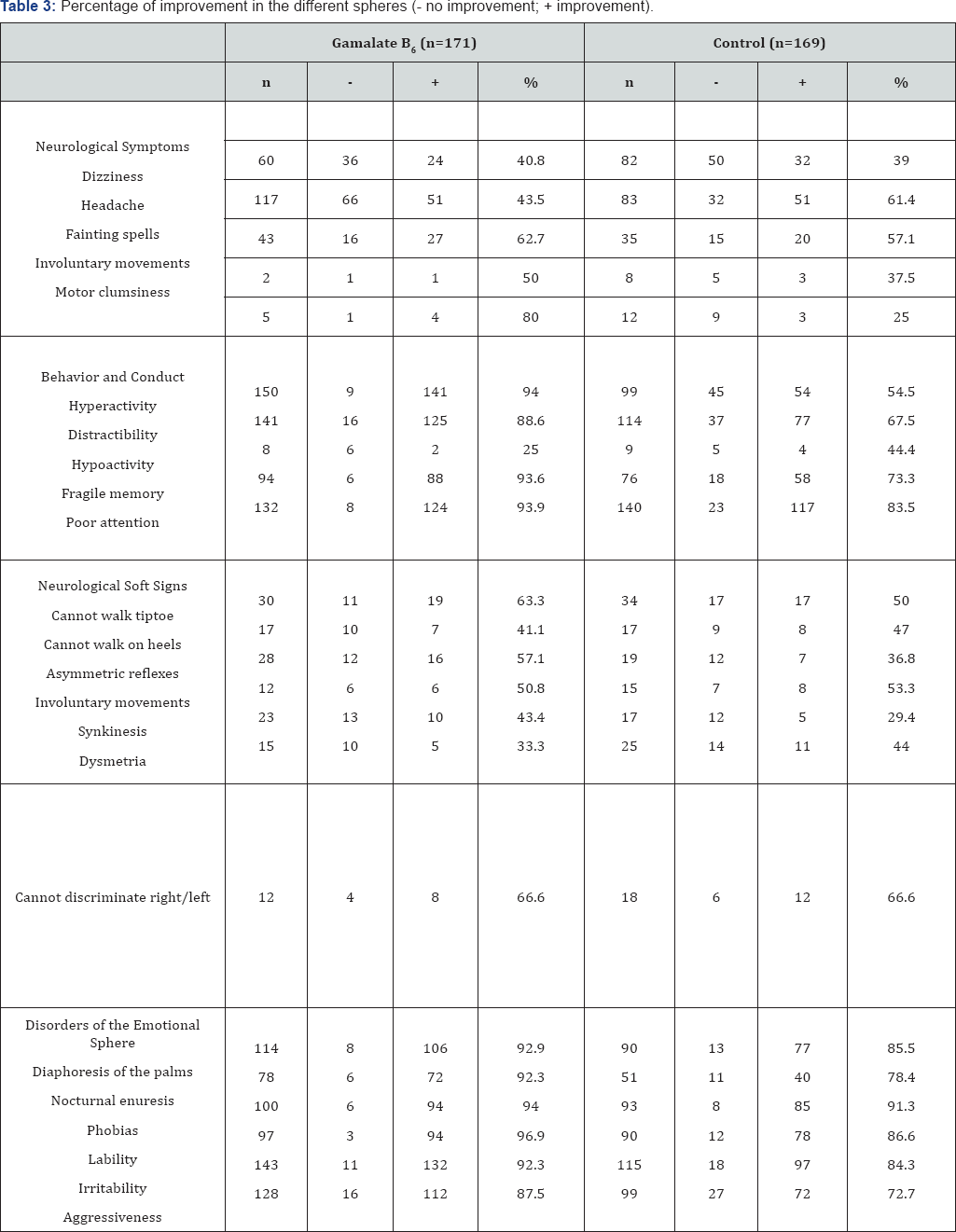
In 1991, Martinez et al. [48] published their open and comparative study on neurological learning disorders of school children in urban-marginal areas of metropolitan Lima (Peru). The study included 340 children, aged 5 to 12 years old with Minimum Brain Dysfunction, currently called Attention Deficit Disorder or ADHD. The group of 340 children was separated indistinctly, according to the day of the visit, into two groups: one that would receive the drug (n=171) and the other (n=169) which was given a neuropsychological treatment without medication (control group).The treatment period was 6 months. In this study it is important to note the percentage improvement in both groups, highlighting the important contribution of neuropsychological therapy to the expectations of dysfunctional syndrome. However, in the group treated with GABAergic precursors, the highest percentages are located in the spheres of behavior and conduct disorders and in the emotional sphere (Table 2 & 3). The authors concluded that the relationship between knowledge, memory and instinctive behavior can be modified by medicinal products that enter the GABA metabolic system. During the study, no safety concerns were detected.
In 1992 Martinez Mendoza published an open study carried out in 100 patients (41 men and 59women) aged between 17 and 70 years (average 41 years). All of them presented a profile of intense or severe anxiety, in spite of the fact that 48 of them had been under treatment with benzodiazepines for longer than one month.
The primary objective of the study was to evaluate the efficacy of Gamalate B6 on subjects' general anxiety symptoms and to determine the efficacy of the product in subjects treated with benzodiazepines and those not receiving this therapy. A secondary objective was to determine whether replacing benzodiazepines with Gamalate B6 would produce positive or undesirable effects. Evaluations were based on the Hamilton's Test for anxiety. Patients were assigned to two groups:
Group A: Patients who were not under treatment with anxiolytic drugs and presented intense or severe symptoms of anxiety. During the 30 days of the study they were administered Gamalate B6 at a dose 6 coated tablets per day (2 coated tablets after each meal).
Group B: Patients who were under treatment with benzodiazepines for a period of "more than one month" and, nevertheless, presented intense symptoms of anxiety. During the first 15 days the dose of benzodiazepine was reduced by half; and associated with Gamalate B6 at a dose of 3 coated tablets a day (1 tablet after each meal). During the second 15 days, to complete the 30 days of the study, all benzodiazepines were suppressed and they were only administered Gamalate B6 at a dose of 6 coated tablets a day (2 coated tablets after each meal).
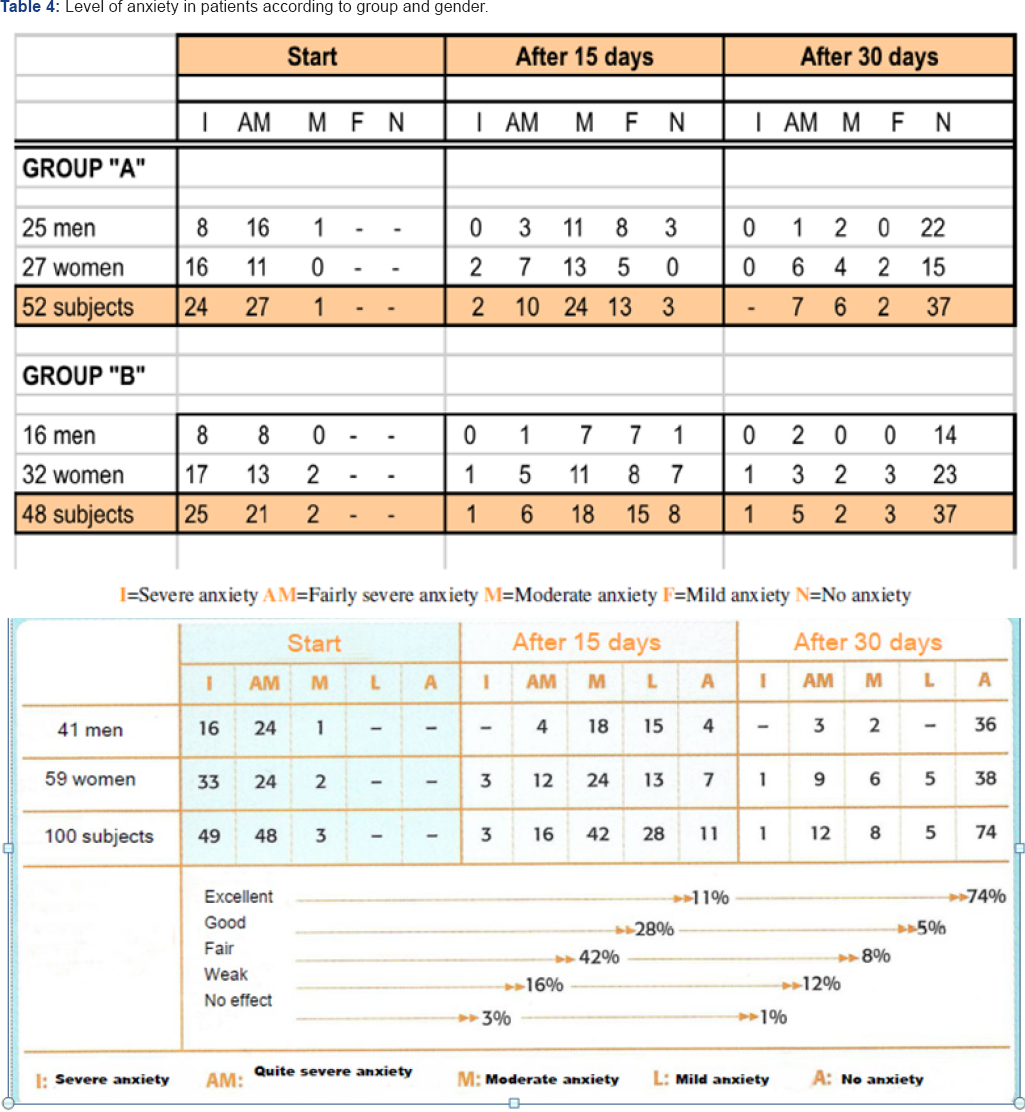
Gamalate B6was shown to be effective in 79% of subjects who were in a severe or fairly-severe anxiety state (Table 4 & 5). The results obtained with Gamalate B6were excellent, as the anxiety-type psychological and psychosomatic symptoms disappeared. Replacement of benzodiazepines with Gamalate B6 caused no anxiety or any other type of disorder. Then the author concluded that Gamalate B6 is a product that is practically free of any adverse reactions and has shown excellent tolerance without any dose adjustment being required at any time [49].
d. In 1998, Novell et al. [50] studied the efficacy and safety of Gamalate B6in the treatment of behavior disorders in slight and moderately mentally retarded children. This was an open-label study on 70 children treated with Gamalate B6 for 3 months. As explained, the primary objective was to evaluate the efficacy and safety of Gamalate B6 in behavioral disorders in children with mild or moderate psychological problems.
The secondary objective was to evaluate the clinical efficacy of Gamalate B6 in cognitive performance (intelligence, memory and attention) and the degree of sociability in these patients.
The trial included patients who presented at least one of the following behavior disorders: hyperactivity, disobedience, irritability, agitation, lethargy, social drop-out, stereotype behavior patterns and excessive loquacity and who complied with DSM-IV and AAMR criteria (American Association on Mental Retardation, 1992) for mild (IQ between 70-55) and moderate (IQ between 40-55) mental retardation in people aged between 6 and 18 years. The patients received Gamalate B6 at a dose of 6 coated tablets a day distributed in three intakes, over a period of three months. The evaluation of the efficacy of the treatment used various tests which were administered by a qualified psychologist during the first and last control visits 90 days after initiating the treatment.
The sample of 70 patients was composed by 43 males (61.5%) and 27 females (38.5%) with a mean age of 10.63±3.26. 74.2% of the patients had slight mental retardation, and the other 25.8%, Moderate mental retardation. The causes of the mental retardation were:
Prenatal: 11.4%
Perinatal: 15.7%
Postnatal: 14.3%
Unknown: 58.6%
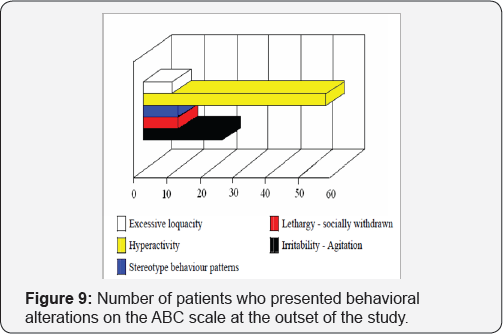
In Table 6 is shown the number of patients with adaptive capacity deficits (AAMR). The number of patients who presented behavior disorders and who scored on one or more of the five ABC sub-scales is shown in Figure 9. Patients mainly scored in the sub-scales for Hyperactivity (n=56) and Irritability and agitation (n=24).


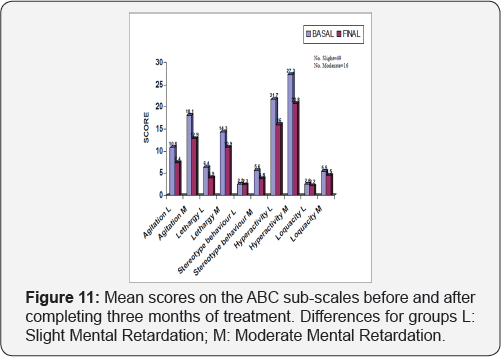
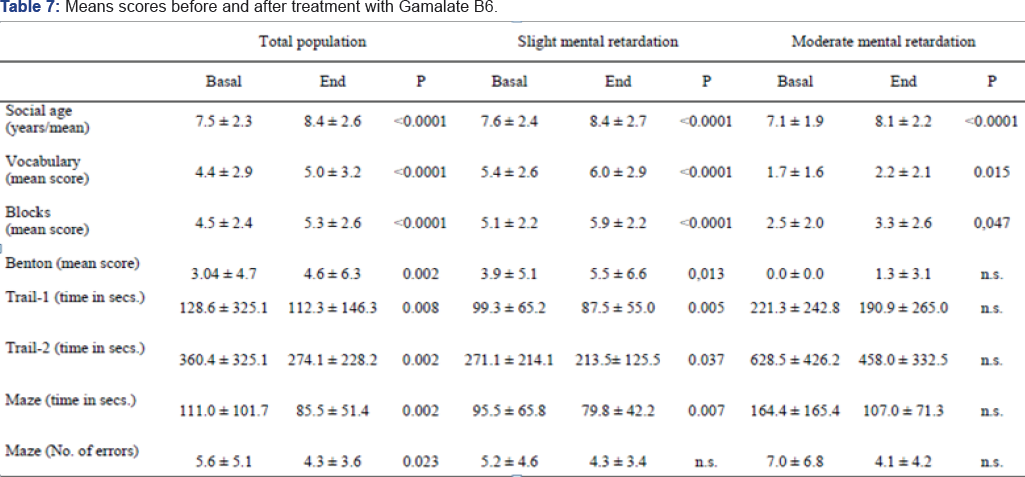
On completion of the treatment period, the mean score obtained in all the sub-scales of the ABC scale dropped in a statistically significant manner (Figure 10). The maximum reduction was obtained in the sub-scale that assessed hyperactive behavior patterns, dropping an average of 5.9 points (Confidence Interval: 4.5, 7.3) after three months treatment with GB6. Agitation dropped an average of 3.6 points (CI: 2.4, 4.9), lethargy2.7 points (CI: 1.6, 3.9), stereotype behavior patterns dropped 1.2 points (CI: 0.5, 1.9) and excessive loquacity dropped 0.5 points (CI: 0.04, 1.03). The social age as determined by the Vineland scale increased an average of 0.8 (CI: 0.6, 1.1) years after the treatment period, with a statistical significance of p<0.0001 (Table 7). An analysis was carried to determine whether treatment with Gamalate B6 could show differences in relation to the intellectual level. The results show that even though there was a reduction in the scores for all the ABC sub-scales, they were higher when analyzing the group with moderate mental retardation:. At the beginning of the treatment the seriousness of the behavior disorders was higher in the more cognitively affected patients. There were no appreciable improvements of the stereotypes in people with moderate affectation, nor on the sub-scale for excessive loquacity in people with slight retardation (Figure 11). Equally, in the group with moderate mental retardation there were no appreciable improvements in
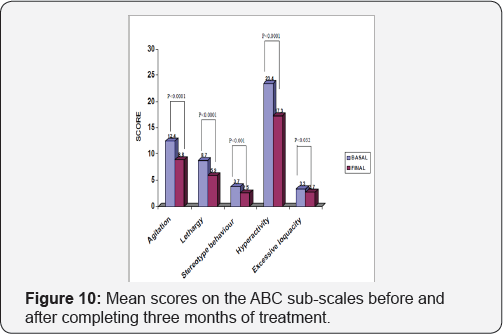
On completion of the treatment period, the mean score obtained in all the sub-scales of the ABC scale dropped in a statistically significant manner (Figure 10). The maximum reduction was obtained in the sub-scale that assessed hyperactive behavior patterns, dropping an average of 5.9 points (Confidence Interval: 4.5, 7.3) after three months treatment with GB6. Agitation dropped an average of 3.6 points (CI: 2.4, 4.9), lethargy2.7 points (CI: 1.6, 3.9), stereotype behavior patterns dropped 1.2 points (CI: 0.5, 1.9) and excessive loquacity dropped 0. 5 points (CI: 0.04, 1.03). The social age as determined by the Vineland scale increased an average of 0.8 (CI: 0.6, 1.1) years after the treatment period, with a statistical significance of p<0.0001 (Table 7). An analysis was carried to determine whether treatment with Gamalate B6 could show differences in relation to the intellectual level. The results show that even though there was a reduction in the scores for all the ABC sub-scales, they were higher when analyzing the group with moderate mental retardation:. At the beginning of the treatment the seriousness of the behavior disorders was higher in the more cognitively affected patients. There were no appreciable improvements of the stereotypes in people with moderate affectation, nor on the sub-scale for excessive loquacity in people with slight retardation (Figure 11). Equally, in the group with moderate mental retardation there were no appreciable improvements in the memory tests (Benton), attention (Trail Making Test) and psychomotricity (Maze) (Table 7).
In 92.9% of cases the tolerance to treatment was excellent, there being no relevant adverse events (7.1%). Only one case had to be withdrawn from treatment for probable intolerance. In two cases there was uneasiness and in one a loss of sleep which could be related to the treatment. In one case there was headache and vomiting of short duration; another case reported diarrhea and abdominal discomfort of mild severity which could not be attributed to the treatment with Gamalate B6 as the patient was concomitantly taking other treatments that could explain this reaction. In three cases the treatment was suspended for inefficacy and replaced by neuroleptic treatment. Other case was withdrawn from the study for non-appearance of the patient for the second control visit.
Thus the results of this open trial suggest that Gamalate B6 could be an efficient treatment in the case of behavior disorders, basically hyperactivity and agitation, in children with slight to moderate intellectual deficiency. Gamalate B6represents an interesting alternative to neuroleptic treatment and favoring performance in functional academic skills.
Up to now, the last clinical study with Gamalate B6 was published in 2002 by Lopez-Pousa et al. [51], assessing the effects of Gamalate B6 on the quality of life of the patients with fibromyalgia. Fibromyalgia is characterized by a profile of generalized, benign, chronic musculoskeletal pain not originating in the joints and of unknown cause. According to the classification criteria of the American College of Rheumatology, the two basic characteristics for the diagnosis of fibromyalgia are the presence of generalized pain of more than three months duration and abnormal sensitivity to pressure with the fingers in specific body areas. Furthermore, there is frequently the presence of other discomforts, such as a feeling of swelling and morning stiffness in the hands, paresthesia and dysesthesia in the hands, difficulty in going to sleep and/or non-restoring sleep. Fibromyalgia is a very frequent complaint that causes a high rate of labor absenteeism with its consequent direct and indirect costs. In spite of the prevalence of the syndrome and its associated psychological and social cost, there are no clear results regarding its etiology or pharmacological treatment. Various approaches have been tried including anti-depressants to increase the levels of serotonin and norepinephrine, nonsteroidal anti-inflammatory drugs that inhibit the release of prostaglandins in inflammatory processes, opiate derivatives, sodium and calcium channel antagonists to stabilize membranes and GABAergic agonist drugs that increase the levels of this neurotransmitter and reduce the excitability of nerve cells.
The purpose of this prospective observational open study is to determine the effectiveness of Gamalate B6in the symptomatic treatment of fibromyalgia over a period of 3 months inpatients attending a neurological clinic. The sample consisted of 24 patients (92% women) with a mean age of 53.33years (SD =6.15; Range=40-67), who attended a neurological visit and complied with the criteria for rheumatic fibromyalgia established by the American College of Rheumatology: persistent muscular-skeletal pain of more than three months duration and the presence of at least eleven specific painful points identified by moderate finger pressure of about 4Kg/cm2. The instruments used to evaluate the efficacy were:
The Fibromyalgia Impact Questionnaire (FIQ): It is a brief, easy to apply specific instrument comprised of a total of 10 items. Its purpose is to evaluate the state of health on the basis of the physical, psychological, social and general affectation of patients with fibromyalgia. It enables distinguishing patients who refer to clinical improvements from those who do not mention them. It is a scale with a Likert-type scoring system from 0 to 10 points, where the higher score indicates a higher degree of negative effects produced by fibromyalgia.
The Brief Pain Inventory (BPI-Sp): It is an instrument comprised of 7 items that assesses the distribution and number of painful points as well as the maximum, minimum and mean severity of the pain over the last 24 hours, the current level of pain and its interference in the performance of various daily activities. It has a Like rt-type score system from 0 to 10 points, and the higher score indicates greater severity or interference of the pain.
A protocolized procedure was used to obtain the main reason for the visit and the medical record. The patient was submitted to an exhaustive general and neurological clinical examination. All patients were then administered the FIQ and BPI-sp. After the examination the patients initiated pharmacological treatment with Gamalate B6 at a dose of 2 tablets every 8 hours together with their usual medication (Table 8). Three months later the patients were gain evaluated using the same questionnaires.
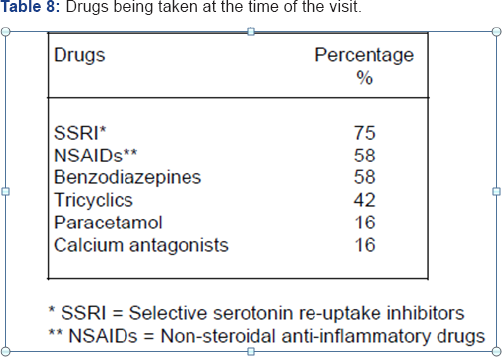
The main reason for visiting the consultancy was headache in 50% of cases, followed by a feeling of vertigo or instability in 25% of cases and memory loss in the remaining 25%. Outstanding among the accompanying symptoms were signs of depression (83%), sleep disorders (75%) and headache (67%) (Table 9).
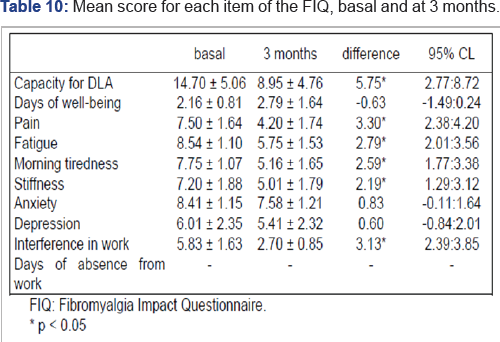
The mean score on the FIQ at the basal time was 67.70 (SD=6.96;Range=55-83) whereas at three months it was 44.33 (SD=6.24; Range=32-57),and there were significant differences between both scores (Z=-4.28; p=0.001). The score for the capacity to perform daily living activities (DLA) assessed by item 1 of the FIQ and comprised of 10 sub-items with a score range from 0(always) to 3 (never) was 14.7 (SD=5.06) at the basal time. Of the remaining items of the FIQ that assess the symptoms associated with fibromyalgia, those with the highest scores at the basal time were fatigue, anxiety, morning tiredness and stiffness, all with scores above 7 points. At three months thescore for the item on the capacity to perform DLA was 8.95 (SD=4.76) and there was a reduction in the score of all items on the FIQ. The difference between both evaluations was significant for all items with the exception of the days of well-being during the last 7 days, anxiety and symptoms of depression (Table 10).
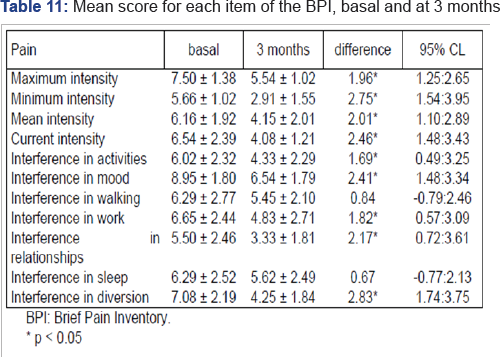
The BIP-sp presented a basal score of 72.79 (SD=8.30; Range=56-90) and 51.66 (SD=6.43; Range=39-63) points at three months, the difference between both evaluations being significant (Z= -4.32; p=0.001). The interference of pain on the mood presented the highest score at the basal time, 8.95 points (SD=1.80), followed of the maximum severity of pain and interference in the capacity for diversion, with scores above 7 points. At three months, the interference produced by pain on sleep and the capacity to walk were the only areas that did not show a significant improvement, whereas all the other areas considered presented significant changes in the scores on the BIP-sp, ranging between 1.69 and 2.83 points (Table 11).
In no case was treatment with Gamalate B6 suspended for intolerance and there were no associated adverse reactions. In two cases there were nauseas and in one case restlessness which were related to the concomitant administration of other treatments, as they were already present when initiating the administration of Gamalate B6.
The results obtained in this study suggest that Gamalate B6 could have beneficial effects in the symptomatic treatment of fibromyalgia, mainly as an add-on treatment to usual medication. The results show a statistically significant clinical improvement ranging between 1 and 3 points at three months in all the evaluation scales used for patients who had not shown evident improvement with their usual treatment.
References
- Bartolomé M. Toxicological and Pharmacological test carried out with the specialty Gamalate B6. Unpublished data.
- (2007) Gamma-aminobutyric acid (GABA), Monograph. Altern Med Rev 12(3): 274-279.
- Gatto CL, Broadie K (2010) Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front Synaptic Neurosci 2: 4.
- Krook-Magnuson E, Varga C, Lee SH, Soltesz I (2012) New dimensions of interneuronal specialization unmasked by principal cell heterogeneity. Trends Neurosci 35(3): 175-184.
- Gonzalez-Burgos G, Fish KN, Lewis DA (2011) GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast 2011(2011): 1-24.
- Lehmann K, Steinecke A, Bolz J (2012) GABA through the ages: regulation of cortical function and plasticity by inhibitory interneurons. Neural Plast 2012(2012): 1-11.
- Scholl J, Kolling N, Nelissen N, Stagg CJ, Harmer CJ, et al. (2017) Excitation and inhibition in anterior cingulate predict use of past experiences. Elife doi: 10.7554/eLife.20365.
- Lee JS, Lee JD, Park HJ, Oh MK, Chun JW, et al. (2013) Is the GABA System Related to the Social Competence Improvement Effect of Aripiprazole? An (18) F-Fluoroflumazenil PET Study. Psychiatry Investig 10(1): 7580.
- Gibson KM, Sweetman L, Nyhan WL, Jakobs C, Rating D, et al. (1983) Succinic semialdehyde dehydrogenase deficiency: an inborn error of gamma-aminobutyric acid metabolism. Clin Chim Acta 133(1): 33-42.
- Pizzarelli R, Cherubini E (2011) Alterations of GABAergic signaling in autism spectrumdisorders. Neural Plast 2011(2011): 297153.
- Robertson CE, Ratai EM, Kanwisher N (2016) Reduced GABAergic Action in the Autistic Brain.Curr Biol 26(1): 80-85.
- Ajram LA, Horder J, Mendez MA, Galanopoulos A, Brennan LP, et al. (2017) Shifting brain inhibitory balance and connectivity of the prefrontal cortex of adults with autism spectrum disorder. Transl Psychiatry 7(5): e1137.
- Inui T, Kumagaya S, Myowa-Yamakoshi M (2017) Neurodevelopmental Hypothesis about the Etiology of Autism Spectrum Disorders.Front Hum Neurosci 11: 354.
- Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, et al. (2013) Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci 7: 60.
- Giacopuzzi E, Gennarelli M, Minelli A, Gardella R, Valsecchi P, et al. (2017) Exome sequencing in schizophrenic patients with high levels of homozygosity identifies novel and extremely rare mutations in the GABA/glutamatergic pathways. PLoS One 12(8): e0182778.
- Wang J, Tang Y, Zhang T, Cui H, Xu L, et al. (2016) Reduced γ-aminobutyric Acid and glutamate+glutamine levels in drug-naive patients with first-episode schizophrenia but not in those at ultrahigh risk. Neural Plast 2016(2016): 3915703.
- Yee JY, Nurjono M, Teo SR, Lee TS, Lee J (2017) GAD1 gene expression in blood of patients with first-episode psychosis. PLoS One 12(1): e0170805.
- Haussleiter IS, Wandinger KP, Juckel G (2017) A case of GABAR antibodies in schizophrenia.BMC Psychiatry 17(1): 9.
- Gilpin NW (2012) Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol 46(4): 329-337.
- Zhang Z, Fan Q, Bai Y, Wang Z, Zhang H, et al. (2016) Brain Gamma- Aminobutyric Acid (GABA) concentration of the prefrontal lobe in unmedicated patients with obsessive-compulsive disorder: a research of magnetic resonance spectroscopy. Shanghai Arch Psychiatry 28(5): 263-270.
- Levar N, van Leeuwen JMC, Puts NAJ, Denys D, van Wingen GA (2017) GABA concentrations in the anterior cingulate cortex are associated with fear network function and fear recovery in humans. Front Hum Neurosci 11: 202.
- Skorzewska A, Wislowska-Stanek A, Lehner M, Turzynska D, Sobolewska A, et al. (2017) Corticotropin releasing factor receptor 1 antagonist differentially inhibits freezing behavior and changes gamma-aminobutyric acidergic activity in the amygdala in low- and high-anxiety rats. J Physiol Pharmacol 68(1): 35-46.
- Szczepanska-Sadowska E, Cudnoch-Jedrzejewska A, Ufnal M, Zera T (2010) Brain and cardiovascular diseases: common neurogenic background of cardiovascular, metabolic and inflammatory diseases. J Physiol Pharmacol 61(5): 509-521.
- Campos AC, Fogafa MV, Scarante FF, Joca SRL, Sales AJ, et al. (2017) Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 8: 269.
- Zhu Z, Wang G, Ma K, Cui S, Wang JH (2017) GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression. Oncotarget 38(22):35933-35945.
- Vaiva G, Thomas P, Ducrocq F, Fontaine M, Boss V, et al. (2004) Low posttrauma GABA plasma levels as a predictive factor in the development of acute posttraumatic stress disorder. Biol Psychiatry. 55(3): 250-254.
- Vaiva G, Boss V, Ducrocq F, Fontaine M, Devos P, et al. (2006) Relationship between posttrauma GABA plasma levels and PTSD at 1-year follow-up.Am J Psychiatr 163(8): 1446-1448.
- Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, et al. (2016) Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry Cogn Neurosci Neuroimaging 2(1): 38-44.
- Contestabile A, Magara S, Cancedda L (2017) The GABAergic Hypothesis for Cognitive Disabilities in Down Syndrome. Front Cell Neurosci 11: 54.
- Kim YS, Yoon BE (2017) Altered GABAergic signaling in brain disease at various stages of life. Exp Neurobiol 26(3): 122-131.
- Hayashi T, Nagai K (1964) Complete cure of epilepsy in dogs following intraventricular injection of 4-amino-3-hydroxybutyric acid. Prensa Med Argent 51: 286.
- Tadokoro Y, Han K, Okada T, Kank, Hirano C (1962) Clinical use of gamma-amino-beta-hydroxybutyric acid in epilepsy, with special reference to its effect on brain waves. Sogo Igaku 19: 462-465.
- Yamamoto S, Haraoka T, Hashimoto Y (1962) On the therapeutic effectiveness of gamma-aminobutyric acid and gamma-amino-beta- hydroxybutyric acid for cerebrovascular disorders and hypertension. Sogo Igaku 19: 445-447.
- Colucci D'Amato F (1968) Therapeutic effect of gabob in a group of patients with character disorders. Osp Psichiatr 36(3): 478-484.
- Mille T, Scamoni C, Presazzi A (1973) Clinical and electroencephalographic studies of Gabob-Diazepam effects on young children with behavior disorders.Minerva Pediatr 25(13): 593-603.
- Amore F, Cavazzuti GB (1971) Clinical and statistical evaluation of GABOB in behavior disorders in young children. Minerva Med 62(29): 1503-1513.
- Heinze H (1978) Long-term treatment with psychoverlan in children and adolescents with behavior disorders. Fortschr Med 24: 96(32).
- (2017)”Vitamin B6”. Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR, USA.
- Technical Information (fact sheet) of Gamalate B6.
- Espadaler Medina JM, Pujol J, Balcells M, Jubert-Gruart J (1996) Clinical experience with a new medicamental association GB6 in neurologic practice. MMW (spanished) 1: 40-57.
- FolchCamarasa L, Folch L, Folch J (1968) Experiences carried out to determine the influence of Gamalate B6 on the thought automatisms in mental defective patients. Rapports de Psicologia y Psiquiatria Pediatricas 10: 1-6.
- Knobel M (1972) Clinical experience with Gamalate B6 in some infantile mental deficiencies. 1972 (Unpublished data).
- Vendrell J, Amoros MC, Vendrell DP (1973) An approach to the study of evolution of speech disturbances in children, on combining the logotherapeutic treatment with a drug association (GB6). N Engl J Med (spanished) 7(82): 51-61.
- Espadaler JM (1982) Our experience in the treatment of minimum cerebral dysfunctions with GB6. Med Klin (spanish edition) 249: 39
- Foch Soler J (1983) Gamalate B6 in paidopsychiatry. Med Klin (spanished) 254: 29-36.
- Zamora A, Perez LM (1990) Magnesium glutamate hydrobromide and GABA as treatment of anxiety. Phronesis 11: 5-8.
- Nunez Sintas D, Lopez-Pousa (1990) Tension headaches response to medication with GAMALATE-B6. Phronesis 12(6): 1-4.
- Martínez M, Alvarado M, Tagle I, Orrillo E (1991) Gabaergic precursors and neurological learning disorders comparative study of school children in urban-marginal areas of metropolitan Lima. Revista de Neuro-Psiquiatri'a 54: 113-120.
- Martínez Mendoza M (1992) Gamalate-B6 (G-B6) in the treatment of anxiety. Phronesis 13: 33-38.
- Novell R, Aguas S, Franco M, Ampudia M, Roig JM, Gutierrez JR et al. (1998) Treatment of behaviour disorders in slight and moderately mentally retarded children. Efficacy and safety of Gamalate B6. Psiquis 19(7): 265-273.
- López-Pousa S, Lombardia C, Ortega E, Novell R (2002) Efficacy of a GABAergic drug (Gamalate-B6®) on the quality of life of the patients with fibromyalgia. Psiquis 23(1): 27-34.






























