Music and Noise Exposure Increases Immobile Timein the Forced Swim Task in Male, but not Female, Rats
Emily Hensleigh Engel S and Laurel M Pritchard*
Department of Psychology, University of Nevada Las Vegas, USA
Submission: March 27, 2017; Published: June 02, 2017
*Corresponding author: Laurel Pritchard, Department of Psychology, University of Nevada Las Vegas 4505 S. Maryland Parkway, Box 455030, Las Vegas, NV 89154, USA, Tel: (702]-895-4759; Email: laurelpritchard@unlv.edu
How to cite this article: Emily Hensleigh Engel S, Laurel M P.Music and Noise Exposure Increases Immobile Time in the Forced Swim Task in Male, but not 004 Female, Rats. Open Access J Neurol Neurosurg. 2017; 4(1): 555629. DOI: 10.19080/OAJNN.2017.04.555629
Abstract
Music exposure in development can lead to long-lasting changes in behavior in adult animals. Both music and noise can additionally impact physiology and behavior in adult animals. Here, we aimed to determine whether music or noise exposure in adult male and female rats impacted performance in the forced swim task. Adult Long-Evans rats (PND 60) were first habituated to a sound-attenuated chamber, then exposed to one of four stimulus conditions for 75 minutes per day for seven days. The stimulus conditions were control (speakers were turned on but no stimulus was present), music (The Beatles Revolver and Magical Mystery Tour), fast music (same music altered to increase tempo by 100%), and noise (broad-band noise containing all frequencies represented in the music stimuli ~1-20kHz). One day after the last stimulus exposure, rats underwent two days of forced swim test. Results revealed males spent significantly more time immobile and less time swimming after exposure to music, fast music, and noise relative to control. There were no differences between groups found in females. These results suggest auditory stimuli acted as stressors and increased depressive-like behaviors in male rats. These findings are relevant to laboratory animal care protocols, as noise exposure may alter behavioral findings.
Keywords: Music; Noise; Forced swim task; Sex differences
Introduction
Most humans exhibit an inherent favorability for rhythmic patterns or music [1]. Music evokes emotional, cognitive, and behavioral reactions, but less is known on the effects of music in animals. Animals can detect different types of rhythmic patterns and various music genres. Rats and primates can differentiate between tunes and across octaves and intensities in an operant task [2]. Rats can also distinguish between Mozart and music from the Beatles and determine the sequence of tones in Frere Jacques and from the same sequence played in reverse [3,4]. Non-human primates can discriminate between childhood songs and tonal melodies but not atonal melodies or random melodies [5]. These studies suggest animals have the capability to discriminate between various rhythmic patterns and simple melodies. Additionally, primates show preference for music with slower tempos, but overall prefer silence relative to other noise [6]. Although animals can detect music and distinguish it from other sounds, they may not find it rewarding or inherently pleasurable.
Music exposure has been shown to affect certain behaviors in animals. Studies demonstrate enhanced working memory [7,8] and increased spatial learning after exposure to the music of Mozart early in development [9,10]. However, some could not replicate these effects and prior studies were criticized because rats could not hear the majority of notes played; therefore the results may not have been due to a 'Mozart Effect' [11,12]. Although it is not clear if rodents demonstrate a clear 'Mozart Effect', animals still respond physiologically to music. Exposure to both classical and rock music can lead to alterations in red blood cells in the periphery, and classical music exposure decreases blood pressure and renal sympathetic activity [13,14]. Furthermore, classical music exposure increases brain derived neurotrophic factor (BDNF) levels but decreases nerve growth factor levels in the hypothalamus [15]. These studies indicate exposure to music can lead to behavioral, physiological, and neurological alterations.
However, not all studies use non-melodic noise as a control, and white noise can additionally have effects on physiology and behavior. Noise exposure after birth decreases auditory exploration in adult animals [16]. It has also been demonstrated that exposure to noise can lead to decreased feeding behaviors and altered circadian cycles [17,18]. However, white noise does not seem to affect blood pressure [14]. As above, these studies demonstrate not only music, but white noise affects animal behavior and physiology. Most studies of the effects of music exposure on rodent behavior have used only classical music or have compared different musical genres without taking into account tempo differences between genres. Here we set out to determine how exposure to popular music at different tempos or white noise affected behavior in the forced swim task, a procedure typically used as a measure of depressive-like behavior.
Methods
Animals
All experimental procedures were approved by the UNLV Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals. Male and female Long-Evans rats were used for this experiment. These rats were bred in the UNLV animal care facility. Animals were sexed on the day after birth, and then left undisturbed until weaning at post-natal day (PND) 21. After weaning, subjects were housed in same sex pairs in standard tub cages with ad libitum access to standard rat chow and water throughout the duration of the experiment [19-22].
Equipment
Stimulus exposure was carried out in open-field chambers (77.47 x 50.17 x 48.90cm) constructed of white, laminated PVC foam. Each chamber was illuminated by a 15W light bulb and had a fan for ventilation and masking of ambient noise. Speakers were mounted in the corners of each open field chamber and kept at a standard volume. The forced-swim apparatus consisted of a cylindrical tank constructed of transparent plastic, approximately 23cm in diameter and 30cm deep. The tank was filled to a depth of approximately 20cm with 25 °C water.
Experimental procedures
At post-natal day (PND) 60, rats were randomly assigned to four groups (n = 8/group). One group was exposed to no specific auditory stimulus (control), one was exposed to white noise (noise), one to music (music), and one to the same music played at a faster tempo (fast music). Each rat was transported in its home cage to the testing facility and allowed to habituate for 30 minutes. After the habituation time, animals were placed in the open-field chamber for 75 minutes, the duration of the CD used to present auditory stimuli. This session was for habituation to the chamber only, and no auditory stimuli were presented. After the habituation session, subjects were returned to their home cages and taken back to the animal care facility. On PND 61-69, each subject was transported to the testing facility each day, allowed to rest in the home cage for 30 minutes, and placed in the open-field chamber and exposed to the assigned stimulus for 75 minutes. After each stimulus exposure session, rats were returned to their home cages and taken back to the animal care facility. Stimulus exposure occurred during the light phase of the light-dark cycle and occurred at approximately the same time every day [23-27].
Stimuli
Stimuli were presented at 75 dB using computer speakers mounted inside the sound attenuating chamber. For the control group, speakers were turned on, but no stimulus was presented. The music groups were exposed to music by The Beatles (Revolver, 1966 and Magical Mystery Tour, 1967), played at the tempo at which it was originally recorded. The same music was digitally altered to increase the tempo of each piece by 100%, and was presented to the "fast music" group. For both music conditions, stimuli were digitally altered to remove any frequencies below 1kHz. This was done because rats are relatively insensitive to frequencies below 1kHz. The "noise" group was exposed to broad-band noise containing all frequencies represented in the music stimuli (approximately 1kHz-20 kHz).
Forced swim task
One day after the last stimulus exposure (PND 70), each rat began the forced swim test. This test consisted of two, five-minute sessions of swimming on consecutive days. At the start of each session, the rat was removed from its home cage and placed in the center of the tank. Each session was recorded using a digital video camera. At the end of the session, each rat was removed from the tank, dried with a towel and warmed under a heating lamp. After each session, water temperature was measured and adjusted as needed by replacement with warm water. Any solid waste in the water was removed between rats. The tank was emptied and cleaned with hot, soapy water, followed by 70% ethanol at the end of each day of testing.
Analyses
Behavior in the forced swim test was scored from video recordings using the Macropod software ODlog™. The following behaviors were scored: time spent swimming (paws moving, but not touching sides or bottom of tank), time spent immobile (floating or making minimal movements necessary to keep head above water), and time spent out of the water (jumping out of the water and holding onto the edge of the tank or escaping the tank altogether). Mean durations of swimming, immobility, and time out of water over the two testing sessions were calculated for each rat. Data were analyzed by two-way ANOVA with condition and sex as between-subjects factors. Significant main effects of condition were followed by Fischer's LSD post-hoc tests. Significant condition x sex interactions were followed by oneway ANOVAs to determine for which sex the effect of condition was significant.
Results
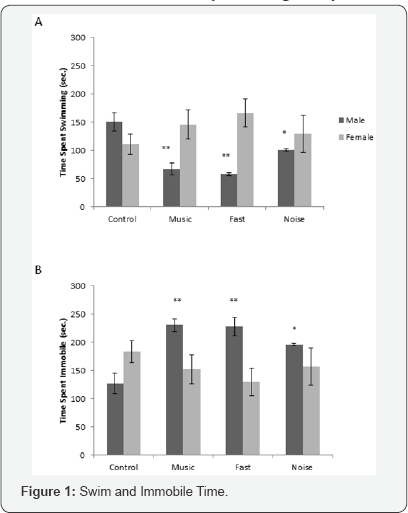
A two-way ANOVA for swim time revealed no significant main effect of condition (F3,29= 0.369, P=0.776), a significant effect of sex (F1,29=5.299, P=0.029), and a significant condition x sex interaction (F3,29= 3.2 66, P=0.035). One-way ANOVAs revealed that the effect of condition was significant for males (F3,,10=7.702, P=0.006), but not for females (F3,,19=0.852, P=0.483). For males, all groups exposed to auditory stimuli spent significantly less time swimming than did controls (P< 0.05; Figure 1A).
A two-way ANOVA for immobile time revealed no significant main effect of condition (F3,29=0.742, P=0.535), a significant effect of sex (F1,29=4.258, P=0.048), and a significant condition x sex interaction (F3,29=3.739, P=0.022). One-way ANOVAs revealed that the effect of condition was significant for males (F3,10=8.919, P=0.004), but not for females (F3,19=0.744, P=0.539). For males, all groups exposed to auditory stimuli spent significantly more time immobile than did controls (P<0.05; Figure 1B).
There were no significant effects of condition or sex on time spent out of water. Because relatively few animals spent any time out of the water (seven total), we also analyzed likelihood of spending any time out of the water using a chi-square test. There were no significant differences across groups or sexes in likelihood of spending any time out of the water (data not shown).
References
- McDermott J, Hauser M (2005) The origins of music: Innateness, uniqueness, and evolution.
- D'Amato MR, Salmon DP (1982) Tune discrimination in monkeys (Cebus apella) and in rats. Animal Learning & Behavior 10(2): 126134.
- Okaichi Y, Okaichi H (2001) Music discrimination by rats (in Japanese). Japanese Journal of Animal Psychology 51(1): 29-34.
- Poli M, Previde EP (1991) Discrimination of musical stimuli by rats (Rattus norvegicus). International Journal of Comp Psychology 5: 7-18.
- Wright AA, Rivera JJ, Hulse SH, Shyan M, Neiworth JJ (2000) Music perception and octave generalization in rhesus monkeys. Journal of Experimental Psychology 129(3): 291-307.
- McDermott J, Hauser MD (2007) Nonhuman primates prefer slow tempos but dislike music overall. Cognition 104(3): 654-668.
- Aoun P, Jones T, Shaw GL, Bodner M (2005) Long-term enhancement of maze learning in mice via a generalized Mozart effect. Neurological research 27(8): 791-796.
- Rauscher FH, Robinson KD, Jens JJ (1998) Improved maze learning through early music exposure in rats. Neurological Research 20(5): 427-432.
- Kim H, Lee MH, Chang HK, Lee TH, Lee HH, et al. (2006) Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain and Development 28(2): 109-114.
- Rauscher FH, Shaw GL, Ky KN (1993) Music and spatial task performance. Nature 365(6447): 611.
- Steele KM, Bass KE, Crook MD (1999) The mystery of the Mozart effect: Failure to replicate. Psychological Science 10(4): 366-369.
- Steele KM (2003) Do rats show a Mozart effect? Music Perception 21(2): 251-265.
- Erken G, Bor Kucukatay M, Erken HA, Kursunluoglu R, Genc O (2008) Influence of classical and rock music on red blood cell rheological properties in rats. Medical science monitor: international medical journal of experimental and clinical research 14(1): BR28-33.
- Nakamura T, Tanida M, Niijima A, Hibino H, Shen J, et al. (2007) Auditory stimulation affects renal sympathetic nerve activity and blood pressure in rats. Neuroscience letters 416(2): 107-112.
- Angelucci F, Ricci E, Padua L, Sabino A, Tonali PA (2007) Music exposure differentially alters the levels of brain-derived neurotrophic factor and nerve growth factor in the mouse hypothalamus. Neuroscience letters 429(2): 152-155.
- Zhang J, Chen L, Gao F, Pu Q, Sun X (2008) Noise exposure at young age impairs the auditory object exploration behavior of rats in adulthood. Physiology & behavior 95(1): 229-234.
- Krebs H, Macht M, Weyers P, Weijers HG, Janke W (1996) Effects of stressful noise on eating and non-eating behavior in rats. Appetite 26(2): 193-202.
- Rabat A (2007) Extra-auditory effects of noise in laboratory animals: the relationship between noise and sleep. J Am Assoc Lab Anim Sci 46(1): 35-41.
- Animal Research Review Panel (2012) Guideline 22: Guidelines for the housing of mice in scientific institutions, pp. 1-143.
- Bourke CH, Neigh GN (2011) Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior 60: 112-120.
- Campbell T, Lin S, DeVries C, Lambert K (2003) Coping strategies in male and female rats exposed to multiple stressors. Physiology & behavior 78(3): 495-504.
- Hong S, Flashner B, Chiu M, ver Hoeve E, Luz S, et al. (2012) Social isolation in adolescence alters behaviors in the forced swim and sucrose preference tests in female but not male rats. Physiol Behav 105(2): 269-275.
- Jennings M, Batchelor GR, Brain PF, Dick A, Elliott H, et al. (1998). Refining rodent husbandry: The mouse: Report of the Rodent Refinement Working Party. Lab Anim 32(3): 233-259.
- McCarthy DO, Ouimet ME, Daun JM (1992) The effects of noise stress on leukocyte function in rats. Research in nursing & health 15(2): 131137.
- National Research Council (2011) Guide for the care and use of laboratory animals. (8th edn), The National Academies Press, Washington DC, USA.
- Russell WMS, Burch RL, Hume CW (1959) The principles of humane experimental technique.
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, et al. (2004) Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. Journal of Neuroendocrinology 16: 516-524.






























