Abstract
Idiosyncratic drug-induced agranulocytosis is a rare but potentially fatal adverse drug reaction characterized by a sudden and profound drop in neutrophil count, predisposing patients to severe infections. Although its overall incidence is low, the condition poses a significant clinical challenge, particularly among older, frail individuals with multiple comorbidities and high levels of polypharmacy. In this vulnerable population, the unpredictable onset and wide spectrum of causative medications further complicate management. Traditional strategies focus on early recognition and prompt discontinuation of the offending drug, but preventive measures have historically been limited. Recent advances in pharmacogenomics have shed light on individual susceptibility, with specific HLA alleles and genetic polymorphisms identified as key risk factors. The integration of genetic, demographic, and clinical characteristics into defined risk clusters supports a more personalized prevention strategy. Moreover, the application of big data analytics and artificial intelligence (AI) holds promise for predictive modeling, real-time signal detection, and improved pharmacovigilance. Technological innovations - such as telemedicine, wearable sensors, and home-based neutrophil monitoring - can facilitate earlier detection and targeted intervention, particularly in geriatric patients at higher risk. Therapeutic patient education (TPE) is essential in raising awareness, reinforcing adherence to monitoring protocols, and empowering older adults in self-care. This review explores how the convergence of scientific, clinical, and technological advances is reshaping the management of idiosyncratic drug - induced agranulocytosis, paving the way toward a more proactive, preventive, and individualized approach - especially relevant in the context of aging populations.
Keywords:Drug-induced agranulocytosis; Elderly patients; Frailty patients; Polypharmacie; Multiples comorbidities; Risk clusters; Big data, Artificial intelligence; Telemedicine; Connected sensors; Therapeutic patient education; Prevention, Prediction
Introduction
Idiosyncratic drug-induced agranulocytosis (IDIA) is a rare but potentially life-threatening adverse drug reaction characterized by a profound reduction in neutrophil count, frequently leading to severe infections and, in some cases, death [1,2]. Although uncommon, IDIA remains a major concern in internal and geriatric medicine due to its unpredictable onset, the large variety of implicated drugs, and the lack of reliable early warning signs. This condition is particularly worrisome in older adults, who often present with frailty, multiple chronic comorbidities, and polypharmacy - all of which can increase susceptibility to adverse drug reactions and delay recognition of evolving neutropenia. While certain medications - such as antithyroid drugs, clozapine, sulfonamides, and some antibiotics - are more frequently associated with IDIA, the idiosyncratic nature of the reaction reflects a multifactorial etiology. This includes genetic predispositions (e.g., HLA variants), immune-mediated mechanisms, and interactions with existing health conditions and complex drug regimens [1,2].
Despite its rarity, IDIA carries high morbidity and a risk of rapid clinical deterioration, particularly in older and vulnerable patients, often requiring immediate drug withdrawal and urgent supportive interventions upon diagnosis [3]. However, early identification remains challenging due to the delayed onset and non-specific initial symptoms. In recent years, advances in pharmacogenomics have begun to elucidate individual susceptibility factors, while the emergence of big data analytics and artificial intelligence (AI) offers new possibilities for predictive modeling and real-time pharmacovigilance. Additionally, the integration of telemedicine, wearable technologies, and home-based blood monitoring opens new perspectives for earlier detection and intervention, especially in geriatric patients with limited mobility or complex care needs [3,4].
This review aims to provide a comprehensive and updated overview of current knowledge and evolving approaches to IDIA, with a particular focus on aging populations [5,6]. It underscores how technological and scientific innovations - combined with a patient-centered, multidisciplinary care model - can support improved risk stratification, early detection, and more personalized prevention and management strategies in internal and geriatric medicine.
Idiosyncratic Drug-Induced Agranulocytosis
Idiosyncratic drug-induced cytopenias result from a decrease in blood cell counts - affecting red blood cells (anemia), neutrophils (neutropenia), platelets (thrombocytopenia), or even multiple hematopoietic lineages, as in drug-induced thrombotic microangiopathy. These hematological abnormalities arise from unpredictable reactions to drugs, unrelated to dosage or known pharmacological properties (Table 1). Their occurrence poses a particular concern in older adults, who often present with polypharmacy, reduced physiological reserves, and multiple comorbidities that may amplify susceptibility and obscure early clinical signs.
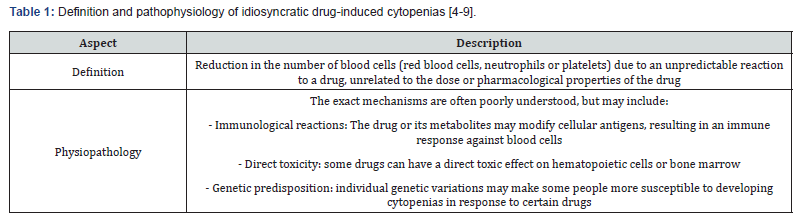
Neutropenia, defined by an abnormally low number of neutrophils - key cells in the defense against bacterial and fungal infections - is typically classified by severity: mild (1000–1500 cells/μL or 1–1.5 × 10⁹/L), moderate (500-1000 cells/μL or 0.5-1 × 10⁹/L), and severe (<500 cells/μL or <0.5 × 10⁹/L). The risk of infection increases significantly as neutrophil counts fall, especially in frail, immunocompromised, or malnourished older patients [7]. Agranulocytosis represents a particularly severe and often abrupt form of neutropenia, defined by neutrophil counts below 500 cells/μL - and in many cases below 100 cells/μL (0.1 × 10⁹/L) - leading to a critically compromised innate immune defense [3].
Idiosyncratic drug-induced agranulocytosis (IDIA) is a rare but severe form of neutropenia, occurring at an estimated incidence of 6-10 cases per million inhabitants per year [1,2]. It is caused by an adverse drug reaction driven by individual-specific factors such as genetic predisposition (e.g., HLA alleles), immune dysregulation, or other poorly understood mechanisms. Unlike general neutropenia, IDIA often results from bone marrow suppression and is not dose-dependent, rendering prediction and prevention particularly challenging - especially in geriatric patients frequently exposed to high-risk drugs due to complex therapeutic regimens.
In older, frail, or multimorbid individuals, the consequences of IDIA may be particularly severe, including prolonged hospitalization, sepsis, and death. A mortality rate between 5% and 20% has been reported for severe drug-induced neutropenia and agranulocytosis [8]. Prompt recognition, early discontinuation of the causative agent, and rapid initiation of supportive therapy - including empirical antibiotics and hematopoietic growth factors - are essential to prevent irreversible complications [9]. In this context, systematic vigilance is required, particularly in elderly patients receiving high-risk medications in the setting of polypharmacy and impaired homeostatic reserves.
IDIA can be triggered by a wide and diverse range of medications, reflecting the unpredictable and multifactorial nature of this adverse drug reaction. Historically, well-established culprit drugs include certain antimicrobials (e.g., trimethoprim-sulfamethoxazole, some cephalosporins), non-steroidal anti-inflammatory drugs (NSAIDs) such as phenylbutazone (with a lower incidence among newer NSAIDs), antithyroid agents (e.g., methimazole, propylthiouracil), and the antipsychotic clozapine, which is associated with a particularly high risk of agranulocytosis and is subject to mandatory hematological monitoring in many countries [10,11].
Other commonly implicated pharmacological classes include anticonvulsants (e.g., carbamazepine), antiarrhythmics (e.g., procainamide), and certain chemotherapeutic agents. While the latter typically cause predictable, dose-dependent myelosuppression, they may also, in rare cases, precipitate idiosyncratic agranulocytosis [12,13]. Emerging therapeutic modalities - such as monoclonal antibodies, targeted biologics, and even advanced cell- and gene-based therapies (including CAR T-cell therapy) - have also been associated with isolated cases of severe neutropenia or agranulocytosis, further expanding the landscape of concern [14,15].
This diversity in causative agents is especially relevant in geriatric medicine. Older adults are more likely to be exposed to multiple high-risk drugs due to polymorbidity and complex therapeutic regimens. They are also more vulnerable to adverse outcomes due to age-related changes in drug metabolism, diminished bone marrow reserve, and impaired immune responses. In this context, careful medication history taking, comprehensive risk-benefit analysis, and systematic pharmacovigilance are essential. Moreover, as the list of potentially offending drugs continues to evolve, clinicians must remain vigilant for newly implicated agents, particularly in polymedicated, frail older patients [16,17].
Understanding Risk: The Concept of Risk Clusters
The traditional approach to drug-induced agranulocytosis has primarily centered on identifying individual high-risk drugs. While this drug-specific perspective remains clinically useful, it fails to account for the multifactorial nature of susceptibility to idiosyncratic drug-induced agranulocytosis (IDIA). In clinical practice - especially in older and frail patients - a more nuanced approach is needed. This has led to the concept of “risk clusters”, which refer to combinations of genetic, demographic, clinical, and pharmacological factors that together increase the likelihood of developing IDIA.
Several key components have been identified within these
risk clusters:
a) Genetic Predisposition: Certain HLA alleles and polymorphisms
in genes involved in immune regulation and drug
metabolism have emerged as important susceptibility factors
[18,19]. Variants in genes encoding drug-metabolizing enzymes
(e.g., CYPs, NATs) or immune regulators (e.g., HLA-B, HLA-DR alleles)
can predispose individuals to aberrant immune responses
following drug exposure. Genetic screening, although not yet standard
practice, may help identify high-risk individuals - particularly
in the context of geriatric patients receiving high-risk drugs.
b) Age and Sex: Advanced age and female sex are both
recognized as risk factors for IDIA, particularly with drugs like
antithyroid agents and clozapine [20,21]. Age-related changes in
pharmacokinetics - such as reduced renal or hepatic clearance
- can result in elevated drug levels in older adults, while immunosenescence
and inflammaging may alter immune reactivity. In
addition, hormonal and immune differences in females may contribute
to increased susceptibility. These factors are particularly
relevant in elderly women with multimorbidity and altered drug
handling capacity.
c) Comorbidities: Chronic conditions such as autoimmune
diseases, chronic infections, hepatic insufficiency, and renal
impairment can increase the risk of IDIA [22]. Autoimmune diseases
often entail baseline immune dysregulation, while hepatic
and renal dysfunction can impair drug clearance, contributing to
drug accumulation and heightened immunogenicity. In geriatric
patients, such comorbidities are common and often co-exist, compounding
the risk.
d) Polypharmacy and Drug–Drug Interactions: Polypharmacy
- highly prevalent in older adults-significantly increases
the complexity of pharmacological management and the risk of
drug interactions [23,24]. Concomitant use of immunomodulatory
agents, myelotoxic drugs, or inhibitors of drug metabolism can
potentiate bone marrow suppression. Some combinations may
have synergistic effects on hematopoietic toxicity, while others
may mask or delay the recognition of agranulocytosis, complicating
timely intervention.
e) History of Previous Idiosyncratic Drug Reactions: A
prior history of idiosyncratic or immune-mediated adverse drug
reactions is a significant risk marker for subsequent reactions, including
IDIA [25]. In elderly patients with extensive therapeutic
histories, cumulative immune sensitization or previous exposure
to structurally similar agents may prime the immune system for
exaggerated responses.
Identifying these risk clusters through comprehensive data integration - combining electronic health records, genetic data, and pharmacological profiles - is crucial for improving predictive accuracy and tailoring preventive strategies (Table 2) [18-25]. In older and multimorbid patients, where clinical complexity is high, this approach may allow for personalized risk stratification, informed consent, preemptive monitoring protocols, and judicious therapeutic decisions. Ultimately, a shift from reactive to proactive and individualized risk management in IDIA - especially in geriatric medicine - offers the promise of reducing morbidity, hospitalizations, and mortality associated with this rare but serious adverse drug reaction.

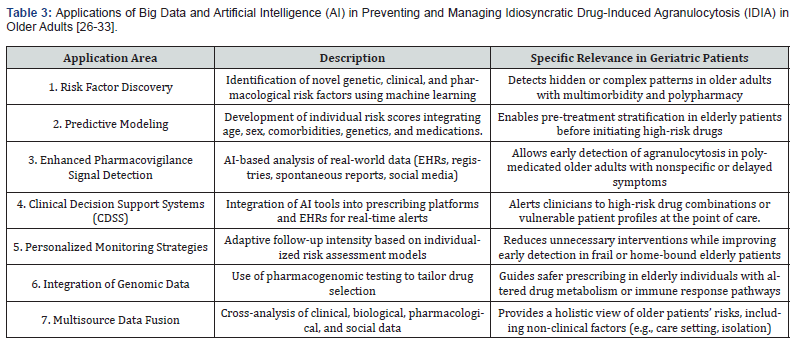
The Power of Big Data and Artificial Intelligence
The vast amounts of health-related data generated from electronic health records (EHRs), pharmacovigilance databases, genomic research, and even non-traditional sources such as social media present unprecedented opportunities to understand and manage the complexities of idiosyncratic drug-induced agranulocytosis (IDIA). The integration of big data analytics and artificial intelligence (AI) holds particular promise in the field of geriatric medicine, where the burden of multimorbidity, polypharmacy, and altered pharmacokinetics creates significant challenges in risk prediction and clinical decision-making.
AI-enhanced data analysis enables a paradigm shift from retrospective recognition of adverse drug reactions toward prospective risk modeling, personalized monitoring, and preventive strategies tailored to older, frail, and vulnerable populations.
Key Applications of Big Data and AI in Managing IDIA Risk in
Older Adults:
a) Identification of Novel Risk Factors and Drug Interactions:
Machine-learning techniques can analyze high-dimensional
data from EHRs, genomic studies, and real-world pharmacovigilance
platforms to uncover new and complex associations.
In older adults with multiple comorbidities and extensive drug
exposure histories, these tools can help identify unexpected
drug-drug interactions or cumulative toxicities associated with
agranulocytosis risk [26,27]. AI may also detect genetic markers
or immunological profiles that confer increased susceptibility in
elderly patients who may otherwise be overlooked using standard
assessment models.
b) Development of Predictive Models for Risk Stratification:
AI algorithms trained on large datasets can generate predictive
models that estimate an individual’s likelihood of developing
IDIA based on integrated variables such as age, sex, renal/hepatic
function, genetic polymorphisms, comorbidities, and pharmacological
exposure [27,28]. In older patients - who often accumulate
multiple risk factors-these models can guide pre-emptive risk
stratification prior to initiating high-risk medications (e.g., antithyroid
drugs, clozapine), facilitating safer prescribing practices.
c) Enhanced Signal Detection in Pharmacovigilance:
Traditional pharmacovigilance systems may miss subtle or delayed-
onset signals, particularly in the elderly, where symptoms
of agranulocytosis can be nonspecific or attributed to underlying
frailty. AI can improve the sensitivity and specificity of signal detection
by analyzing a wide array of structured and unstructured
data - including patient reports, registry data, and even public
sources like social media - for early signs of drug-associated neutropenia
[29,30]. Early signal recognition is especially valuable in
geriatric patients, where delayed intervention may result in rapid
clinical deterioration.
d) Personalized Risk Assessment and Clinical Decision
Support: Perhaps the most transformative contribution of AI lies
in its ability to integrate heterogeneous data sources to deliver
patient-specific risk assessments [31,32]. For older patients, such
tools can combine clinical parameters (e.g., frailty index, organ
function, medication burden), pharmacogenomic data, and social
determinants (e.g., care setting, support network) to tailor monitoring
intensity and therapeutic strategies. This individualized
approach is particularly relevant in geriatrics, where interindividual
variability is high and “one-size-fits-all” guidelines are often
inadequate.
By leveraging these tools, clinicians can move toward a proactive and data-driven approach to IDIA management - especially in older populations disproportionately affected by this complication. The integration of AI into clinical workflows also opens avenues for real-time clinical decision support, alerting prescribers to elevated risks based on a patient’s evolving profile.
As these technologies mature, they are poised to redefine pharmacovigilance and personalized medicine for IDIA, contributing to safer prescribing, earlier detection, and improved outcomes for high-risk groups, including frail older adults with complex medication regimens [33] (Table 3).
Innovations in Biological Understanding
Recent advancements in biology are providing deeper insights
into the complex mechanisms underlying drug-induced agranulocytosis
(IDIA), insights that are particularly relevant for improving
prevention, diagnosis, and treatment in older, frail patients.
These innovations hold promise for tailoring safer therapeutic
approaches in populations with increased vulnerability due to
age-related immune changes, comorbidities, and polypharmacy.
a) Pharmacogenomics: Ongoing research into genetic
markers associated with susceptibility to IDIA continues to refine
our understanding of individual risk profiles [34,35]. In geriatric
populations, where physiological heterogeneity is high, the integration
of pharmacogenomic testing prior to initiating high-risk
medications may enable personalized prescribing strategies that
minimize adverse effects and optimize efficacy.
b) Immunopathogenesis: Investigations into immune
mechanisms involved in IDIA - such as drug-dependent antibody
formation and cytotoxic T cell activation against neutrophils - are
critical for developing targeted therapies [35,36]. Older adults often
exhibit altered immune regulation (immunosenescence) that
may modify these pathogenic processes, underscoring the need
for age-adapted therapeutic interventions that modulate harmful
immune responses while preserving immune competence.
c) Neutrophil Biology: Breakthroughs in understanding
neutrophil development, survival, and clearance mechanisms offer
new therapeutic avenues [37,38]. In elderly patients, where
bone marrow reserves and neutrophil regenerative capacity may
be impaired, these insights could inform novel treatments aimed
at accelerating recovery from neutropenia and reducing infection-
related morbidity.
d) Biomarkers: The identification of early biomarkers
predictive of impending agranulocytosis is a major goal for proactive
monitoring and timely intervention [39,40]. For older and
frail patients, sensitive and specific biomarkers detectable in peripheral
blood or bone marrow could transform clinical management
by enabling earlier diagnosis and reducing the risk of severe
infectious complications through prompt treatment adjustments.
Together, these biological innovations are essential for advancing our understanding of IDIA and for developing more effective, personalized strategies to prevent and manage this serious adverse drug reaction - particularly in aging populations at heightened risk due to complex clinical profiles [41].
Integrating Telemedicine and Connected Sensors for Enhanced Monitoring
Telemedicine and connected sensors represent promising
innovations for enhancing patient monitoring, particularly in the
early detection phase of drug-induced agranulocytosis (IDIA).
These technologies facilitate more proactive and personalized
care, which is especially crucial for older adults who may have
limited mobility, cognitive challenges, or difficulties accessing frequent
in-person medical evaluations.
a) Remote Symptom Monitoring: Telehealth platforms
enable elderly patients to report symptoms such as fever, fatigue,
or sore throat-early indicators of infection and potential complications
of agranulocytosis-from their homes [42,43]. Regular,
remote symptom tracking supports earlier identification of concerning
clinical changes and allows healthcare providers to intervene
more swiftly, a critical advantage for frail older adults at
higher risk of rapid deterioration.
b) Wearable Sensors: Continuous monitoring devices
measuring vital signs (e.g., body temperature, heart rate, oxygen
saturation) provide an additional safety net for at-risk patients
[44,45]. These sensors can automatically alert healthcare professionals
to abnormal trends, enabling timely clinical reassessment.
This real-time data stream is particularly beneficial for elderly patients,
in whom subtle physiological changes might otherwise go
unnoticed until more severe illness develops.
c) Home-Based Blood Cell Counts: Emerging point-ofcare
testing (POCT) technologies for home monitoring of white
blood cell (WBC) counts, especially neutrophils, offer a transformative
tool for managing IDIA risk [46,47]. Designed for ease of
use and reliability, these devices allow high-risk older patients -
such as those newly prescribed agranulocytosis-associated drugs
or those within identified risk clusters - to perform frequent blood
count checks without clinic visits. Early detection of neutropenia
in the home setting can drastically reduce time to diagnosis and
initiation of treatment, thereby preventing severe infectious complications.
In summary, the integration of telemedicine and connected health technologies into routine care pathways can markedly strengthen early monitoring and management of IDIA [48,49]. These tools promote a shift toward patient-centered, accessible care tailored to the needs of elderly and frail patients, ultimately enabling earlier diagnosis, prompt treatment, and improved clinical outcomes (Table 4).

The Crucial Role of Therapeutic Patient Education
Therapeutic patient education (TPE) plays a pivotal role in
reducing the risks associated with idiosyncratic drug-induced
agranulocytosis (IDIA), especially among older adults who may
be more vulnerable due to multimorbidity, cognitive decline, or
complex medication regimens. By equipping patients with relevant
knowledge and self-management tools, TPE promotes safer
medication use, enhances early symptom recognition, and facilitates
timely medical intervention - key factors for preventing severe
complications in this high-risk population.
a) Medication Awareness: A core objective of TPE is to
ensure that patients fully understand the medications they take,
particularly those with a known risk of IDIA [50,51]. For elderly
patients, this involves clear communication about potential side
effects, including agranulocytosis, and the critical importance of
promptly reporting any early symptoms such as fever, sore throat,
or oral ulcers, which may signal neutropenia or infection.
b) Early Symptom Recognition: TPE empowers older
adults and their caregivers to identify early clinical signs of agranulocytosis.
Given that older patients may present atypically or
have diminished symptom perception, education about warning
signs increases the likelihood of timely medical consultation, improving
chances of early diagnosis and recovery [52,53].
c) Adherence to Monitoring Protocols: Regular blood
monitoring is essential for patients on high-risk medications.
TPE can improve adherence to scheduled white blood cell (WBC)
counts and clinical follow-ups by clarifying the rationale behind
testing and highlighting the risks of missed appointments [54,55].
This is particularly important in geriatrics, where cognitive or logistical
barriers may impair compliance.
d) Self-Management Strategies: TPE supports infection
prevention through teaching effective hygiene practices, avoidance
of high-risk environments during neutropenia, and vigilance
for infection signs [56]. These behaviors are crucial in older adults
who face increased infectious risks and complications from agranulocytosis.
Tailored TPE programs delivered via digital platforms and mobile applications further enhance patient engagement and retention of critical information [57,58]. Such tools can be adapted to individual literacy levels, cognitive capacities, and risk profiles, offering features like symptom trackers, medication reminders, educational videos, and direct communication with healthcare providers. This interactive, patient-centered approach can significantly improve the management of IDIA in elderly populations.
In summary, therapeutic patient education is a cornerstone of preventive care for IDIA [59]. By fostering medication awareness, early symptom detection, adherence to monitoring, and proactive self-care behaviors, TPE enhances safety and clinical outcomes - particularly for older, frail patients at elevated risk of this serious adverse drug reaction.
Prevention and Prediction: Towards a Proactive Approach
The ultimate goal in managing idiosyncratic drug-induced agranulocytosis (IDIA) is its prevention, especially critical in older adults who face heightened vulnerability due to multimorbidity, polypharmacy, and physiological changes. Achieving effective prevention demands a comprehensive, multifaceted strategy that combines clinical vigilance, technological innovation, and patient and public engagement.
Key Components of a Preventive Strategy for IDIA in Older
Adults:
a) Evidence-Based Prescribing: Clinicians must carefully
assess the risk-benefit balance of medications known to carry
IDIA risk, particularly in elderly patients with complex health
profiles [60,61]. For those identified as high risk through genetic,
clinical, or pharmacological factors, safer therapeutic alternatives
should be prioritized. This cautious approach is essential to minimize
preventable cases of agranulocytosis in frail populations.
b) Targeted Monitoring Strategies: Prevention relies
on risk-stratified monitoring protocols customized to individual
patient risk clusters. In older adults, this often involves more
frequent blood count surveillance, enhanced by telemedicine and
connected sensor technologies that allow remote symptom and
vital sign monitoring [62,63]. Such innovations enable earlier detection
of neutropenia and timely clinical intervention, mitigating
severe complications.
c) Pre-prescription Risk Assessment: Artificial intelligence-
powered predictive models are transformative tools for
prevention. By integrating genetic data, comorbidities, medication
histories, and other relevant factors, these models can estimate
an individual’s IDIA risk prior to therapy initiation [64,65].
This facilitates personalized prescribing and monitoring plans,
reducing adverse drug reaction incidence among elderly patients.
d) Pharmacovigilance Enhancement: Strengthening
pharmacovigilance systems is critical for real-time identification
and evaluation of IDIA cases. The incorporation of big data analytics
and AI improves signal detection sensitivity, allowing earlier
recognition of emerging drug safety concerns and enabling
prompt regulatory responses [66,67]. Enhanced reporting mechanisms
support a more precise understanding of IDIA epidemiology
and risk factors in geriatric populations.
e) Public Awareness Campaigns: Educating healthcare
professionals and the public about IDIA risk factors, early symptom
recognition, and the importance of rapid reporting is vital
[68]. Targeted campaigns can improve awareness among older
adults and caregivers, fostering quicker diagnosis, cessation of offending
drugs, and initiation of appropriate treatment.
In conclusion, preventing IDIA in older adults requires a coordinated, multi-dimensional approach encompassing personalized clinical decisions, patient empowerment, technological integration, and public health communication (Table 5). Aligning these efforts promises to significantly reduce the incidence and severity of this serious adverse drug reaction, ultimately enhancing patient safety and clinical outcomes in vulnerable elderly populations [69-70].

Conclusion
Idiosyncratic drug-induced agranulocytosis (IDIA) remains a significant clinical challenge due to its unpredictable onset, potential severity, and the wide variety of implicated medications. This challenge is especially acute in older adults, who are more vulnerable due to comorbidities, polypharmacy, and altered physiological responses. However, the convergence of advances across multiple disciplines offers a transformative opportunity to shift from reactive to proactive, personalized care.
Progress in identifying risk clusters - through comprehensive analysis of genetic predispositions, patient demographics, comorbid conditions, and medication profiles - has laid the foundation for more targeted prevention strategies tailored to high-risk groups such as the elderly. Concurrently, the integration of big data analytics and artificial intelligence (AI) facilitates the development of sophisticated predictive models capable of estimating an individual’s risk of developing IDIA prior to exposure to highrisk drugs. These models promise personalized prescribing and monitoring protocols that can reduce adverse outcomes, particularly in fragile geriatric populations.
Innovations in biological research - including pharmacogenomics, neutrophil biology, and immunopathogenesis - are deepening our understanding of IDIA mechanisms. Such insights may enable the development of targeted therapies and early detection biomarkers, which are vital for improving prognosis in older patients who often present with atypical symptoms. Mean while, connected health technologies - such as wearable sensors, telemedicine platforms, and home-based blood count monitoring devices - are revolutionizing patient surveillance, allowing earlier intervention and reducing healthcare burdens, especially beneficial for elderly individuals with limited mobility or access to healthcare facilities.
By embracing this multidisciplinary toolkit, clinicians can transition toward a personalized, predictive, and preventive approach to managing IDIA, ultimately enhancing patient safety and outcomes in internal medicine and geriatrics.
However, further research is critical to fully realize these advances. This includes validating risk clusters in diverse populations, refining and clinically validating AI-driven predictive tools, and assessing the real-world effectiveness of connected monitoring technologies in elderly and multimorbid patients. Only through rigorous study and careful implementation can scientific and technological progress be translated into tangible benefits for patients at risk of this rare but serious adverse drug reaction.
Acknowledgment
We gratefully acknowledge the use of bibliographic databases such as PubMed, Embase, and Google Scholar for accessing the scientific literature essential to this manuscript. We also utilized information and communication technology tools, including Zotero for reference management and ChatGPT to ensure the manuscript’s consistency with the journal’s style and to assist with English language review. Finally, Microsoft Office software was used for document formatting and finalization.
References
- Andrès E, Cottet MR, Maloisel F, Séverac F, Keller O, et al. (2017) Idiosyncratic drug-induced neutropenia & agranulocytosis. QJM 110(5): 299-305.
- Johnston A, Uetrecht J (2015) Current understanding of the mechanisms of idiosyncratic drug-induced agranulocytosis. Expert Opin Drug Metab Toxicol 11(2): 243-257.
- Shad MU (2023) Pharmacogenomic screening for agranulocytosis and efficacy with clozapine. J Transl Genet Genom 7: 141-165.
- Dsouza VS, Leyens, L, Kurian JR, Brand A, Brand H (2025) Artificial intelligence (AI) in pharmacovigilance: A systematic review on predicting adverse drug reactions (ADR) in hospitalized patients. Research Social Administrative Pharmacy 21(6): 453-462.
- Uetrecht J (2007) Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol 47: 513-539.
- Andrès E, Hajjam EHA, Maloisel F, Ortiz AMB, Bailón MM, et al. (2025) Artificial Intelligence (AI) and Drug-Induced and Idiosyncratic Cytopenia: The Role of AI in Prevention, Prediction, and Patient Participation. Hematol Rep 17(3): 24.
- Liang L, Hu J, Sun G, Hong N, Wu G, et al. (2022) Artificial Intelligence-Based Pharmacovigilance in the Setting of Limited Resources. Drug Saf 45(5): 511-519.
- Rattay B, Benndorf RA (2021) Drug-Induced Idiosyncratic Agranulocytosis - Infrequent but Dangerous. Front Pharmacol 12: 727717.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, et al. (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30(2): 239-245.
- Uetrecht J (2007) Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol 47: 513-539.
- Mijovic A, MacCabe JH (2020) Clozapine-induced agranulocytosis. Ann Hematol 99(11): 2477-2482.
- Andrès E, Maloisel F (2008) Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol 15(1): 15-21.
- Curtis BR (2017) Non-chemotherapy drug-induced neutropenia: key points to manage the challenges. Hematology Am Soc Hematol Educ Program 2017(1): 187-193.
- Andrès E, Villalba NL, Zulfiqar AA, Serraj K, Cottet RM, et al. (2019) State of Art of Idiosyncratic Drug-Induced Neutropenia or Agranulocytosis, with a Focus on Biotherapies. J Clin Med 8(9): 1351.
- Lin H, Cheng J, Mu W, Zhou J, Zhu L (2021) Advances in Universal CAR-T Cell Therapy. Front Immunol 6.
- Duwez M, Szymanski G, Carre M, Mallaret M, Lepelley M (2020) Idiosyncratic drug-induced agranulocytosis: 7 year-analysis in a French university hospital. Ann Pharm Fr 78(3): 230-241.
- Shamim AM, Shamim AM, Arora P, Dwivedi P (2024) Artificial intelligence and big data for pharmacovigilance and patient safety. J Med Surg Public Health 3: 100139.
- Andersohn F, Konzen C, Garbe E (2007) Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 146(9): 657-665.
- Chen WT, Chi CC (2019) Associations of HLA genotypes with antithyroid drug-induced agranulocytosis: A systematic review and meta-analysis of pharmacogenomics studies. Br J Clin Pharmacol 85(9): 1878-1887.
- Ibáñez L, Vidal X, Ballarín E, Laporte JR (2005) Population-based drug-induced agranulocytosis. Arch Intern Med 165(8): 869-874.
- Villalba LN, Ortiz AMB, Maouche Y, Zulfiqar AA, Andrès E (2020) Idiosyncratic Drug-Induced Neutropenia and Agranulocytosis in Elderly Patients. J Clin Med 9: 1808.
- Vial T, Pofilet C, Pham E, Payen C, Evreux JC (1996) Acute drug-induced agranulocytosis: experience of the Regional Center of Pharmacovigilance of Lyon over 7 years. Therapie 51(5): 508-515.
- Sommer J, Viviani R, Wozniak J, Stingl JC, Just KS (2024) Dealing with adverse drug reactions in the context of polypharmacy using regression models. Sci Rep 14(1): 27355.
- Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H (2008) Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals Drug Saf 31(9): 789-798.
- Curtis BR (2014) Drug-induced immune neutropenia/agranulocytosis. Immunohematology 30(2): 95-101.
- Huang X, Xie X, Huang S, Wu S, Huang L (2024) Predicting non-chemotherapy drug-induced agranulocytosis toxicity through ensemble machine learning approaches. Front Pharmacol 14: 1431941.
- Chen WT, Chi CC (2019) Associations of HLA genotypes with antithyroid drug-induced agranulocytosis: A systematic review and meta-analysis of pharmacogenomics studies. Br J Clin Pharmacol 85(9): 1878-1887.
- Iverson S, Zahid N, Uetrecht JP (2002) Predicting drug-induced agranulocytosis: characterizing neutrophil-generated metabolites of a model compound, DMP 406, and assessing the relevance of an in vitro apoptosis assay for identifying drugs that may cause agranulocytosis. Chem Biol Interact 142(1-2): 175-199.
- Hughes JH, Tong DMH, Burns V, Daly B, Razavi P, et al. (2023) Clinical decision support for chemotherapy-induced neutropenia using a hybrid pharmacodynamic/machine learning model. CPT Pharmacometrics Syst Pharmacol 12(11): 1764-1776.
- Pizarro GA, Peyrony O, Chumbita M, Gallo PM, Aiello TF, et al. (2024) Improving management of febrile neutropenia in oncology patients: the role of artificial intelligence and machine learning. Expert Rev Anti Infect Ther 22(4): 179-187.
- Choo H, Yoo SY, Moon S, Park M, Lee J, et al. (2023) Deep-learning-based personalized prediction of absolute neutrophil count recovery and comparison with clinicians for validation. J Biomed Inform 137.
- Sheehy J, Gallanagh M, Sullivan C, Lane S (2025) Clinical prediction models for febrile neutropenia and its outcomes: a systematic review. Support Care Cancer 33(7): 537.
- Salas M, Petracek J, Yalamanchili P, Aimer O, Kasthuril D, et al. (2022) The Use of Artificial Intelligence in Pharmacovigilance: A Systematic Review of the Literature. Pharmaceut Med 36(5): 295-306.
- Islam F, Men X, Yoshida K, Zai CC, Müller DJ (2021) Pharmacogenetics-Guided Advances in Antipsychotic Treatment. Clin Pharmacol Ther 110(3): 582-588.
- De With SAJ, Pulit SL, Staal WG, Kahn RS, Ophoff RA (2017) More than 25 years of genetic studies of clozapine-induced agranulocytosis. Pharmacogenomics J 17(4): 304-311.
- Arndt PA, Leger RM (2014) Introduction to immunohematology special edition on drug-induced immune cytopenias. Immunohematology 30(2): 43.
- Curtis BR (2017) Non-chemotherapy drug-induced neutropenia: key points to manage the challenges. Hematology Am Soc Hematol Educ Program 2017(1): 187-193.
- Bhatt V, Saleem A (2004) Drug-induced neutropenia--pathophysiology, clinical features, and management. Ann Clin Lab Sci 34(2): 131-137.
- Matzner Y (1997) Neutrophil pathophysiology. Semin Hematol 34: 265-266.
- Sun C, Zhao L, Yuan Y, Xiang Y, Liu A (2023) Detection of drug safety signal of drug-induced neutropenia and agranulocytosis in all-aged patients using electronic medical records. Pharmacoepidemiol Drug Saf 32(4): 416-425.
- Casique MN, Tong HY, Borobia AM, Carcas AJ, Frías J, et al. (2016) Nonchemotherapy drug-induced agranulocytosis in children detected by a prospective pharmacovigilance program. Pediatr Hematol Oncol 33(7-8): 441-456.
- Nystazaki M, Alevizopoulos G (2021) Clozapine treatment: Ensuring ongoing monitoring during the COVID-19 pandemic. Psychiatriki 32(2): 165-166.
- Coppo P, Corre E, Rondeau E, Benhamou Y, Bachet A, et al. (2016) Centre de référence des microangiopathies thrombotiques. Telemedicine in thrombotic microangiopathies: A way forward in rare diseases requiring emergency care. Rev Med Interne 37: 514-520.
- Steig A, Miller F, Shreim S, Wilcox J, Sykes C, et al. (2024) Remote management of anaemia in patients with end-stage kidney disease using a wearable, non-invasive sensor. Clin Kidney J 18(1).
- Issom DZ, Henriksen A, Woldaregay AZ, Rochat J, Lovis C, et al. (2020) Factors Influencing Motivation and Engagement in Mobile Health Among Patients With Sickle Cell Disease in Low-Prevalence, High-Income Countries: Qualitative Exploration of Patient Requirements. JMIR Hum Factors 7(1).
- Rothberg GBE, Quest TE, Yeung SJ, Pelosof LC, Gerber DE, et al. (2022) Oncologic emergencies and urgencies: A comprehensive review. CA Cancer J Clin 72(6): 570-593.
- Haeusler GM, Gaynor L, The B, Babl FE, Orme LM, et al. (2021) Home-based care of low-risk febrile neutropenia in children-an implementation study in a tertiary pediatric hospital. Support Care Cancer 29(3): 1609-1617.
- Brands MR, Gouw SC, Beestrum M, Cronin RM, Fijnvandraat K, et al. (2022) Patient-Centered Digital Health Records and Their Effects on Health Outcomes: Systematic Review. J Med Internet Res 24(12).
- May JE, Irelan PC, Boedeker K, Cahill E, Fein S, et al. (2020) Systems-based hematology: highlighting successes and next steps. Blood Adv 4(18): 4574-4583.
- Tesi B, Boileau C, Boycott KM, Canaud G, Caulfield M, et al. (2023) Precision medicine in rare diseases: What is next? J Intern Med 294(4): 397-412.
- Sumner M, Ray W, Fiedler S, Nguyen T, Rethemeyer RK (2019) A Mixed-Method Study of Practitioners' Perspectives on Issues Related to EHR Medication Reconciliation at a Health System. Qual Manag Health Care 28(2): 84-95.
- Marien S, Krug B, Spinewine A (2017) Electronic tools to support medication reconciliation: a systematic review. J Am Med Inform Assoc 24(1): 227-240.
- Fraczkowski D, Matson J, Lopez KD (2020) Nurse workarounds in the electronic health record: An integrative review. J Am Med Inform Assoc 27(7): 1149-1165.
- Mettler C, Daguzan A, Lagouanelle MC, Briantais A, Ducros P, et al. (2021) Expectation of patients and caregivers about patient education for immune thrombocytopenia. Rev Med Interne 42(1): 3-10.
- Grover S, Chaurasiya N, Chakrabarti S (2023) Clinician Reasons for Stopping Clozapine: A Retrospective Cohort Study. J Clin Psychopharmacol 43(5): 403-406.
- Aguado JM, Cruz JJ, Virizuela JA, Aguilar M, Carmona A, et al. (2017) Management of Infection and Febrile Neutropenia in Patients with Solid Cancer. Enferm Infecc Microbiol Clin 35(7): 451-460.
- Mattsson OT, Lindhart CL, Schöley J, Hansen FL, Herrstedt J (2020) Patient self-testing of white blood cell count and differentiation: A study of feasibility and measurement performance in a population of Danish cancer patients. Eur J Cancer Care (Engl) 29.
- Mak WC, Ching YSS (2015) Effect of an education program on knowledge, self-care behavior and handwashing competence on prevention of febrile neutropenia among breast cancer patients receiving Doxorubicin and Cyclophosphamide in Chemotherapy Day Centre. Asia Pac J Oncol Nurs 2(4): 276-288.
- Huebner H, Wurmthaler LA, Goossens C, Ernst M, Mocker A, et al. (2025) A Digital Home-Based Health Care Center for Remote Monitoring of Side Effects During Breast Cancer Therapy: Prospective, Single-Arm, Monocentric Feasibility Study. JMIR Cancer 11.
- Takenaka S, Moro H, Shimizu U, Koizumi T, Nagano K, et al. (2023) Preparing of Point-of-Care Reagents for Risk Assessment in the Elderly at Home by a Home-Visit Nurse and Verification of Their Analytical Accuracy. Diagnostics (Basel), 13(14): 2407.
- Garbe E (2007) Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf 6(3): 323-335.
- He Y, Li J, Zheng J, Khan Z, Qiang W, et al. (2017) Emphasis on the early diagnosis of antithyroid drug-induced agranulocytosis: retrospective analysis over 16 years at one Chinese center. J Endocrinol Invest 40(7): 733-740.
- Assimacopoulos A, Alam R, Arbo M, Nazir J, Chen DG, et al. (2008) A brief retrospective review of medical records comparing outcomes for inpatients treated via telehealth versus in-person protocols: is telehealth equally effective as in-person visits for treating neutropenic fever, bacterial pneumonia, and infected bacterial wounds? Telemed J E Health 14(8): 762-768.
- Pizarro GA, Peyrony O, Chumbita M, Gallo MP, Aiello TF, et al. (2024) Improving management of febrile neutropenia in oncology patients: the role of artificial intelligence and machine learning. Expert Rev Anti Infect Ther 22(4): 179-187.
- Mithoowani S, Cameron L, Crowther MA (2022) Neutropenia. CMAJ 194(14).
- Casique MN, Tong HY, Borobia AM, Carcas AJ, Frías J, et al. (2016) Nonchemotherapy drug-induced agranulocytosis in children detected by a prospective pharmacovigilance program. Pediatr Hematol Oncol 33(7-8): 441-456.
- Cuevas DLC, Sanz EJ, De Leon J (2024) Pharmacovigilance in Action: Utilizing VigiBase Data to Improve Clozapine Safety. Patient Prefer Adherence 18: 2261-2280.
- Mijovic A, MacCabe JH (2020) Clozapine-induced agranulocytosis. Ann Hematol 99(11): 2477-2482.
- Njue L, Porret N, Kaufmann SAS, Varra LF, Andres M, et al. (2024) Isolated Severe Neutropenia in Adults, Evaluation of Underlying Causes and Outcomes, Real-World Data Collected over a 5-Year Period in a Tertiary Referral Hospital. Medicina (Kaunas)., 60(10): 1576.
- Wu S, Huang L, Chen J, Xie X, Huang S, et al. (2025) Non-chemotherapy drugs inducing agranulocytosis: a disproportionality analysis based on the FAERS database. Front Pharmacol 16.






























