Cognitive Impairment and Related Factors Among Non-Diabetic Patients Over 60 Years in a Tertiary Hospital in Lagos, Nigeria
Ogbonna Adaobi Nneamaka1*, Akujuobi Obianuju Mirian2 and Ayonote Uzoramaka Angela1
1Department of Family Medicine, Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria
2Department of Counselling Psychology, Yorkville University, New Brunswick, Canada
Submission: July 14, 2022; Published: August 16, 2022
*Corresponding author: Ogbonna Adaobi Nneamaka, Department of Family Medicine, Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria
How to cite this article: Ogbonna A N, Akujuobi O M, Ayonote U A. Cognitive Impairment and Related Factors Among Non-Diabetic Patients Over 60 Years in a Tertiary Hospital in Lagos, Nigeria. OAJ Gerontol & Geriatric Med. 2022; 6(5): 555700. DOI: 10.19080/OAJGGM.2022.06.555700
Abstract
Background
Globally, there is an increase in the elderly population, and cognitive impairment, a common geriatric syndrome is expected to increase. This study sought to determine the prevalence and identify factors associated with cognitive impairment among non-diabetic elderly patients receiving care in Lagos University Teaching Hospital (LUTH) Lagos.
Method
Using a descriptive cross-sectional study design, 126 randomly selected non-diabetic elderly patients were investigated using a pre-tested, semi-structured, interviewer-administered questionnaire. Data included sociodemographic information, medical and family history, physical activity, social habits, and cognitive function which was assessed using the 6-item cognitive impairment test. Scores of 0-7 from the 6-CIT indicated normal cognition; 8-14 Mild Cognitive Impairment (MCI) and 10-28 was Significant Cognitive Impairment (SCI). The participants with MCI and SCI were combined to get the prevalence of impaired cognitive function. The relationship between cognitive impairment and study variables were explored using Chi-square test and binary logistic regression analysis. Level of significance was set at p<0.05.
Results
Of the 126 participants, about two-thirds (64.3%) were women and one-third (35.7%) men. The mean age was 65.6±5.4years. Majority were married (67.5%), Christians (70.6%) with post-primary educational attainment (67.4%). The prevalence of impaired cognition was 28.6% (MCI -13.5%; SCI - 15.1%). A positive family history of cognitive impairment (aOR-3.82; p-0.028) and level of education (No formal education: aOR=6.31;p-0.017; primary education:aOR-3.01; p-0.031) significantly increased the odds of cognitive impairment.
Conclusion
The prevalence of cognitive impairment is high among the elderly in this locality. Early screening for cognitive function in elderly and mental exercise which can aid cognitive stimulation may be helpful in halting or slowing cognitive decline.
Keywords: Cognitive impairment; Non-diabetic; Elderly people; Nigeria; Cognitive aging; Dementia; Degenerative diseases; Depression; Caregiver distress; Neuropsychiatric illness
Introduction
Cognitive impairment is a gerontological syndrome and one of the most common health problems in the elderly [1]. The United Nations defines an elderly person as an individual aged 60 years and above [2]. The elderly population is on a steady increase globally with 962 million older persons in 2017 which is more than twice of what it was in 1980 [2]. By 2050, it is expected to double again, when the elderly population is projected to reach nearly 2.1 billion [2]. Africa is expected to witness the fastest upsurge in the coming decades, where the elderly population is projected to increase by more than threefold between 2017 and 2050 from 69 to 226 million [2]. In Nigeria, the elderly make up about 4.3% of the population according to the 2006 population census [3]. The prevalence of chronic diseases in the elderly is on the increase due to population aging [4]. This imminent population transition poses the challenge of effective healthcare delivery to elderly people [5].
Cognitive functioning refers to multiple mental abilities including learning, thinking, reasoning, remembering, problem solving, decision making and attention [6]. It is typically classified into five domains which are learning and memory, language, visuospatial, executive and psychomotor [7]. Changes that occur in cognitive domains have been found to have a close relationship with physiological and social aspects of life [8]. Successful cognitive aging has been associated with preservation of the hippocampal function combined with a high responsiveness in the frontal area [8]. Neuropsychological tests have found a reduction in cerebral electrical power in cortical areas such as the frontal, parietal and occipital lobes causing a decline in sensory aspects, processing, motor performance and some cognitive functions.8 Gradual reduction in cerebral blood flow and gray matter volume mainly in the prefrontal cortex and temporal convolutions of the putamen and occipital regions have also been implicated as an explanation for normal age-related cognitive decline [8-9]. The social aspects that have been described include education, social participation, lifestyle, overall health status and genetic factors [8,10]. Women are also said to be less vulnerable to age related brain changes than men [11]. Being female is therefore considered a modulating factor in cognitive aging [11].
Cognitive impairment is one of the non-communicable diseases associated with aging [12]. It is characterized by global and irreversible cognitive decline [13-14]. Mild Cognitive Impairment (MCI) is defined as a syndrome of cognitive complaints, measurable mild declines in cognition but no change in functional abilities, including instrumental activities of daily living [15]. Mild cognitive impairment can involve one or more cognitive domains which include learning and memory, visuospatial, language, executive and psychomotor domains [15]. In mild cognitive impairment, daily function and independence is not undermined whereas in significant cognitive impairment also known as dementia, there is significant impairment of daily function and independence [13-15]. Dementia is heralded by MCI in about a third of cases [13].
Disability and dependency result most times from dementia in the elderly [13-14]. Dementia is established when cognitive impairment has become severe enough to compromise social and/ or occupational functioning [15-16]. It has a significant impact not only on individuals but also on their caregivers, families, communities and societies [17-18]. This enormous impact on the individuals and societies, makes all effort at preventing the onset of cognitive impairment and its progression of immense benefits to public health and the society at large [17-18].
The prevalence of dementia increases with age and is therefore expected to rise with the aging of populations worldwide [19]. Cognitive decline progresses slowly from normal cognitive function through mild cognitive impairment to dementia. A systematic review conducted,. revealed that the prevalence of cognitive impairment in people aged 60 and older in indigenous populations varied between 4.4% and 17.7% while that of dementia varied between 0.5% and 26.8% [20]. A study done by Prince et al. [21] in 2010 estimated 35.6million people worldwide living with dementia [21].
Few studies to determine the prevalence of cognitive impairment and dementia have been conducted in Sub-Saharan Africa (SSA) [13,22-23]. However, it is expected that the impact of population ageing in sub-Saharan Africa will progressively augment the burden of cognitive impairment and degenerative diseases in this region [13]. In 2010, 57.7% of all people with dementia lived in Low- and Middle-Income Countries (LMIC), projected to rise to 63.4% in 2030 and 70.5% in 2050 [21]. The health and social burden of cognitive impairment and dementia will therefore rise dramatically in these regions [13]. The prevalence of cognitive impairment ranged from 6.3% in Nigeria to 25% in the Central African Republic while that for dementia was up to 10.1% in Nigeria as reported in a systematic review done by Mavrodaris et al. [13] Okokon et al. [24] found the prevalence of cognitive impairment to be 49.1% in south-south Nigeria [13,24]. According to Ogunniyi et al. [23] the age-adjusted prevalence estimates were 18.4% and 2.9% for mild cognitive impairment and dementia respectively [23]. Mild cognitive impairment occupies the intermediate stage in the continuum of cognition and is considered as the leading edge for preventive strategies [23]. Knowledge of the burden of MCI just like dementia is therefore important. The strain imposed by MCI and dementia on caregivers is quite considerable with loss of income and psychosocial stress as well as significant social and economic implications in terms of direct medical and social care costs and the costs of informal care [23,25]. The total global societal cost of dementia was estimated to be US $818 billion in 2015 which is equivalent to 1.1% of global Gross Domestic Product (GDP) [25].
The recognition of cognitive impairment as a public health priority has led to the development of several tools for assessment of cognitive function in the elderly over the years [26-27]. These tools include the Mini Mental State Examination (MMSE), Mini Cog, Six item Cognitive Impairment Test (6-CIT), Montreal Cognitive Assessment test (MoCA) amongst others [27-32]. Most of the tools are easy to use, with high sensitivity and specificity in identifying cognitive impairment. However, the 6-1tem CIT, is easy to use in the elderly population. It is also widely validated and used in Nigeria [15].
Factors that affect cognitive function can be modifiable or nonmodifiable factors. The non-modifiable risk factors include age, family history, female sex and genetic susceptibility genes such as the Apolipoprotein E ε4 allele [16,33-34]. Modifiable risk factors include current smoking, depression, mid-life hypertension, obesity, dyslipidemia, and diabetes mellitus [16-17,33-35]. It is expected that reduction of these risk factors will reduce the risk of cognitive decline [16]. However, years of formal education, cognitive training, Mediterranean diet, physical activity, moderate alcohol consumption and social engagement have been shown to be protective [4,15,33-34].
Risk factors for dementia in American and European countries have been well investigated, but little research has been carried out in West Africa where environmental, socio-economic, and modifiable risk factors may differ [36]. This is significant because majority of older adults in the world reside in developing countries and projections suggest that by 2040, 71% of the people with dementia in the world will reside in developing countries [37]. In Nigeria, as well as in many other African countries, cognitive impairment is often seen as a sign of aging and little is done to explore the potential treatment of such impairment.35 This leads to a missed opportunity for early intervention. In Africa, caregiving generally takes place within the family setting. Therefore, late recognition is associated with caregiver distress [35].
Since there is currently no cure for dementia, primary prevention through identification of modifiable risk factors as well as interventions that aim at preventing the progression of mild cognitive impairment to dementia should be encouraged [34]. This study was carried out to determine the cognitive function among non-diabetic elderly patients accessing care at outpatient clinics of Lagos University Teaching Hospital (LUTH) and to identify risk factors associated with cognitive impairment in this group of the elderly. We chose to study non-diabetic elderly patients since diabetic mellitus has been strongly linked to increased prevalence, early onset, and increased severity of cognitive impairment [38-40]. We posit that the risk factors among non-diabetic elderly would differ from their diabetic counterpart and should be investigated.
Methodology
Study Area
The study was carried out at the General Outpatient Clinic (GOPC) of the department of Family Medicine, Lagos University Teaching Hospital (LUTH) Lagos. Lagos is a metropolitan state located in South-West Nigeria with a population of about 21 million which makes it the largest city in Africa [41]. It is multi-ethnic and urban though the Yorubas constitute the major ethnic group [41]. The Lagos University Teaching Hospital was established in 1962 to train both undergraduate and postgraduate medical, dental and paramedical students while providing care in the major specialties of medicine to patients within and around Lagos as well as all over Nigeria. Currently, it has above 800 bed spaces for inpatient care and operates several outpatient clinics. The Department of Family Medicine provides health care services to undifferentiated cases while running 3 of the medical outpatient clinics in the hospital. These clinics include the General Outpatient Clinic, the National Health Insurance Scheme (NHIS) clinic and the LUTH Staff clinic. The clinics run every Monday to Friday between 8am to 4pm and runs a geriatric outpatient consultation every weekday. The GOPC serves as a primary care clinic within a tertiary hospital setting and is run by consultant Family Physicians, Resident Doctors in Family Medicine, various cadres of Medical Officers and House Officers.
Study Design
The study was a hospital-based, descriptive, cross-sectional study.
Study Population
The study population consisted of non-diabetic elderly patients aged 60 years and above attending the General Outpatient Clinic in LUTH within the period of study. The United Nations definition for elderly, persons aged 60 years and above, was adopted for the study [2].
Selection criteria
Consenting non-diabetic elderly patients aged 60 years and above were eligible to be recruited for the study. Exclusion criteria, included acutely ill patients, [42] elderly with history suggestive of secondary cause of cognitive impairment (recent head injury, thyroid disease, HIV, ongoing depression, psychoactive substance use and stroke within the past six months), history suggestive of neuropsychiatric illness, delirium and hearing impairment [17,43].
Sample Size Determination
Sample size for the study was calculated from the formula for estimating a single proportion in a population stated below: [44]
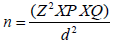
Where Z is the normal standard deviate at 95% confidence interval given as 1.96, P is the proportion of the disease or health event in the population of interest, which is impaired cognitive function in this study reported as 7.5% (0.075) by Tiwari et al. [40] while Q is the complementary proportion given as 1 - P, which is 92.5% (0.925) and d is the margin of error (level of precision) in this study set as 5% (0.05). After substitution and correcting for non-response with a non-response rate of 10%, a sample size of 117 was obtained. However, a total of 132 eligible participants were recruited meanwhile 126 participants completed the study
Sampling Technique
Participants were recruited from GOPC LUTH over a period of two months between January and February 2021. The GOPC had an average monthly attendance of 163 and therefore an average two monthly attendance of 326 (163x2) of non-diabetic patients aged 60 years and above. The sample interval was determined by dividing the study population by the total sample size (326/117= 2.78), which was approximately 3 in order to achieve the minimum sample size. Using systematic sampling method, a random start was done on each clinic day as follows: the first individual was chosen among the first three patients aged 60 years and above by simple random sampling using balloting method. Afterwards, in order of their arrival at the clinic, every third individual was recruited. The process was continued every clinic day until the minimum sample size was achieved.
Any individual that did not consent or meet the selection criteria to participate in the study was exempted and the next individual was recruited while maintaining the sample interval. After recruitment, the folders of the selected participants were tagged at the health information registration desk and sent to the designated consulting rooms for the study.
Study Instruments
A pre-tested, semi-structured, interviewer administered questionnaire was used for data collection. The study instrument was made up of five sections namely Section A which explored socio-demographic information like participant’s age, gender, level of education, occupation, average monthly income, marital status, religion, and tribe. Section B investigated the medical, medication and family history of participants. This was assessed by participants’ self-report while Section C examined the lifestyle characteristics, which included physical and social activity and tobacco use among participants. Section D was adopted from the 6-item cognitive impairment test which is a short and simple test of cognition with the capacity to assess cognitive impairment in the elderly within 2-3minutes. It consists of six questions designed to test concentration and memory using a reverse scoring technique [45]. It has a least score of zero and a maximum score of 28. Scores 0- 7 indicates normal cognition; 8- 9 suggests mild cognitive impairment and 10-28 is considered severe cognitive impairment [46]. The last section, Section E, recorded the clinical and laboratory measurements which include weight, height, Body Mass Index (BMI), blood pressure, fasting blood glucose and HbA1c levels.
The study instrument was pretested by administering it on 25 elderly patients in the GOP clinic of Lagos State University Teaching Hospital (LASUTH) another tertiary health facility in Lagos state. This was done to check the applicability of the questionnaire in a similar tertiary hospital setting and to confirm the clarity of the questionnaire.
Data collection
Two newly qualified, post-internship medical doctors were trained by the principal investigator for two consecutive days to help with data collection, before the commencement of the study. They were selected based on their background medical knowledge, their venepuncture skills and their proficiency in English and Yoruba languages. They were trained on the process of interview which included greeting, establishing a rapport, introduction, assuring confidentiality, explaining the data collection process, taking informed consent, ensuring a comfortable and private environment, and administering the questionnaire. They were also trained on brief counselling techniques, and how to give the participants feedback on their test results. On each research day, after introduction and explanation of the purpose of the study, informed consent was obtained from each respondent in a private consulting room. Participants were assured of confidentiality and interviewed using the semi-structured study questionnaire. Thereafter, participants’ weight, height, and blood pressure were measured. Weight of the participants was measured using Seca® weighing scale while height was measured with the Seca® stadiometer following strict measurement protocols [47]. Blood pressure was measured with Accoson® mercury sphygmomanometer and Littman’s stethoscope after explanation of the procedure to the participant. Sufficient time of five to ten minutes was allowed for rest.
Blood pressure was measured using an appropriately sized cuff in the sitting position with participant’s back supported and the first (K1) and fifth (K5) phases of Korotkoff sounds were taken as indicative of the systolic and diastolic blood pressures respectively according to WHO specifications to the nearest 2mmHg. The mean of two blood pressure measurements taken after a three-minute interval was recorded as the blood pressure to ensure accuracy.
Fasting blood glucose of the participants was measured after an overnight fast of 8-10 hours using capillary blood loaded on an Accu-check Active® glucometer which estimates the blood glucose using the hexokinase method. A fasting blood sugar of ≥126mg/ dl was indicative of DM and this excluded such participants from the study [48]. HbA1c of all participants was measured with the Infopia Clover A1c analyzer® using 1-2ml of venous blood [49] collected following standard WHO guidelines on venipuncture [50]. Results of the HbA1c were available within 15-20 minutes of sample collection and a HbA1c of ≥ 6.5% was considered elevated for the non-diabetic participants [51-52].
Data Analysis and Management
Each filled questionnaire was cross-checked for completeness by the principal investigator after every interview. Data entry and analysis was performed using Statistical Package for Social Sciences (SPSS) version 26. Frequencies and proportions summarized the socio-demographic characteristics, risk factors and clinical features in participants. Continuous variables were summarized by the mean and standard deviation. Monthly income was categorized using the national poverty line in Nigeria which is placed at 137,430 naira per capita per year [53]. This brings the poverty line to N11,456 (approximately N11,500) per month. Participants living below the poverty line (≤ 11,500 naira monthly) were considered poor and those living above the poverty line were classified as non-poor. Body Mass Index was calculated by dividing the weight (kg) of each participant by the square of his/ her height (m2) [54]. Body mass index was thereafter classified as follows: underweight < 18.5 kg/m2; normal range 18.5- 24.9kg/ m2; overweight 25.0 to 29.9kg/m2 and obese ≥ 30 kg/m2 [55].
The primary outcome variable (dependent variable) was the level of cognition in the elderly. Scores from the 6-item CIT indicated the level of cognition. The scores were categorized into three groups as normal cognition (0-7); mild cognitive impairment (8-14) and significant cognitive impairment (≥ 15) [32]. To assess the association between cognitive function and the independent variables, cognitive function was dichotomized as impaired cognitive function (which comprises of mild and significant cognitive impairment) and normal cognitive function. Using the Chi-square test of proportion, the relationship between the dichotomized cognition variable and risk factors for impaired cognitive function was explored. A binary logistic regression analysis was further carried out to investigate the strength of association and identified significant predictors of cognitive impairment in the study population. Univariate regression quantified the strength of association while the multivariate regression identified the significant predictors of cognitive impairment. The level of statistical significance was set at pValue <0.05.
Ethical Consideration
Ethical approval for the study was obtained from LUTH Health Research and Ethical Committee of Lagos University Teaching hospital. Written signed informed consent was obtained from each participant at the point of recruitment. Participants were assured of confidentiality on all information obtained from them and the questionnaires were safely and securely stored for research purpose only. The completed questionnaires were serially coded with numbers without any links to identifying the participants. The study posed no potential risk to the participants although very minimal pain and discomfort was felt during blood sample collection. The participants were informed of their right to participate and to withdraw their participation at any point in the study. They were assured that their refusal to participate or their withdrawal from the study will not compromise their care.
Results
Sociodemographic characteristics and anthropometric measurement of study participants
One hundred and twenty-six participants were selected and completed the study. Of the 126 participants, 81 (64.3%) were women and 45 (35.7%) were men. Slightly above half (53.6%) were aged between 60 – 64 years, while less than a tenth (5.6%) were 75 years and above (Table 1). Majority were married (67.5%) while about a quarter of study participants were widowed (27.0%). Most of the participants were Christians (70.6%) with post-primary educational attainment (67.4%).
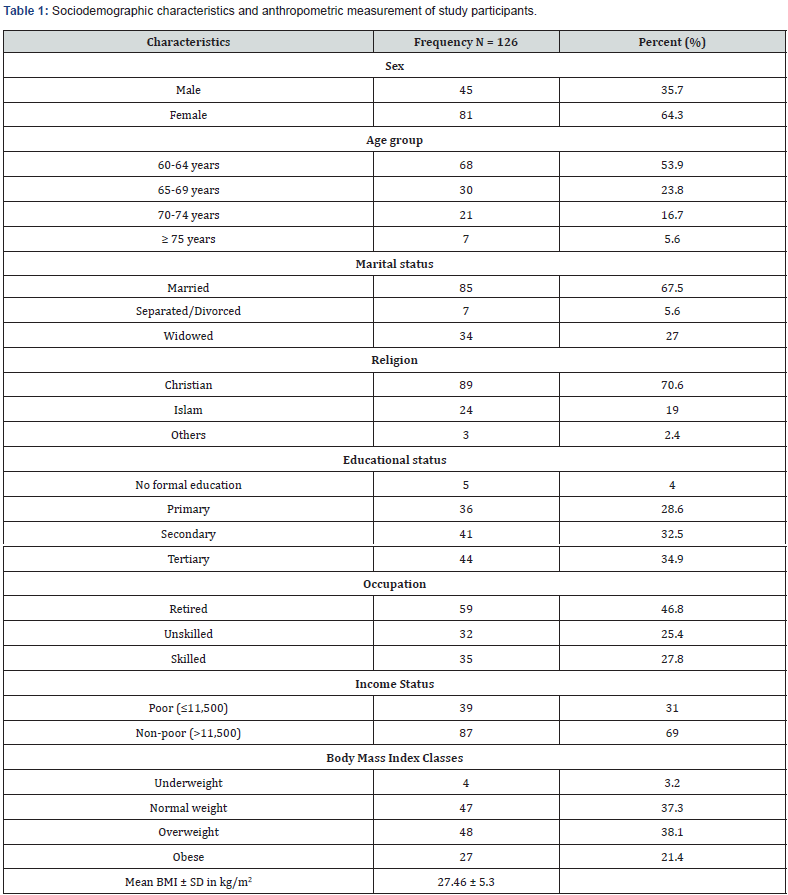
Table 1 also show that 59 participants (46.8%) were retired, while 87 participants were classified as non-poor considering their income status. Twenty-seven participants (21.4%), 47 (37.3%) and 48 (38.1%) were obese, normal weight and overweight, respectively. The mean body mass index was 27.5kg/ m2 with a standard deviation of 5.3kg/m2 (Table 1).
Co-morbid conditions and Lifestyle of study participants
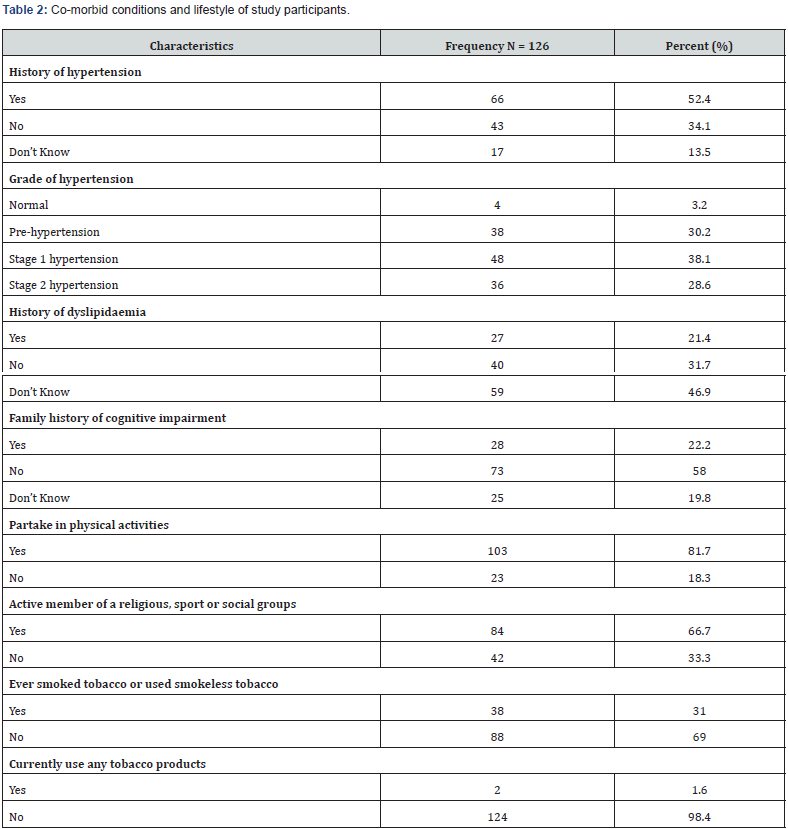
Table 2 shows that about half of the study participants (52.4%) were known hypertensive patients, while 17 participants do not know their blood pressure status (13.5%). From the categorization of blood pressure readings, 48 participants (38.1%) were categorized as Stage 1 hypertension. Other categories include prehypertension (30.2%), and Stage 2 hypertension (28.6%). About 1 in every 5 participant had history of dyslipidaemia (21.4%) and family history of cognitive impairment (22.2%). One hundred and three participants (81.7%) were still partaking in physical activities while 84 participants (66.7%) were active members of religious, sports and social groups. Only 2 participants were still current users of tobacco products as at the time of the study (Table 2).
Prevalence of Cognitive Impairment among participants in the study
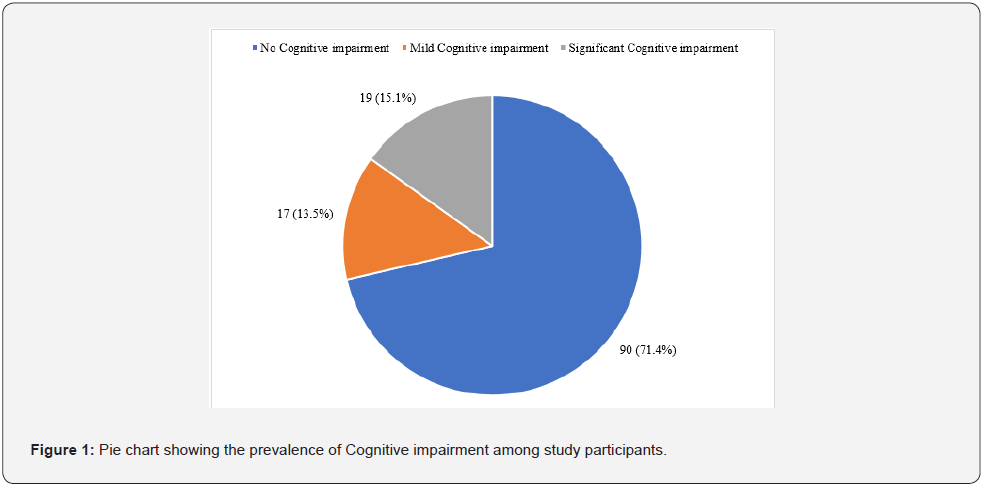
A total of 36 participants (28.6%) had different levels of cognitive impairment in the study. Seventeen participants (13.5%) had mild cognitive impairment, while 19 (15.1%) had significant cognitive impairment (Figure 1).
fig 1
Association between Cognitive Impairment and Sociodemographic characteristics, anthropometric measurement of study participants
Table 3 shows that there was a statistically significant association between level of education attained and cognitive impairment among the elderly (χ2 = 8.57; p- 0.036). The participants with no formal education (OR- 9.00; p- 0.045) and those with only primary education (OR- 3.38; p- 0.013) were 9 times and 3 times, respectively, more likely to develop cognitive impairment when compared to their counterpart with tertiary education. The association between cognitive impairment and the other socio-demographic variables like gender, age, marital status, and occupation was not statistically significant (p>0.05). The socioeconomic variable, income status (χ2 = 0.12; p- 0.738) and anthropometric categories (χ2 = 5.75; p- 0.124) were also not significantly associated with the development of cognitive impairment (Table 3).
Association Between Cognitive Impairment and Medical Characteristics, Lifestyle Characteristics, Clinical Characteristics among Participant
A positive family history of cognitive impairment was significantly associated with developing cognitive impairment (χ2 = 12.16; p- 0.002). As presented in Table 4, the odd of developing cognitive impairment was 4 times higher among participants with positive family history of cognitive impairment (OR-4.05; p-0.028). The presence (χ2 = 0.82; p-0.968) and grade of hypertension χ2 = 1.70; p-0.637) were not significantly associated with the development of cognitive impairment in the study population (Table 4). History of dyslipidaemia, partaking in physical and social activities, and smoking as a current or past habit were not significantly associated (p > 0.05) with the development of cognitive impairment among study participants (Table 4).Predictors of Cognitive impairment among nondiabetic elderly patients
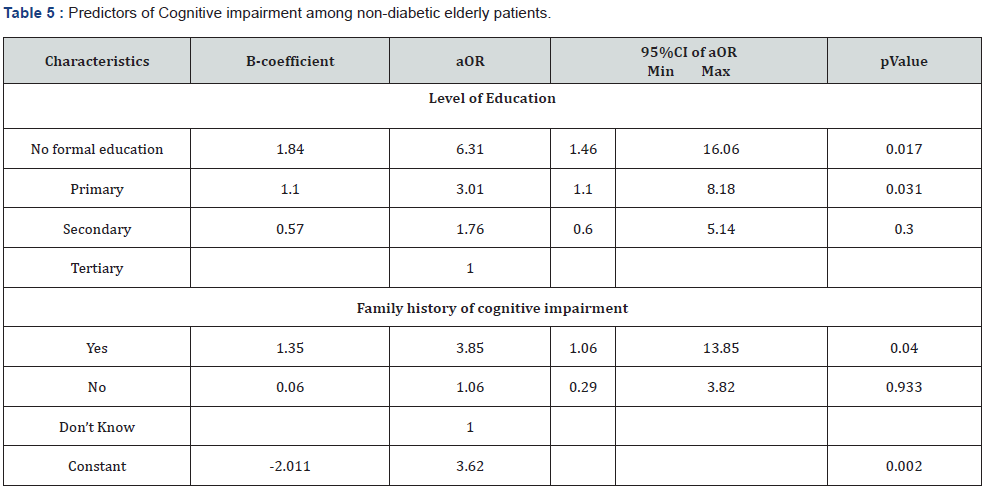
Two factors, level of education and family history of cognitive impairment, were identified in this study as independent predictors of impaired cognitive function among elderly nondiabetic participants in the study (Table 5). With reducing level of educational attainment, the odd of impaired cognitive function increases significantly (p<0.05). However, the increased likelihood of impaired cognitive function among participants with secondary education (OR- 1.76; p- 0.300) was not significant statistically (Table 5).
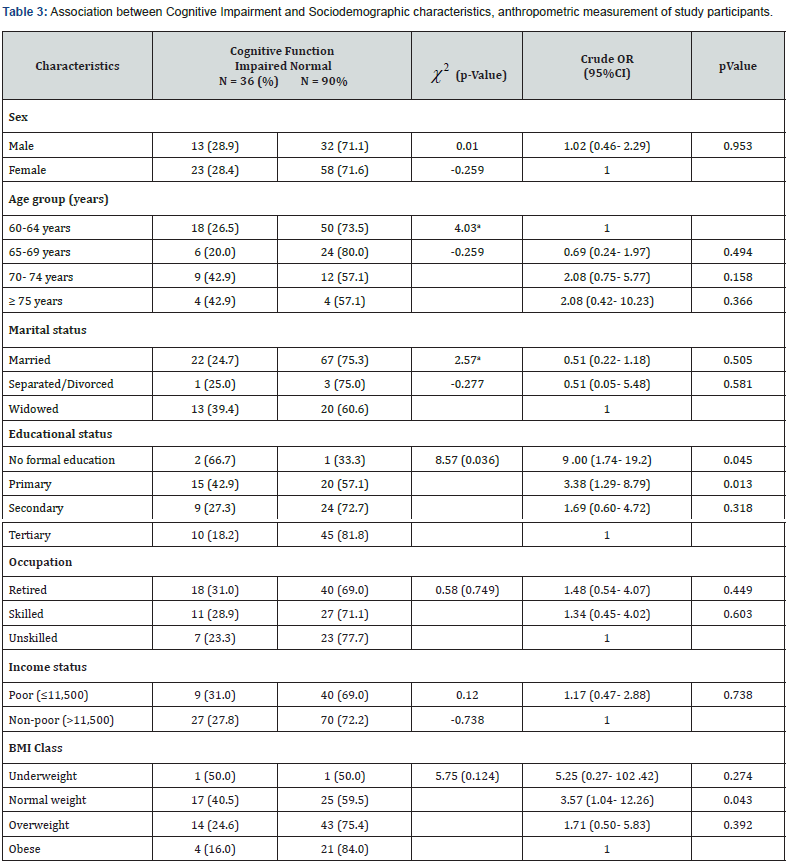
χ2 – Chi square; *Statistically significant; aFisher’s exact test; OR- Odd ratio; CI - Confidence interval
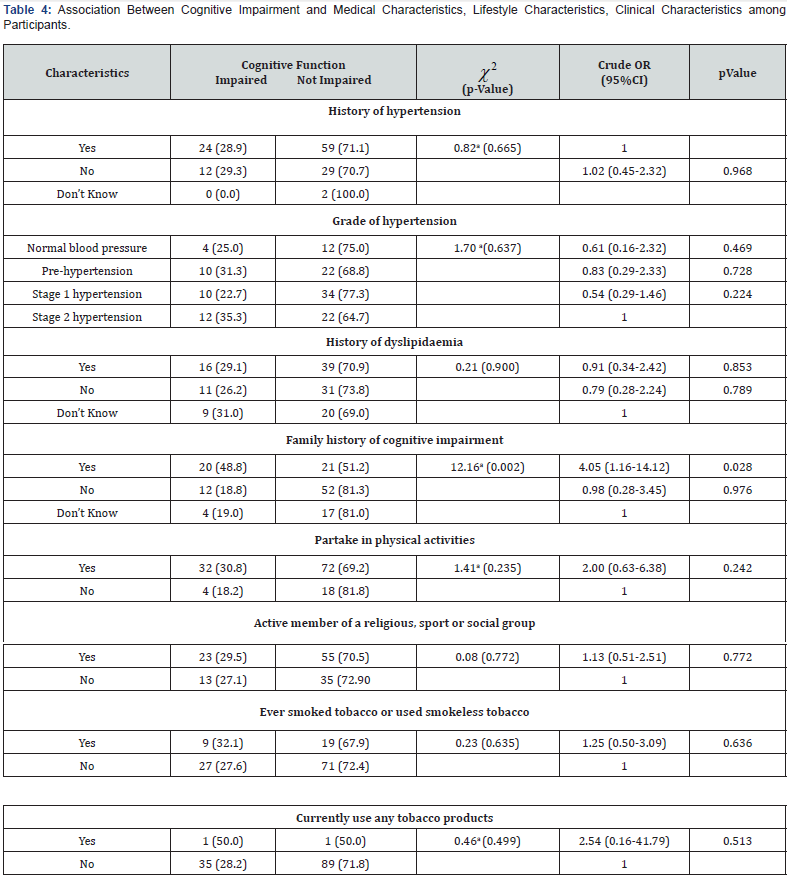
χ2 – Chi square; *Statistically significant; aFisher’s exact test; OR – Odd ratio; CI – Confidence interval
Discussion
This study was conceptualized to determine the prevalence of cognitive impairment among non-diabetic elderly population and to uncover associated factors associated with cognitive impairment in this population. The prevalence of cognitive impairment was 28.6% and it was significantly associated with lower schooling years (low level of education and a positive family history of cognitive impairment in the study population.
The prevalence of 28.6% in this study is like findings by Tianyi et al [56] in Cameroun who reported 33.3% [56]. Baye et al [57] obtained 31.5% from a study in Ethiopia [57] and in rural area of central Africa republic found a prevalence of 25.0% [58]. Similar findings were also seen outside the African continent; in Korea, Choi et al [59] reported 32.91% while Boyle and his colleague found 28.1% in the USA [60]. This similarity was observed despite the difference in the study tools used in these studies. Most studies referenced above used the Mini Mental State Examination (MMSE) in their assessment of cognitive function [56,58-59]. while the index study used the 6-item cognitive impairment test (6-item CIT). The 6-item CIT is a brief and easily administered test designed for use in primary care settings. It consists of six questions which tests memory, orientation, and concentration [32]. It can easily be adapted linguistically and culturally and take 3-5 minutes to perform. The MMSE on the other hand is made of 5 domains namely orientation, registration, attention and calculation, recall and language [61]. The MMSE is particularly affected by the level of educational attainment of the respondents [56].
However, this disadvantage can be overcome when educationspecific cut-off values are used to set thresholds for categorization while using the tool [60]. Education-specific cut-off values are known to improve the sensitivity and specificity of the tool while screening for cognitive impairment. Different scholars like Crum et al. [62] and Kochhann et al. [63] have developed recommended cut-off scores for different duration of schooling [60,62-63]. The application of education-specific cut-off in the rural setting of Cameroun where formal education is low, [60] could explain the similarity between the findings in this study carried out in an urban setting where educational level was expectedly high. This suggests that tools in the assessment of cognitive function require further research and modification for continuous improvement in their diagnostic properties.
There were other studies that found even higher prevalence of impaired cognitive function than what was reported in this study, Yarube et al. [64] conducted a similar study in northern Nigeria [64] and reported a prevalence of 64.0% among nondiabetic participants and Artero et al. [65] reported 42.0% in France [65]. While unlike this study, some works like Uwakwe et al. [66] and Guerchet et al. [67] study in the rural areas of Nigeria and Benin reported 10.4% and 11.8%, respectively [66-67]. Different sociodemographic and socioeconomic characteristics in the different study settings could explain the disparities in the results. However, the findings from the index and all other studies indicated a high prevalence of cognitive impairment among the non-diabetic elderly. This implies that with an aging population globally, the occurrence of impaired cognitive function would increase with its attendance health manpower, health financing and psychosocial challenges. The increasing burden of cognitive impairment is associated with enormous social and economic consequences with respect to medical and social cost especially informal cost for regions like Africa where the care of the elderly is non-institutionalized. It is pertinent that our health systems begin to plan and prepare for this gradually rise in the burden of cognitive impairment and dementia. Furthermore, in this study almost half of the 28.6% who had cognitive impairment were classified as mild cognitive impairment. This phase shows a unique opportunity for intervention strategies to either halt or slow down the progression to significant cognitive impairment or dementia. Programmes instituting screening for early recognition of mild cognitive changes in the elderly would be vital to the success of this intervention strategies.
Level of education and family history of cognitive impairment were the only factors identified as significant predictors of declining cognitive function among the study population in this locality. The odds of impaired cognitive function were 6 times higher in participants with no formal education and 3 times more among participants with a family history of cognitive impairment. This significant association between educational level and cognitive impairment in the study participants implies that the lower the educational level (less years of schooling), the higher the risk of cognitive impairment in non-diabetic individuals. This can be likened to findings by Yusuf et al. [43] in the Northwest, Okokon et al. [24] in the south-south and Oghagbon et al. [68] from north-central zone of the Nigeria which showed that low education was significantly associated with dementia [43]. Several other studies within and outside Africa also corroborate this finding linking low level of education with impaired cognitive function [69-73].
The possible explanation linking high level of education with lower risk of cognitive impairment is that individuals with higher education readily maintain or increase cognitive functional development through frequent cognitive stimulation.8 Scholars have also postulated that higher childhood educational levels and lifelong higher educational attainment are protective against cognitive impairment and dementia [74-75]. This suggests that cognitive stimulation is more important in early life and much of the apparent later effect might be due to people of higher cognitive function seeking out cognitively stimulating activities and education [75]. Factors such as fetal and infant poor nutrition with consequent poor brain development and less mental stimulation in childhood accounts for cognitive impairment in later life among persons with low level of education [27].
As regards family history of cognitive impairment, this study found that family history of cognitive impairment is a strong predictor of cognitive impairment in the elderly in this setting. This is in line with a longitudinal study by Jia et al. [71] which revealed a statistically significant association between parental history of dementia and cognitive impairment [71]. Another study conducted among African Americans by Wessel et al. [76] also found a significant association between family history of dementia and cognitive impairment [76]. Although, unlike our result, Ramlall et al. [77] found no relationship between family history of dementia and cognitive impairment [77]. Literature has shown that family history of dementia indicates a genetic predisposition to the disease and the APOE*4 is a risk factor for AD [8,71]. However, the role of APOE*4 has been controversial as it is reported as having a protective effect in some cases [78]. The study showed that there was no statistically significant association between age and cognitive function in the study participants even though the prevalence of cognitive impairment was more with increasing age. A study by Ucheagwu et al. [79] carried out in South-east Nigeria revealed similar findings as age was not statistically significantly associated with cognitive impairment [79]. The inability of both studies to demonstrate a statistically significant relationship between age and cognitive impairment, despite an obvious increase in the proportion of cognitive impairment as age increases may not be unconnected to the sample sizes in both studies. Several previous studies have however reported a significant association between age and cognitive impairment [23,56,70-71]. Increasing age has been penciled as the strongest risk factor for impaired cognitive function, which is almost always present after the eight decade of life [16,23,72,74]. As people age, brain tissue volume decreases, white brain matter hyperintensities increase, and related deficits are seen in working memory, attention, and executive function [80]. With increasing age chronic medical condition like hypertension and diabetes are also more common and they are identified as risk factors for impaired cognitive function [81].
The relationship between sex and cognitive impairment has been reported with mixed findings. This study found no statistically significant association between sex and cognitive impairment among the study population. This is comparable to a South-African study conducted by Ramlall et al. [77] and an Indian study conducted by Mohan et al. [82] which showed that there was no association between sex and cognitive impairment [82]. However, studies by Okokon et al. [24] and Ogunniyi et al. [23] in southern Nigeria, Yusuf et al. [43] in north-west Nigeria Tianyi et al. [56] in Cameroon, Guerchet et al. [58] in Central African Republic and Ren et al. [1] in China found a significant association between sex and cognitive impairment [23-24,43,56,58,1]. These studies reported that a higher chance of impaired cognition in elderly female participants exist. They posit that estrogens, progesterone, and testosterone may have neuroprotective effects, and decrease in these hormones (estrogen and progesterone) in postmenopausal women may be responsible for increased cognitive impairment while the presence of testosterone in elderly men reduces the chance of impaired cognitive function [83]. The effect of education with respect to sex and cognitive impairment cannot also be overlooked as women are not exposed to education as much as their male counterpart in early life, especially in African communities [84,24].
Marital status was not statistically associated with cognitive impairment in this study. This is in line with a study by Baye et al. [57] which revealed that there was no significant association between marital status and cognitive impairment [57]. Conversely, a study done in South-south Nigeria by Okokon et al. [24] and Tianyi et al. [56] in Cameroun found a significant association between marital status and cognitive impairment [24,56]. These studies revealed an increased risk of cognitive impairment among those with single or widowed status. Marital relationship exposes partners to social challenges that are believed to have protective effect against impaired cognitive function [56,85]. There was no statistically significant association between occupation and cognitive impairment among participants. Similarly, a cohort study by Pais et al. [70] found no significant association between employment status and cognitive impairment [70]. The finding in this study however differed from that of Okokon et al. [24] which showed that individuals who did not engage in any form of occupation were found to have increased likelihood of having cognitive impairment [24].
This study revealed that there was no significant association between history of hypertension or grade of hypertension and cognitive impairment in the non-diabetic elderly. In the same vein, studies by Baye et al. [54] and Paddick et al. [57] showed that there was no significant association between hypertension and cognitive impairment [54,57]. Conversely, a Cameroonian study by Tianyi et al. [56] and Ghanian study by Nutakor et al. [86] revealed that systolic hypertension was significantly and independently associated with cognitive impairment [56,86]. Nutakor et al. [86] further demonstrated association between hypertension and better cognitive functioning [86].
History of dyslipidemia among the elders in this study was not associated with declining cognitive function. This finding is in consonance with a Chinese study by Ren et al. [1] which revealed that dyslipidemia was not associated with cognitive impairment [1]. In contrast, another Chinese study by Jia et al. [71] found that dyslipidemia was significantly associated with cognitive impairment [71]. There was no significant association between body mass index and cognitive impairment in study participants. Similarly, cross-sectional studies by Adebiyi et al. [35] in Southwestern Nigeria and Tianyi et al. [56] in Cameroon showed no significant association between body mass index and cognitive impairment [35,56]. Conversely, a study conducted by Ucheagwu et al. [79] in South-east Nigeria concluded that body mass index was a significant predictor of cognition [79].
The index study did not find a significant relationship between physical activity and cognitive impairment in the study population. A cohort study amongst community dwelling elders by Gureje et al. [37] reported a similar finding while on the contrary, a Chinese study by Ren et al. [1] found that physical activity significantly lowered the risk of cognitive impairment in the elderly [37,1]. As regards to social activity, the study showed that there was no statistically significant association between social activity and cognitive impairment in the elderly. A cross-sectional study on prevalence and factors associated with mild cognitive impairment among an urban elderly population by Mohan et al. [82] revealed a similar finding [82]. However, in contrast to finding by the index study, Wang et al. [86] showed that social activity was protective against cognitive impairment [86]. The index study also showed that past or current smoking was not statistically related to cognitive impairment [87]. Similarly, in a Chinese study by Ren et al. [1] smoking was not significantly associated with cognitive impairment as there was no difference in the cognitive scores of past smokers, current smokers and in those who never smoked [1]. This finding was unlike a Malaysian study which showed a significant association between smoking status and cognitive impairment [88]. Interestingly, the Malaysian study reported lower rates of cognitive impairment among current smokers suggesting a protective effect of smoking among elderly with respect to declining cognitive function [88].
The index study is not without its limitations. First being a hospital-based study in a tertiary health facility, participants in the study were likely to be elderly who were well-to-do (as demonstrated by income status) and have developed good health seeking behaviour which may have impacted on their cognition, hence leading to an underestimation of the prevalence of cognitive impairment in this study. Cognitive impairment may be higher among the general population in the communities where these individuals live. The cross-sectional design also makes it difficult to study the progression in severity of cognitive impairment and its associated factors. Our sample size was also limited, hence despite demonstrating relationships between cognitive impairment and some variables (especially age), these relationships were not statistically significant.
Lastly, the self-reporting aspect of the participants’ response may have introduced a recall bias into the study. Despite these limitations, the study filled a knowledge gap of declining cognitive impairment among non-diabetic elderly. It demonstrates a high prevalence of impaired cognitive function even among the non-diabetic elderly and identified factors associated with its development. The study further shows the validity and reliability of the 6-item CIT tool in our locality. There is a need to incorporate routine cognitive assessment in the care of the elderly at all levels of care in the health system of Nigeria. Furthermore, there is need for community based, longitudinal and/or experimental studies investigating cognitive function among the elderly to gain a better understanding of its risk factors and help in designing and evolving an innovative, evidence based preventive strategies for cognitive impairment in our aging population.
References
- Ren L, Zheng Y, Wu L, Gu Y, He Y, et al. (2018) Investigation of the prevalence of Cognitive Impairment and its risk factors within the elderly population in Shanghai, China. Sci Rep 8(1): 3575-3583.
- UN Department of and Social Affairs. World Population Ageing 2017: Highlights 2017.
- Adebowale SA, Atte O, Ayeni O (2012) Elderly Well-being in a Rural Community in North Central Nigeria, Sub-Saharan Africa. Public Heal Res 2(4): 92-101.
- Kim EJ, Jung SW, Kim YE, Go DS, Yoon SJ (2018) Assessing the Impact of Aging on Burden of Disease. Iran J Public Health 47(Suppl 1): 33-38.
- Animasahaun VJ, Chapman H (2017) Psychosocial health challenges of the elderly in Nigeria: a narrative review. Afr Heal Sci 17(2): 575-583.
- Fisher GG, Chacon M, Chaffee DS (2020) Theories of cognitive aging and work. In: Work Across the Lifespan. Elsevier, p. 17-45.
- Knopman DS, Petersen RC (2014) Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc 89(10): 1452-1459.
- Mendoza RNM, Arias Merino ED, Flores Villavicencio ME, Rodríguez-Díaz M, Díaz-García IF (2021) Cognitive Aging. In: Gerontology. InTech; 2018.
- Kujawski S, Kujawska A, Gajos M, Topka W, Perkowski R, et al. (2018) Cognitive Functioning in Older People. Results of the First Wave of Cognition of Older People, Education, Recreational Activities, Nutrition, Comorbidities, Functional Capacity Studies (COPERNICUS). Front Aging Neurosci 10: 421-428.
- Kelly ME, Duff H, Kelly S, McHugh Power JE, Brennan S, et al. (2017) The impact ofsocial activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Syst Rev 6(1): 259-266.
- Rut C, Jose B, Antonieta N (2018) Age-Related Cognitive Changes: The Importance of Modulating Factors. J Geriatr Med Gerontol 4(2): 48-57.
- Jaul E, Barron J (2017) Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front public Health 5: 335-342.
- Mavrodaris A, Powell J, Thorogood M (2013) Prevalences of dementia and cognitive impairment among older people in sub-Saharan Africa: a systematic review. Bull World Health Organ 91(10): 773-783.
- Yusuf AJ, Baiyewu O, Sheikh TL, Shehu AU (2011) Prevalence of dementia and dementia subtypes among community-dwelling elderly people in northern Nigeria. Int psychogeriatrics 23(3): 379-386.
- Murman DL (2015) The Impact of Age on Cognition. Semin Hear 36(3): 111-121.
- Hugo J, Ganguli M (2014) Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 30(3): 421-442.
- World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines (2019).
- Rakesh G, Szabo ST, Alexopoulos GS, Zannas AS (2017) Strategies for dementia prevention: latest evidence and implications. Ther Adv Chronic Dis 8(9): 121-136.
- Arntzen KA, Schirmer H, Wilsgaard T, Mathiesen EB (2011) Impact of cardiovascular risk factors on cognitive function: The Tromsø Eur J Neurol 18(5): 737-743.
- de Souza-Talarico JN, de Carvalho AP, Brucki SMD, Nitrini R, Ferretti-Rebustini RE de. L (2016) Dementia and Cognitive Impairment Prevalence and Associated Factors in Indigenous Populations. Alzheimer Dis Assoc Disord 30(3): 281-287.
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, et al. (2013) The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 9(1): 63-75.
- Olayinka OO, Mbuyi NN (2014) Epidemiology of Dementia among the Elderly in Sub-Saharan Africa. Int J Alzheimers Dis 1-15.
- Ogunniyi A, Adebiyi AO, Adediran AB, Olakehinde OO, Siwoku AA (2016) Prevalence estimates of major neurocognitive disorders in a rural Nigerian community. Brain Behav 6(7): e00481.
- Okokon IB, Asibong UE, Ogbonna UK, John EE (2018) Psychogeriatric Problems Seen in the Primary Care Arm of a Tertiary Hospital in Calabar, South-South Nigeria. Int J Geriatr Gerontol 1-9.
- World Health Organisation. Dementia fact sheets (2019).
- Lekoubou A, Echouffo-Tcheugui JB, Kengne AP (2014) Epidemiology of neurodegenerative diseases in sub-Saharan Africa: a systematic review. BMC Public Health 14: 653.
- Arizaga RL, Gogorza RE, Allegri RF, Baumann PD, Morales MC, et al. (2014) Cognitive impairment and risk factor prevalence in a population over 60 in Argentina. Dement Neuropsychol 8(4): 364-370.
- Dinesh G, Gursimran K, Akriti G (2019) Geriatric syndromes 2016.
- Hayden KM, Reed BR, Manly JJ, Tommet D, Pietrzak RH, et al. (2011) Cognitive decline in the elderly: An analysis of population heterogeneity. Age Ageing 40(6): 684-689.
- Palsetia D, Rao G, Tiwari S, Lodha P, De Sousa A The clock drawing test versus mini-mental status examination as a screening tool for dementia: A clinical comparison. Indian J Psychol Med 40(1):1-10.
- Sheehan B (2012) Assessment scales in dementia. Ther Adv Neurol Disord 5(6): 349-358.
- Abdel-Aziz K, Larner AJ (2015) Six-item cognitive impairment test (6CIT): pragmatic diagnostic accuracy study for dementia and MCI. Int psychogeriatr 27(6): 991-997.
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, et al. (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11(6): 718-726.
- George-Carey R, Adeloye D, Chan KY, Paul A, Kolčić I, Campbell H, et al. (2012) An estimate of the prevalence of dementia in Africa: A systematic analysis. J Glob Health 2(2):020401.
- Adebiyi AO, Ogunniyi A, Adediran BA, Olakehinde OO, Siwoku AA (2015) Cognitive Impairment Among the Aging Population in a Community in Southwest Nigeria. Health Educ Behav 43(1 Suppl): 93S-9S.+
- Guerchet M, Mouanga AM, M’belesso P, Tabo A, Bandzouzi B, et al. (2012) Factors Associated with Dementia Among Elderly People Living in Two Cities in Central Africa: The EDAC Multicenter Study. J Alzheimers Dis 29(1): 15-24.
- Gureje O, Ogunniyi A, Kola L, Abiona T (2011) Incidence of and Risk Factors for Dementia in the Ibadan Study of Aging. J Am Geriatr Soc 59(5): 869-874.
- Saedi E, Gheini MR, Faiz F, Arami MA (2016) Diabetes mellitus and cognitive impairments. World J Diabetes 7(17): 412-422.
- Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. (2014) Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement 10(1): 18-26.
- Tiwari SC, Tripathi RK, Farooqi SA, Kumar R, Srivastava G, et al. (2012) Diabetes mellitus: A risk factor for cognitive impairment amongst urban older adults. Ind Psychiatry J 21(1): 44-48.
- Lagos Population 2020 (Demographics, Maps, Graphs). World Population Review. 2020. Available at: http://worldpopulationreview.com/world-cities/lagos-population/. Accessed on January 3rd
- Sakusic A, O’Horo JC, Dziadzko M, Volha D, Ali R, et al. (2018) Potentially Modifiable Risk Factors for Long-Term Cognitive Impairment After Critical Illness: A Systematic Review. Mayo Clin Proc 93(1): 68-82.
- Yusuf AJ, Baiyewu O, Bakari AG, Garko SB, Jibril ME-B, et al. (2018) Low education and lack of spousal relationship are associated with dementia in older adults with diabetes mellitus in Nigeria. Psychogeriatrics 18(3): 216-223.
- Charan J, Biswas T (2013) How to calculate sample size for different study designs in medical research. Indian J Psychol Med 35(2): 121-126.
- Ogunrin AO (2014) Effect of Vinpocetine (CognitolTM) on Cognitive Performances of a Nigerian Population. Ann Med Health Sci Res 4(4): 654-661.
- Six Item Cognitive Impairment Test ( 6CIT ) (2019).
- Nutrition Screening as as A guide to completing the Mini Nutritional Assessment ( MNA ®) (2020).
- World Health Organization: Definition and Diagnosis of Diabetes Mellitus And Intermediate Hyperglycemia (2019).
- Infopia Co. L. Clover A1c (HbA1c Measuring System). 2020.
- Best practices in phlebotomy - WHO Guidelines on Drawing Blood - NCBI Bookshelf (2021).
- Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. (2019) 42(Suppl 1): S13-S28.
- Chentli F, Azzoug S, Mahgoun S (2015) Diabetes mellitus in elderly. Indian J Endocrinol Metab 19(6): 744-752.
- Nigerian Bureau of Statistics. NBS 2019 (2018) Poverty and Inequality in Nigeria: Executive Summary. Natl Bur Stat p.1-26.
- Paddick SM, Kisoli A, Samuel M, Higginson J, Gray WK, et al. (2015) Mild cognitive impairment in rural Tanzania: Prevalence, profile, and outcomes at 4-year follow-up. Am J Geriatr Psychiatry 23(9): 950-959.
- World Health Organization. Global Database on Body Mass Index (2020).
- Tianyi FL, Agbor VN, Njamnshi AK, Atashili J (2019) Factors associated with the prevalence of cognitive impairment in a rural elderly cameroonian population: A community-based study in Sub-Saharan Africa. Dement Geriatr Cogn Disord 47(1-2): 104-113.
- Baye D, Amare DW, Andualem M (2017) Cognitive impairment among type 2 diabetes mellitus patients at Jimma University Specialized Hospital, Southwest Ethiopia. J Public Heal Epidemiol 9(11): 300-308.
- Guerchet M, Mouanga AM, M’belesso P, Tabo A, Bandzouzi B, et al. (2012) Factors associated with dementia among elderly people living in two cities in Central Africa: the EDAC multicenter study. J Alzheimers Dis 29(1): 15-24.
- Choi SJ, Jung SS, You YS, Shin BS, Kim JE, et al. (2008) Prevalence of Alzheimer’s dementia and its risk factors in community-dwelling elderly Koreans. Psychiatry Invest 5(2): 78-85.
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA (2006) Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology 67(3): 441-445.
- Palsetia D, Rao G, Tiwari S, Lodha P, De Sousa A (2018) The clock drawing test versus mini-mental status examination as a screening tool for dementia: A clinical comparison. Indian J Psychol Med 40(1): 1-10.
- Crum RM, Anthony JC, Bassett SS, Folstein MF (1993) Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 269(18): 2386-2391.
- Kochhann R, Varela JS, Lisboa CS, Chaves ML (2010) The Mini Mental State Examination: review of cutoff points adjusted for schooling in a large Southern Brazilian sample. Dement Neuropsychol 4(1): 35-41.
- Yarube IU, Mukhtar IG (2018) Impaired cognition and normal cardiometabolic parameters in patients with type 2 diabetes in Kano, Nigeria. Sub-Saharan Afr J Med 5(2): 37-44.
- Artero S, Ancelin ML, Portet F, Dupuy A, Berr C, et al. (2008) Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry 79(9): 979-984.
- Uwakwe R (2000) The pattern of psychiatric disorders among the aged in a selected community in Nigeria. Int J Geriatr Psychiatry 15(4): 355-62.
- Guerchet M, Houinato D, Paraiso MN, von Ahsen N, Nubukpo P, et al. (2009) Cognitive impairment and dementia in elderly people living in rural Benin, west Africa. Dement Geriatr Cogn Disord 27(1): 34-41.
- Oghagbon EK, Giménez-Llort L (2019) Short height and poor education increase the risk of dementia in Nigerian type 2 diabetic women. Alzheimers Dement 11: 493-499.
- Hale JM, Schneider DC, Gampe J, Mehta NK, Myrskylä M (2020) Trends in the Risk of Cognitive Impairment in the United States, 1996–2014. Epidemiology 31(5): 745-754.
- Pais R, Ruano L, Moreira C, Carvalho OP, Barros H (2020) Prevalence and incidence of cognitive impairment in an elder Portuguese population (65–85 years old). BMC Geriatr 20(1): 470-479.
- Jia L, Du Y, Chu L, Zhang Z, Li F, et al. (2020) Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Heal 5(12): 661-671.
- Ojagbemi A, Okekunle AP, Babatunde O (2021) Dominant and Modifiable Risk Factors for Dementia in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Front Neurol 12: 627761.
- Nutakor JA, Dai B, Zhou J, Larnyo E, Gavu AK, et al. (2021) Association between socioeconomic status and cognitive functioning among older adults in Ghana. Int J Geriatr Psychiatry 36(5): 756-765.
- Roberts R, Knopman DS (2013) Classification and epidemiology of MCI. Vol. 29, Clin Geriatr Med 29(4): 753-772.
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, et al. (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (10248): 413-446.
- Wessels AM, Lane KA, Gao S, Hall KS, Unverzagt FW, et al. (2011) Diabetes and cognitive decline in elderly African Americans: A 15-year follow-up study. Alzheimers Dement 7(4): 418-424.
- Ramlall S, Chipps J, Pillay BJ, Bhigjee AI (2013) Mild cognitive impairment and dementia in a heterogeneous elderly population: Prevalence and risk profile. Afr J Psychiatry 16(6): 456-465.
- Fan J, Tao W, Li X, Li H, Zhang J, et al. (2019) The contribution of genetic factors to cognitive impairment and dementia: Apolipoprotein E gene, gene interactions, and polygenic risk. Int J Mol Sci 20(5): 1177.
- Ucheagwu V, Ajaelu C, Okoli P, Ossai J, Ofojebe P (2019) Roles of demographics, anthropometric and metabolic syndrome on cognition among mid adults from rural population in Nigeria. Ann Alzheimers Dement Care 3(1): 003-010.
- Sunwoo MK, Jeon S, Ham JH, Hong JY, Lee JE, et al. (2014) The burden of white matter hyperintensities is a predictor of progressive mild cognitive impairment in patients with Parkinson’s disease. Eur J Neurol 21(6): 922-e50.
- Myers JS (2008) Factors associated with changing cognitive function in older adults: implications for nursing rehabilitation. Rehabil Nurs 33(3): 117-123.
- Mohan D, Iype T, Varghese S, Usha A, Mohan M (2019) A cross-sectional study to assess prevalence and factors associated with mild cognitive impairment among older adults in an urban area of Kerala, South India. BMJ Open 9(3): e025473.
- Li R, Singh M (2014) Sex differences in cognitive impairment and Alzheimer’s disease. Front Neuroendocrinol 35(3): 385-403.
- Laws KR, Irvine K, Gale TM (2016) Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry 6(1): 54-65.
- Håkansson K, Rovio S, Helkala EL, Vilska AR, Winblad B, et al. (2009) Association between mid-life marital status and cognitive function in later life: population based cohort study. BMJ 339(2): b2462.
- Nutakor JA, Dai B, Zhou J, Larnyo E, Gavu AK, et al. (2021) Association between socioeconomic status and cognitive functioning among older adults in Ghana. Int J Geriatr Psychiatry 36(5): 756-765.
- Wang H-X, Jin Y, Hendrie HC, Liang C, Yang L, et al. (2013) Late Life Leisure Activities and Risk of Cognitive Decline. J Gerontol A Biol Sci Med Sci 68(2): 205-213.
- Momtaz YA, Ibrahim R, Hamid TA, Chai ST (2015) Smoking and Cognitive Impairment Among Older Persons in Malaysia. Am J Alzheimers Dis Other Demen 30(4): 405-411.






























