Review of Pain in Parkinson’s Disease
Watt I1*, Phyu E1, Rukavina K2, Chaudhuri KR2,3 and Gangadharan S1,2
1Sunshine Coast Hospital and Health Service, Australia
2Basic and Clinical Neuroscience, King’s College London, UK
3NIHR Maudsley Biomedical Research Centre, UK
Submission: March 25, 2022; Published: May 16, 2022
*Corresponding author: Isabel Watt, Sunshine Coast Hospital and Health Service, Australia
How to cite this article: Watt I, Phyu E, Krbot K, Chaudhuri R, Gangadharan S. Review of Pain in Parkinson’s Disease. OAJ Gerontol & Geriatric Med. 2022; 6(4): 555695. DOI: 10.19080/OAJGGM.2022.06.555695
Abstract
Pain is one of the most common and bothersome non-motor symptoms in Parkinson’s Disease (PD), with a significant effect on Quality of Life (QoL), yet it remains largely underrecognized and subsequently undertreated. The present review aims to discuss existing evidence on pain in PD, including its epidemiology, pathophysiology, classification and presentation, measurement and management.
Keywords: Pain; Parkinson’s disease; Neurodegeneration; Management; Non motor symptoms; Dystonia; Musculoskeletal pain; Carpal tunnel syndrome
Background
Parkinson’s disease is the second most common neurodegenerative condition after Alzheimer’s disease [1]. It is characterized by the cardinal motor features of tremor, rigidity, bradykinesia and postural instability, as well as various non-motor symptoms, many of which were described by James Parkinson himself more than two centuries ago [2,3]. Pain is an important and prevalent non-motor symptom of PD which has gained increasing attention in the literature in recent years. Pain is prevalent in 30 to 85% of patients with PD and may vary in aetiology, location, duration and quality [1,4-6]. It frequently predates the PD diagnosis and may be more disturbing for PD patients than their motor symptoms [7,8].
Current guidelines for the diagnosis and management of pain in PD are based mainly on information obtained from case reports, small observational studies and expert opinion, while the availability of level I evidence (evidence from Randomized Controlled Trials - RCTs) remains limited. There is an urgent need to raise awareness, improve recognition and further develop treatment strategies for PD pain.
Epidemiology
The reported prevalence of pain in PD varies considerably between studies and regions, which may be due to its heterogeneity and to the lack of a clear definition [6]. It is agreed that the prevalence is high, between 30 and 85% [6]. Indeed, in a large clinical study including 1957 PD patients conducted by Silverdale and colleagues, 85% of PD patients reported pain, with 42% describing moderate or severe pain [8].
Female gender, dyskinesia, painful medical comorbidities, and postural abnormalities secondary to rigidity and bradykinesia have all been identified as potential additional factors contributing to the appearance of spontaneous pain in predisposed subjects [5,9-10]. Notably, pain prevalence and severity seem to be irrespective of the severity of motor symptoms and of disease progression [8]. Furthermore, there is a correlation between pain in PD and other non-motor symptoms. Particularly, pain is associated with gastrointestinal and cardiovascular symptoms, reduced sleep quality as well as affective and autonomic symptoms [8,11-14].
Pathophysiology
The pathophysiology of pain in PD is complex and not fully understood but likely relates to abnormalities throughout the central and peripheral nervous system including the basal ganglia and dopamine-dependent pathways, non-dopaminergic structures and epidermal nerve fibres.
There is mounting evidence pointing towards an important role of the basal ganglia in the experience of pain [15-17]. The basal ganglia are component of what is collectively known as the ‘salience network’. The salience network is an intrinsically connected large-scale network anchored in the anterior insula and dorsal anterior cingulate cortex, which includes three key subcortical structures, specifically, the amygdala, ventral striatum and substantia nigra. This network is essential for the detection and integration of emotional and sensory stimuli [17-19].
The importance of dysregulation of dopamine-dependent pathways to the experience of pain in PD patients is now well acknowledged. In a study by Brefel-Courbon cerebral activity was investigated with positron emission tomography during experimental nociceptive stimulation. During the off phase, there was a significant increase in pain-induced activation in the right insula and prefrontal and left anterior cingulate cortices in PD patients compared with their non-PD counterparts [20]. Levodopa significantly reduced pain-induced activation in these areas [20]. Additionally, Braak and colleagues recognised Lewy bodies in the non-dopaminergic structures involved in pain processing well before the substantia nigra was involved [21]. This may be an explanation for the temporal discrepancies between pain and motor manifestations in early-stage PD [17]. Deficits in multiple non-dopaminergic neurotransmitter systems and pathways (cholinergic, noradrenergic, and serotonergic) may be another important modulator of pain perception in PD patients [3].
In PD, peripheral transmission of nociceptive inputs is impaired as well. Patients with PD showed an increase in tactile and thermal thresholds, reduction in mechanical pain perception and loss of epidermal nerve fibres and Meissner corpuscles compared with non-PD controls [22]. However, a more recent study using deep brain stimulation supports the view that the role of these peripheral mechanisms is not as important as that of central mechanisms [8].
Classification and Clinical Presentations
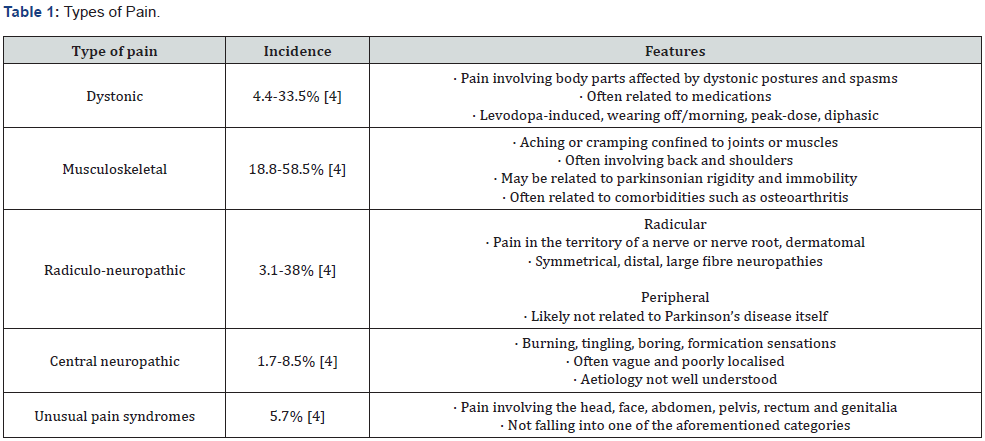
Classification of pain in PD has been approached in different ways, for example, by aetiology or by symptomology [23]. The most cited method of classification remains that proposed by Ford in 2010, which has subsequently been reviewed and modified [24- 25]. Ford’s updated classification divides pain into dystonic and non-dystonic, with non-dystonic encompassing neuropathic and musculoskeletal pain as well as unusual pain syndromes which includes those symptoms not fitting another category [4,25-26].
Defazio and colleagues summarised the frequency of these varying PD pain types in a meta-analysis, which found that dystonic, musculoskeletal and radiculo-neuropathic pain were particularly common [4]. Back and shoulder pain were also quite prevalent [4].
Alternate classification of pain type include those based on aetiology such as, nociceptive, neurogenic, and psychogenic pain, or nociceptor (subdivided into musculoskeletal, visceral and cutaneous) and neuropathic (peripheral and central) [23]. Chaudhuri and colleagues suggest classification of PD pain into musculoskeletal, PD related chronic pain, fluctuation related, nocturnal, coat-hanger, orofacial, peripheral limb and abdominal pain [27].
Dystonic pain
Dystonic pain may be due to the underlying disease itself or as a result of on/off fluctuations under dopaminergic treatment. It manifests as dystonic spasms which are usually paroxysmal and spontaneous, or triggered by activity, and may occur in the extremities, the face and the pharyngeal muscles [28]. Long term Dopaminergic therapy has been associated with dystonia with almost 30% of patients on long term Levodopa affected [29]. Most Levodopa induced dyskinesias are choreiform, not sustained and painless. Dystonic dyskinesias have a sustained twisting nature and can be quite uncomfortable [24]. Dystonic pain can be further classified as beginning-of-dose, end-of-dose and wearing-off pain [30]. The most frequent manifestation is early morning dystonia due to dopaminergic deficiency, accompanied by akinesia and rigidity. This is a focal dystonia, usually presenting as an involuntary plantar flexion and inversion of the foot, with intensity decreasing after dopaminergic medication [30-31]. Dystonic pain is a frequent complaint in early-onset PD, where it may be associated with mutations of the Parkin or PINK gene [30].
Musculoskeletal pain
The most common form of PD pain, unexplained musculoskeletal pain, typically seems to be related to rigidity, akinesia, postural abnormalities or dystonia [30]. Clinically, patients report cramp-like pain, aching or tightness appearing typically in the neck, arm, paraspinal or calf muscles [9,13]. Some patients report rather vague (although sometimes intense) painful sensations such as ill-defined muscular or articular pain [32]. Joint pain occurs most frequently in shoulders, followed by the hips, knees, and ankles [9,30,33-35].
Radiculo-neuropathic pain
A proportion of patients also report painful, sometimes superficial and cutaneous, paraesthesias like burning, painful pins and needles or electrical charge-like sensations well localized to the territory of a nerve root [30,36]. Neurological deficits in the affected nerve root, such as numbness or weakness may also occur [24]. Postural abnormalities and dystonia can cause lumbar disc herniation and nerve or root compression leading to this kind of pain [37]. Radicular pain as an accompaniment to back pain is more prevalent in patients with PD compared with controls [38]. Carpal tunnel syndrome and compressive radial neuropathy have also been reported to have higher prevalence in patients with PD [39-41].
Central pain
Central pain in PD patients results directly from abnormalities in central pain processing. Affected PD patients usually describe it as a bizarre and unexplained painful sensation (tingling, numbness, shooting pain), intermittent or persistent [30,33,42- 43]. Central pain can occur in different areas of the body including the mouth, rectum, vagina, abdomen, chest and testes [44-46]. In some patients, central PD pain occurs predominantly in the side more affected by motor symptoms and in the off state, and may be modified by dopaminergic medication [24]. However, sometimes there is no correlation between central pain and motor symptoms or dopaminergic medication [30].
Akathisia / Restless Leg syndrome
PD patients may also experience painful sensations likened to akathisia or Restless Legs Syndrome (RLS) [30]. Akathisia is usually described as subjective restlessness or the painful impulse to move continually. It improves after administration of dopaminergic medication [24]. Patients with RLS usually report intense and disagreeable sensations (paraesthesia and dysesthesia) of the extremities, more pronounced in the lower extremities, and at night, improving with movement [47]. Reported prevalence of RLS in patients with PD ranges from 8% to 20%, significantly higher than the 1% prevalence found in the general population [48-49].
Measurement of pain
Pain is a difficult construct to define and assess. It is subjective and heterogeneous. The King’s PD Pain Scale (KPPS) is a well validated and widely used tool in the literature [5-6]. A patientcompleted questionnaire, the King’s PD Pain Questionnaire (KPPQ), proposed by the same group, has also been validated [50]. The Visual Analogue Scale (VAS) and Short-Form McGill Pain Questionnaire (SFMPQ) are other pain scores that has been used alongside the KPPS in a large-scale clinical study of pain in PD [8].
Management of pain in PD
Dystonic Pain
Dystonia can occur as a wearing off effect, a peak dose phenomenon or diphasic (early dose and late dose); therefore, the management varies depending on its type [51]. In general, the management of dystonic pain implicates maintenance of stable levels of dopaminergic drugs, which may be achieved by preemptive levodopa dosing, controlled-release levodopa, or a longacting dopamine agonist [2,28,51-53]. Continuous dopaminergic infusion with duodenal levodopa or dopamine agonists such as subcutaneous apomorphine reportedly relieve off dystonia, but there is an urgent need for level I evidence to evaluate this [54- 56]. Paradoxically, withdrawal of levodopa sometimes relieves patients of their early-morning dystonia, suggesting that the severity of dystonia results from a rebound as brain dopamine levels fluctuate [29].
Various other drugs have been reported to improve off period dystonia although RCTs are still missing. Among the available agents, benzodiazepines, baclofen and lithium are most commonly used [57-58]. Injections of botulinum toxin may also be helpful to treat focal dystonia in PD [59-60]. Both subthalamic nucleus stimulation and globus pallidus interna stimulation have been found to help dystonia in PD [61]. Intrathecal baclofen, effective for spasticity of spinal or cerebral origin, has shown little effect on the dystonia associated with parkinsonism [62]. Kodama and colleagues reported a case of improvement of ‘off period’ dystonia with repetitive transcranial magnetic stimulation over the contralateral primary motor area [63].
For peak-dose dystonia, reduction of levodopa dosage may be considered. For diphasic dystonia that occurs at the beginning or end of the medication dose interval, increasing plasma concentrations by using higher doses of levodopa, or substituting regular for controlled-release levodopa, may be helpful [64]. Amantadine has been found to have antidyskinetic properties, as well as low doses of clozapine [65-67]. High-frequency stimulation of the subthalamic nucleus or the globus pallidus interna provides an alternative approach [68].
Musculoskeletal pain
If the musculoskeletal pain occurs secondary to Parkinsonian rigidity, it is essential to optimize the dopaminergic medication. Oxycodone/naloxone prolonged release is reportedly “possibly useful” for PD patients with chronic pain [69]. Despite its adverse effects, including dizziness, headache, fatigue, worsening cognitive dysfunction, and gastrointestinal tract symptoms such as nausea, vomiting, and constipation, oxycodone-naloxone is considered to pose an “acceptable risk without specialized monitoring” [56,69]. Non-pharmacological interventions such as physiotherapy (physical therapy, warming-up, stretching) and physical activity in general are essential for PD patients suffering from muskuloskeletal pain of different origins [6].
Peripheral and Radicular Neuropathic Pain
Tricyclic antidepressants (Amitriptyline, Nortriptyline, Desipramine), selective noradrenaline reuptake inhibitors (Venlafaxine), agents that act on the voltage gated Calcium channels (Gabapentin, Pregabalin) and opioid analgesics are effective [70].
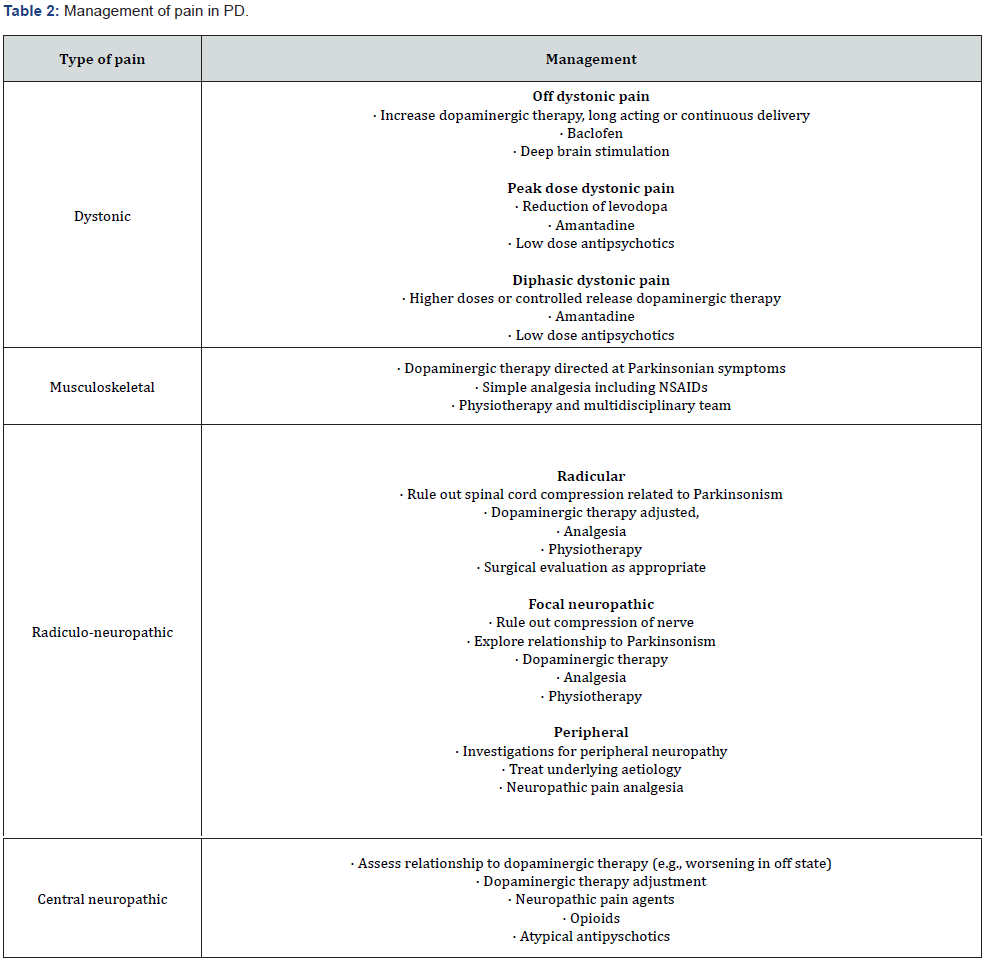
Central Pain
Dopaminergic therapy is somewhat effective and remains the first line therapy of choice in central pain syndromes in PD. Conventional analgesics, opioids, tricyclic antidepressants and atypical antipsychotics, including clozapine, may be helpful [62].
Conclusion
Pain in PD is a distressing non-motor symptom, diminishing the QoL of those experiencing it. Our understanding of exact mechanisms of pathological pain processing in PD patients remains limited. Other sources of pain should be ruled out before attributing it to PD. The validated scores and scales quantify the pain and certainly assist follow up, particularly in cognitively intact patients. The musculoskeletal dynamics and the correlation with on-off symptomatology, guide Levodopa dose evaluation and adjustments. The management of pain in Parkinson’s Disease can be quite challenging and has a telling effect on the health related QoL of sufferers. Identifying the type of pain-dystonic or non-dystonic is the first step in management and guides pharmacotherapy to be tailored accordingly.
Conflict of interest
None to declare.
References
- Broen MP, Braaksma MM, Patijn J, Weber WE (2012) Prevalence of pain in Parkinson's disease: a systematic review using the modified QUADAS tool. Mov Disord 27(4): 480-484.
- Parkinson J (2002) An essay on the shaking palsy 1817. J Neuropsychiatry Clin Neurosci 14(2): 223-236.
- Sauerbier A, Jenner P, Todorova A, Chaudhuri KR (2016) Non motor subtypes and Parkinson's disease. Parkinsonism Relat Disord 22 Suppl 1: S41-46.
- Defazio G, Gigante A, Mancino P, Tinazzi M (2013) The epidemiology of pain in Parkinson’s disease. J Neural Transm (Vienna) 120(4): 583-586.
- Chaudhuri KR, Rizos A, Trenkwalder C, Rascol O, Pal S, et al. (2015) King's Parkinson's disease pain scale, the first scale for pain in PD: an international validation. Mov Disord 30(12): 1623-1631.
- Antonini A, Tinazzi M, Abbruzzese G, Berardelli A, Chaudhuri KR, et al. (2018) Pain in Parkinson's disease: facts and uncertainties. Eur J Neurol 25(7): 917-e69.
- Martinez‐Martin P, Rodriguez‐Blazquez C, Kurtis MM, Chaudhuri KR, NMSS Validation Group (2011) The impact of non‐motor symptoms on health‐related quality of life of patients with Parkinson's disease. Mov Disord 26(3): 399-406.
- Zambito-Marsala S, Ero R, Bacchin R, Fornasier A, Fabris F, et al. (2017) Abnormal nociceptive processing occurs centrally and not peripherally in pain-free Parkinson disease patients: A study with laser-evoked potentials. Parkinsonism Relat Disord 34: 43-48.
- Defazio G, Berardelli A, Fabbrini G, Martino D, Fincati E, et al. (2008) Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch Neurol 65(9): 1191-1194.
- Braga M, Pederzoli M, Antonini A, Beretta F, Crespi V (2014) Reasons for hospitalization in Parkinson’s disease: a case-control study. Parkinsonism Relat Disord 20(5): 488-492.
- Bugalho P, Lampreia T, Miguel R, Mendonça MD, Caetano A, et al. (2016) Non-Motor symptoms in Portuguese Parkinson’s Disease patients: correlation and impact on Quality of Life and Activities of Daily Living. Sci Rep 6: 32267.
- Rana AQ, Qureshi AR, Shamli Oghli Y, Saqib Y, Mohammed B, et al. (2018) Decreased sleep quality in Parkinson’s patients is associated with higher anxiety and depression prevalence and severity, and correlates with pain intensity and quality. Neurol Res 40(8): 696-701.
- Nègre-Pagès L, Regragui W, Bouhassira D, Grandjean H, Rascol O, DoPaMiP Study Group (2008) Chronic pain in Parkinson's disease: the cross-sectional French DoPaMiP survey. Mov Disord 23(10): 1361-1369.
- Ehrt U, Larsen JP, Aarsland D (2009) Pain and its relationship to depression in Parkinson disease. Am J Geriatr Psychiatry 17(4): 269-275.
- Muller B, Larsen JP, Wentzel-Larsen T, Skeie GO, Tysnes OB, Parkwest Study G (2011) Autonomic and sensory symptoms and signs in incident, untreated Parkinson's disease: frequent but mild. Mov Disord 26(1): 65-72.
- Borsook D, Upadhyay J, Chudler EH, Becerra L (2010) A key role of the basal ganglia in pain and analgesia-insights gained through human functional imaging. Mol Pain 6: 27.
- Tseng MT, Lin CH (2017) Pain in early-stage Parkinson's disease: Implications from clinical features to pathophysiology mechanisms. J Formos Med Assoc 116(8): 571-581.
- Juri C, Rodriguez-Oroz M, and Obeso JA (2010) The pathophysiological basis of sensory disturbances in Parkinson's disease. J Neurol Sci 289(1-2): 60-65.
- Menon V, Salience network (2015) In: Toga AW, ed. Brain Mapping: An Encyclopedic Elsevier Inc: Stanford, CA, 597–611.
- Brefel-Courbon C, Payoux P, Thalamas C, Ory F, Quelven I, et al. (2005) Effect of Levodopa on Pain Threshold in Parkinson’s Disease: A Clinical and Positron Emission Tomography Study. Mov Disord 20(12): 1557-1563.
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, et al. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24(2): 197-211.
- Nolano M, Provitera V, Estraneo A, Selim MM, Caporaso G, et al. (2008) Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain 131(7):1903-1911.
- Toda K, Harada T (2010) Prevalence, classification, and etiology of pain in Parkinson's disease: association between Parkinson's disease and fibromyalgia or chronic widespread pain. Tohoku J Exp Med 222(1): 1-5.
- Ford B (2010) Pain in Parkinson's disease. Mov Disord 25(S1): S98-S103.
- Ha AD, Jankovic J (2012) Pain in Parkinson's disease. Mov Disord 27(4): 485-491.
- Valkovic P, Minar M, Singliarova H, Harsany J, Hanakova M, et al. (2015) Pain in parkinson’s disease: A cross-sectional study of its prevalence, types, and relationship to depression and quality of life. PLoS One 10(8): e0136541.
- Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5): 464-474.
- Tolosa E, Compta Y, Gaig C (2007) The premotor phase of Parkinson's disease. Parkinsonism Relat Disord 13: S2-S7.
- Kidron D, Eldad Melamed (1987) Forms of dystonia in patients with Parkinson's disease. Neurology 37(6): 1009-1011.
- Wasner G, Deuschl G (2012) Pains in Parkinson disease-many syndromes under one Nat Rev Neurol 8(5): 284-294.
- Quinn NP, Koller WC, Lang AE, Marsden CD (1986) Painful Parkinson’s disease. Lancet 1: 1366-1369.
- Vaserman-Lehuede N, Verin M (1999) Shoulder pain in patients with Parkinson’s Rev Rhum Engl Ed 66: 220-223.
- Snider SR, Fahn S, Isgreen WP, Cote LJ (1976) Primary sensory symptoms in Neurology 26(5): 423-429.
- Hanagasi HA, Akat S, Gurvit H, Yazici J, Emre M (2011) Pain is common in Parkinson's disease. Clin Neurol Neurosurg 113(1): 11-13.
- Lee MA, Walker RW, Hildreth TJ, Prentice WM (2006) A survey of pain in idiopathic Parkinson's disease. J Pain Symptom Manage 32(5): 462-469.
- Bouhassira D, Attal N, Fermanian J (2004) Development and validation of the Neuropathic Pain Symptom Inventory. Pain 108(3): 248-257.
- Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P (2000) Mechanical initiation of intervertebral disc degeneration. Spine. 25(13): 1625-1636.
- Broetz D, Eichner M, Gasser T, Weller M, Steinbach JP (2007) Radicular and nonradicular back pain in Parkinson's disease: a controlled study. Mov Disord 22(6): 853-856.
- Preston DN, Grimes JD (1985) Radial compression neuropathy in advanced Parkinson's disease. Arch Neurol 42(7): 695-696.
- Rajabally YA, Martey J (2011) Neuropathy in Parkinson disease: prevalence and Neurology 77(22): 1947-1950.
- Kurlan R, Baker P, Miller C (1985) Severe compression neuropathy following sudden onset of parkinsonian immobility. Arch Neurol 42(7): 720.
- Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, et al. (2004) Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 62(12): 2171-2175.
- Schestatsky P, Kumru H, Valls-Solé J, Valldeoriola F, Marti MJ, et al. (2007) Neurophysiologic study of central pain in patients with Parkinson Neurology 69(23): 2162-2169.
- Clifford TJ, Warsi MJ, Burnett CA (1998) Burning mouth in Parkinson's disease sufferers. Gerodontology 15(2): 73-78.
- Quigley EM (1996) Gastrointestinal dysfunction in Parkinson’s disease. Semin Neurol 16: 245-250.
- Ford B, Louis ED, Greene P, Fahn S (1996) Oral and genital pain syndromes in Parkinson's disease. Mov Disord 11(4): 421-426.
- Walters AS (1995) Toward a better definition of the restless legs syndrome the International Restless Legs Syndrome Study Group. Move disord 10(5): 634-642.
- Krishnan PR, Bhatia M, Behari M (2002) Restless legs syndrome in Parkinson’s disease: a case controlled study. Mov Disord 18(2): 181-185.
- Ondo WG, Vuong KD, Jankovic J (2002) Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol 59: 421-424.
- Martinez-Martin P, Rizos AM, Wetmore J, Antonini A, Odin P, et al. (2018) First comprehensive tool for screening pain in Parkinson's disease: the King's Parkinson's Disease Pain Questionnaire. Eur J Neurol 25(10): 1255-1261.
- Juncos JL, Fabbrini G, Mouradian MM (1987) Controlled release levodopa-carbidopa (CR-5) in the management of parkinsonian motor fluctuations. Arch Neurol 44(10): 1010-1012.
- Pastor P, Tolosa E (2003) Cabergoline in the treatment of Parkinson's disease (in Spanish). Neurologia 18(4): 202-209.
- Rascol O, Zesiewicz T, Chaudhuri KR, Asgharnejad M, Surmann E, et al. (2016) A Randomized Controlled Exploratory Pilot Study to Evaluate the Effect of Rotigotine Transdermal Patch on Parkinson's Disease-Associated Chronic Pain. J Clin Pharmacol 56(7): 852-861.
- Karlsborg M, Korbo L, Regeur L (2010) Duodopa pump treatment in patients with advanced Parkinson's disease. Dan Med Bull 57(6): A4155.
- Lees AJ (1993) Dopamine agonists in Parkinson's disease: a look at apomorphine. Fundam Clin Pharmacol 7(3-4): 121-128.
- Seppi K, Chaudhuri RK, Coelho M, Fox SH, Katzenschlager R, et al. (2019) Update on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. Mov Disord 34(2): 180-198.
- Poewe WH, Lees AJ, Stem GM (1988) Dystonia in Parkinson's disease: clinical and pharmacological features. Ann Neurol 23(l): 73-78.
- Simuni T, Lyons KE, Pahwa R, Robert A Hauser, Cynthia Comella, et al. (2009) Treatment of early Parkinson's disease: part Eur Neurol 61(4): 206-215.
- Cordivari C, Misra VP, Catania S (2001) Treatment of dystonic clenched fist with botulinum toxin. Mov Disord 16(5): 907-913.
- Pacchetti C, Albani G, Martignoni E (1995) Off painful dystonia in Parkinson's disease treated with botulinum toxin. Mov Disord 10(3): 333-336.
- Limousin P, Krack P, Pollak P (1998) Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 339(16): 1105-1111.
- Ford B, Greene P, Louis ED (1996) Use of intrathecal baclofen in the treatment of patients with dystonia. Arch Neurol 53(12): 1241-1246.
- Kodama M, Kasahara T, Hyodo M (2011) Effect of low-frequency repetitive transcranial magnetic stimulation combined with physical therapy on L-dopa-induced painful off-period dystonia in Parkinson's disease. Am J Phys Med Rehabil 90(2): 150-155.
- Fabbrini G, Brotchie JM, Grandas F (2007) Levodopa-induced dyskinesias. Mov Disord 22(10): 1379-1389.
- Thomas A, Iacono D, Luciano AL (2004) Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry 75(l): 141-143.
- Metman LV, Del Dotto P, LePoole K (1999) Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol 56(11): 1383-1386.
- Durif F, Debilly B, Galitzky M (2004) Clozapine improves dyskinesias in Parkinson disease: a double-blind, placebo-controlled study. Neurology 62(3): 381-388.
- Anderson VC, Burchiel KJ, Hogarth P (2005) Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 62(4): 554-560.
- Trenkwalder C, Chaudhuri KR, Martinez-Martin P, Rascol O, Ehret R, et al. (2015) Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson's disease (PANDA): a double-blind, randomised, placebo-controlled trial. The Lancet Neurology 14(12): 1161-1170.
- O'Connor AB, Dworkin RH (2009) Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 122(10 Suppl): S22-S32.






























