Improved Nutrition Status is Associated with Attenuated Decrease in Circulating Myostatin After Hip Fracture Surgery in Elderly Patients
Torbjörn Åkerfeldt1, Mahtia Adinda1, Anna-Karin Gunnarsson2, Lena Gunningberg3, Sune Larsson2, Michael Svensson4 and Anders Larsson1*
1 Department of Medical Sciences, Uppsala University, Uppsala, Sweden
2 Department of Orthopedics, Uppsala University, Uppsala, Sweden
3 Department of Public Health and Caring Sciences, Uppsala University, Uppsala, Sweden
4 Department of Surgical and Perioperative Science, Sports Medicine, Umeå University, Sweden
Submission: February 03, 2020; Published: February 25, 2020
*Corresponding author: Anders Larsson, Department of Medical Sciences, University Hospital, Sweden
How to cite this article: Torbjörn Å, Mahtia Adinda, Anders Larsson, et al. Improved Nutrition Status is Associated with Attenuated Decrease in Circulating Myostatin After Hip Fracture Surgery in Elderly Patients. OAJ Gerontol & Geriatric Med. 2020; 5(3): 555665. DOI: 10.19080/OAJGGM.2020.05.555665
Abstract
Aim: Myostatin is a potent regulator of muscle growth as well as bone regeneration. The aim of this study was to investigate the effect of orthopedic surgery on myostatin levels in a nutritional intervention study. Materials and Methods: Patients with hip fractures treated at the Uppsala University Hospital were enrolled in the study. Additional nutrition was given to an intervention group (n=46) while a control group were given standard nutrition (n=42). The additional support was provided in the form of two preoperative carbohydrate drinks, an intravenous glucose infusion preoperatively, and drinks three times a day during the first five postoperative days. Results: 88 patients completed the study. Compared to preoperative values (median 2338 pg/mL, interquartile range 1258-3905 pg/mL) there were significant decreases in myostatin values after surgery (median 1471 pg/mL, interquartile range 783-2273 pg/mL); p<0.000001). The decrease from preoperative to postoperative values was smaller in the intervention group (p=0.020). Conclusions: There is a highly significant decrease in myostatin values after orthopedic surgery. Increased nutritional support significantly attenuated the decrease
Keywords: Hip fracture; Human; Muscle loss; Myostatin; Surgery
Introduction
Wound healing and the mobilization of the patient after surgery are important factors for successful outcomes of orthopedic surgical procedures. The surgical trauma causes a metabolic response that mobilizes nutrients to ensure wound healing and strengthen the resistance to infections. A central part in this process is the redistribution of proteins and amino acids from skeletal muscles. This leads to a rapid loss of lean body mass and a change in body composition. The process is mediated by the release of cytokines and hormones such as growth hormone, cortisol and insulin. Orthopedic patients are often elderly individuals. These patients have reduced muscle mass and they are at increased risk of being malnourished [1,2] and this malnutrition is associated with increased risk of postoperative complications [1-6]. Thus, they are at an increased risk during this catabolic period. The initial low muscle mass in combination with an additional loss may complicate the mobilization of the patients after surgery. It is thus important to study the regulation of muscle mass in the post-surgical phase.
Myostatin (also known as growth differentiation factor 8) belongs to the Bone Morphogenetic Protein (BMP) family and the TGF beta superfamily [7,8]. Most normal cells display moderate to strong cytoplasmic staining for myostatin (www.proteinatlas.org). Circulating myostatin acts on muscle tissue, by binding to the cell-bound receptor activin type II receptor (ActRIIB) [9]. Myostatin is a potent negative regulator of skeletal muscle growth. Inhibition of myostatin promotes muscle regeneration after injury and increases bone strength and mineralization of the skeleton [10]. Myostatin is highly expressed in the fracture callus area indicating a direct role for myostatin in bone repair and bone remodulation after fractures [11,12].
We have previously shown that additional energy administered before and for five days after surgery decreased postoperative complications measured as pressure ulcers, hospital-acquired infections and length of hospital stay [1,13]. Considering the different potential roles of myostatin we decided to explore the effects of orthopedic surgery on myostatin levels in the same study cohort and investigate if the additional energy intake was associated with an effect on the myostatin levels after surgery. Hip surgery is a suitable model for studying the effects of orthopedic surgical trauma in humans.
Materials and Methods
Study population
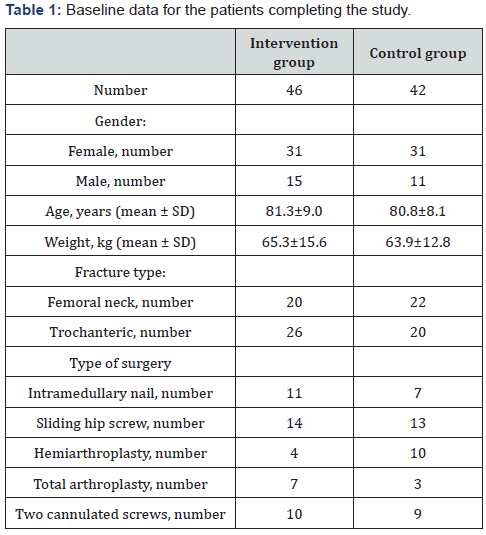
Patients with hip fractures treated at the Uppsala University Hospital were included in the study. The intervention group received additional nutrition (n=46) while the control group (n=42) were given the standard nutrition regimen at the ward. The age, gender, type of fracture and surgery are provided in (Table 1) The control group received the standard nutritional support pre- and post-operatively while the intervention group was given additional nutritional support in the form of two preoperative carbohydrate drinks, an intravenous glucose infusion preoperatively, and drinks three times a day during the first five postoperative days. All patients gave informed consent prior to inclusion in the study. The study was performed according to the Helsinki declaration and was approved by the ethical review board in Uppsala (2005:150).
Blood samples were collected in Vacutainer tubes without additives. After clotting and centrifugation, the serum was transferred to new tubes and frozen. The samples were kept frozen at -70°C until analysis.
Myostatin ELISA
Serum levels of myostatin were measured by sandwich ELISA (Quantikine kit DGDF80, R&D Systems, Minneapolis, MN, USA). The intraassay CV was 6%.
Statistical calculations
Myostatin values before and after surgery were analyzed using Wilcoxon matched pairs test, Mann-Whitney U-test and Statistica 10 (StatSoft, Tulsa, OK, USA). Figures were created in Excel 2000 (Microsoft Corporation, Seattle, WA, USA).
Results
Effects of surgery on myostatin values
1. There were no significant differences in age, gender, body weight, fracture type or type of surgery between the intervention group and the control group (Table 1).
2. Before surgery the median myostatin value was 2338 pg/mL (interquartile range 1258-3905 pg/mL). After surgery the median myostatin value had decreased to 1471 pg/mL, IQR 783-2273 pg/mL). The decrease was highly significant (p<0.000001).
Effects of additional nutrition on postsurgical values
The differences between preoperative and postoperative myostatin values was less pronounced in the intervention group (p=0.020).
Discussion
There are a number of studies on the role of myostatin for regulation of muscle growth. There are also studies showing the role of myostatin for bone regeneration and architecture. Myostatin gene polymorphisms has been shown to be associated with variations in peak bone mineral density [11,14] and transgenic overexpression of myostatin propeptide, an inhibitor of myostatin signaling, increased bone mineral density in mice [15]. Both muscle and bone strength are of utmost importance in orthopedic surgery patients. Loss of muscle strength is a risk factor for fall related injuries [16]. Patients with hip fractures are usually elderly individuals with an acute fracture. They are often malnourished at the time of admission to the hospital and the European Society of Parenteral and Enteral Nutrition (ESPEN) recommends that nutritional supplementation are given to elderly patients with hip fractures [17]. Improved nutrition has also been shown to improve physical performance in elderly people [18,19]. The biochemical mechanisms behind this is not clear. Myostatin is a potent regulator of muscle mass and myostatin inhibitors have been proposed as promising options for the treatment of sarcopenia [20].
The present study at Uppsala University Hospital enrolled patients with hip fractures to investigate the effects of nutritional supplementation. We have previously reported that the high energy intake administered for 5 days significantly decreased pressure ulcers and hospital acquired infections and reduced the median length of hospital stay. Considering the potential roles of myostatin in orthopedic patients we decided to study the levels of circulating myostatin levels before and after surgery in the same patient group. The postoperative myostatin values in serum are significantly lower than the preoperative values in this group of acute orthopedic surgery patients. This is in line with our previous findings in other surgery populations [21], and in contrast to the study by Mendias et al, who used a much younger surgery population undergoing ACL reconstruction [22]. Interestingly, and somewhat unexpectedly, the nutritional support resulted in an attenuated decrease in postoperative vs preoperative myostatin values. Even if myostatin is not considered as a nutritional biomarker today, the nutritional support thus had a significant effect on circulating myostatin concentrations. The number of pressure ulcers and infections in this material is rather low considering the total number of patients. We have therefore not made a statistical correlation between myostatin levels and specific postoperative complications. Larger studies are warranted that explore the association between myostatin and postsurgical complications.
1. In conclusion, there is a highly significant decrease in myostatin values after orthopedic surgery and the increased nutrition significantly attenuated the decrease.
2. In conclusion, there is a highly significant decrease in myostatin values after orthopedic surgery and the increased nutrition significantly attenuated the decrease.
Acknowledgements
This study was supported by the Uppsala University Hospital Research Fund. Thanks to Carina Brännström for analytical work and to all nurses at the ward for their care of the patients.
Conflict of Interest
The authors certify that they have no conflict of interest.
References
- Gunnarsson AK, Åkerfeldt T, Larsson S, Gunningberg L (2012) Increased energy intake in hip fracture patients affects nutritional biochemical markers. Scand J Surg 101(3): 204-210.
- Lumbers M, Driver LT, Howland RJ, Older MW, Williams CM (1996) Nutritional status and clinical outcome in elderly female surgical orthopedic patients. Clin Nutr 15(3): 101-107.
- Herrmann FR, SafranC, Levkoff SE, Minaker KL (1992) Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch Intern Med 152(1): 125-130.
- Houwing RH, Rozendaal M, Wouters-Wesseling W, Beulens JW, Buskens E (2003) A randomized, double-blind assessment of the effect of nutritional supplementation on the prevention of pressure ulcers in hip-fracture patients. Clin Nutr 22(4): 401-405.
- Patterson BM, Cornell CN, Carbone B, Levine B, Chapman D (1992) Protein depletion and metabolic stress in elderly patients who have a fracture of the hip. J Bone Joint Surg Am 74(2): 51-60.
- Ponzer S, Tidermark J, Brismar K, Söderqvist A, Cederholm T (1999) Nutritional status, insulin-like growth factor-1 and quality of life in elderly women with hip fractures. Clin Nutr 18(4): 241-246.
- Elliott B, Renshaw D, Getting S, Mackenzie R (2012) The central role of myostatin in skeletal muscle and whole-body homeostasis. Acta Physiol 205(3): 324-340.
- Joulia-Ekaza D, Cabello G (2007) The myostatin gene: physiology and pharmacological relevance. Curr Opin Pharmacol 7(3): 310-315.
- Huang Z, Chen X, Chen D (2011) Myostatin: a novel insight into its role in metabolism, signal pathways, and expression regulation. Cell Signal 23(9): 1441-1446.
- Buehring B, Binkley N (2013) Myostatin-the holy grail for muscle, bone, and fat? Curr Osteoporos Rep 11(4): 407-414.
- Elkasrawy MN, Hamrick MW (2010) Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact 10(1): 56-63.
- Hamrick MW, Arounleut P, Kellum E, Cain M, Immel D (2010) Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury. J Trauma 69(3): 579-583.
- Gunnarsson AK, Lönn K, Gunningberg L (2009) Does nutritional intervention for patients with hip fractures reduce postoperative complications and improve rehabilitation? J Clin Nurs 18(9): 1325-1333.
- Zhang ZL, He JW, Qin YJ, Hu YQ, Li M, et al. (2008) Association between myostatin gene polymorphisms and peak BMD variation in Chinese nuclear families. Osteoporos Int 19(1): 39-47.
- Mitchell AD, Wall RJ (2007) In vivo evaluation of changes in body composition of transgenic mice expressing the myostatin pro domain using dual energy X-ray absorptiometry. Growth Dev Aging 70(1): 25-37.
- Malafarina V, Uriz-Otano F, Gil-Guerrero L, Iniesta R, Zulet MA, et al. (2013) Study protocol: High-protein nutritional intervention based on β-hydroxy-β-methylbutirate, vitamin D3 and calcium on obese and lean aged patients with hip fractures and sarcopenia. The HIPERPROT-GER study. Maturitas 76(2): 123-128.
- Volkert D, Berner YN, Berry E, Cederholm T, Coti Bertrand P, et al. (2006) ESPEN guidelines on enteral nutrition: geriatrics. Clin Nutr 25(2): 330-360.
- Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L (2013) Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Dir Assoc 14(1): 10-17.
- Tieland M van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, et al. (2012) Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 13(8): 720-726.
- Morley JE (2016) Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int 98(4): 319-333.
- Åkerfeldt T, Helmersson-Karlqvist J, Gunningberg L, Swenne CL, Larsson A (2015) Postsurgical acute phase reaction is associated with decreased levels of circulating myostatin. Inflammation 38(4): 1727-1730.
- Mendias CL, Lynch EB, Davis ME, Sibilsky Enselman ER, Harning JA, et al. (2013) Changes in circulating biomarkers of muscle atrophy, inflammation, and cartilage turnover in patients undergoing anterior cruciate ligament reconstruction and rehabilitation. Am J Sports Med 41(8): 1819-1826.






























