The Concepts of Biorhythms, Redundancy and Reserve: Impact on Cardiovascular Aging and Disease
Vinod Nikhra*
Consultant Physician and Senior Chief Medical Officer, HinduRao Hospital & NDMC Medical College, India
Submission: August 21, 2019;Published: September 16, 2019
*Corresponding author: Vinod Nikhra, Consultant Physician and Senior Chief Medical Officer, HinduRao Hospital & NDMC Medical College, India
How to cite this article: Vinod Nikhra. The Concepts of Biorhythms, Redundancy and Reserve: Impact on Cardiovascular Aging and Disease. 002 OAJ Gerontol & Geriatric Med. 2019; 5(2): 555656. DO 10.19080/OAJGGM.2019.05.555656
Abstract
Biorhythms, redundancy and reserve: The dynamic equilibrium in the interactions of biorhythms with the rhythms of the surrounding ecosystem forms the basis of life. The biorhythms can be categorized by their relationship to 24-h day cycle as circadian, ultradian and infradian. In addition, there are other rhythmic patterns generated outside the CNS, such as the cardiac rhythm. In addition, there are rhythmic contractions of muscles controlling gastrointestinal motility and uterine contractions. The organ reserve is the ability of an organ to endure recurring stressful conditions and restore the homeostasis and normal physiological function. There occurs age-related decline in physiological functions attributable to loss of organ reserve due to multiple factors.
Molecular basis of reserve and redundancy: The intermediary metabolism comprising of cellular biochemical reactions and metabolites is enriched with biochemical steps or intracellular structures exhibiting excess metabolic capacity than required for normal basic functions. In addition, the mitochondrial DNA (mtDNA) found in multi-copies, serves as a structural excess capacity for cellular energy metabolism. Similarly, the DNA repeats in telomeres serve as structural excess capacity for repair. The organ reserve is, thus, a joint contribution of excess metabolic capacities of several metabolic pathways and biochemical structures enabling a built-in robust metabolism and structural mechanisms for stress response and repair.
Physiology of metabolic reserve and redundancy: The living systems owe their survival to a series of complex biochemical pathways for maintenance and repair, and the defence systems creating the homeo-dynamic space which provides the buffering capacity for a biological system. The aging and the age-related diseases can be held as the consequences of a progressive shrinkage of the homeo-dynamic space, due to failure of maintenance and repair. The senescent changes occur in the cells, tissues and organs, and in turn affect the functioning of the biological systems. At subcellular level, mitochondrial oxidative stress and dysfunction play an important role in aging, and microRNAs (miRNAs) are important regulators of aging and age-related diseases
Impact of senescence of organs and systems: Various stimuli appear to converge on certain pathways that influence cell cycle regulation, DNA repair and apoptosis, and the process of cellular senescence. At the cellular level, these pathways are regulated by the tumor suppressor proteins p53 and pRb. The p53 is a crucial mediator of the cellular response to damaged DNA and dysfunctional telomeres, and in turn activates the cyclin-dependent inhibitor p21. The senescence is induced at cellular level via the p53 pathway in response to DNA damage and telomere dysfunction, whereas the p16/pRb pathway mediates senescence caused by oncogenic stimuli, chromatin disruption, and other cellular stresses. The aging of organs is progressive and irreversible, and associated with increased incidence and prevalence of atherosclerosis, hypertension, coronary artery disease and cardiac failure, cerebrovascular and peripheral vascular disease, and various neurodegenerative disorders
Conclusion-Retarding aging and disease: The age is the most important determinant of health in general and cardiovascular health in particular. But, retarding the rate of progression of CVDs at subclinical level need to be considered before the clinical disease becomes manifest. The modalities to prevent, retard or treat the CV changes with aging have been shown to reduce the prevalence of CVDs. The life-style interventions in form of regular physical exercise, smoking cessation and intervention in sleep disorders are established practical ways. There are promising data about calorie restriction (CR) and calorie restriction mimetics (CRMs) being promising tools for reducing morbidity and mortality related to metabolic, cardiovascular and neurodegenerative diseases
Keywords: Aging; Biorhythms Bioenergetic pathways Cardiovascular disease Functional threshold Homeo dynamic space Redundancy Reserve Degeneracy Plasticity Calorie restriction
Biorhythms, Redundancy and Reserve
The Physiology of Biorhythms
The dynamic equilibrium in the interactions of biorhythms with the rhythms of the surrounding ecosystem forms thebasis of life [1]. The biorhythms can be categorized by their relationship to 24-h day cycle and can be circadian, ultradian and infradian (Figure 1). A major environmental stimulus for the circadian rhythm is a 24-h pattern of light and darknessrepresenting a clock that entrains various aspects of our physiology [2]. The pineal gland is sensitive to light and secretes melatonin, which may serve as the underlying physiological basis for circadian biorhythm. The neural circadian clock is located in the hypothalamic suprachiasmatic nucleus (SCN) and the projections from the SCN influence motor, sensory and autonomic systems [3,4]. Further, the circadian clock appears to regulate maintenance of neural stem cells, whereas energy metabolism modulates both neural stem cell physiology and circadian clock machinery [5]. Apart from the master clock, it appears that every cell expresses a clock-gene and circadian rhythms can be observed at the cellular level. The metabolic patterns also follow a circadian rhythm.
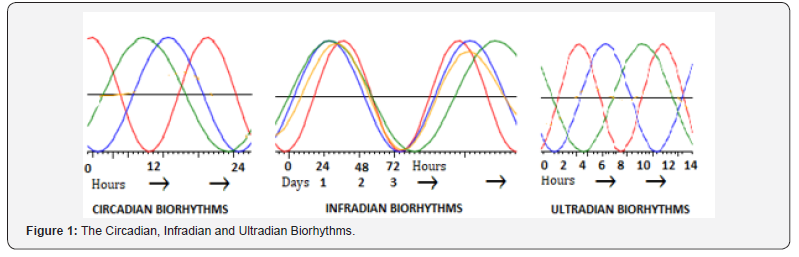
The ultradian rhythms take various forms from the repetitive beating of the heart to the respiratory rhythm. There are shorter duration ultradian rhythms relating to feeding patterns and metabolism. They relate to the cycles in digestive systems and the distribution of food intake throughout the day. The ultradian cycles are linked to circadian rhythms and any alteration in one will lead to disruptions in the other. There are additional ultradian rhythms driven by the CNS and are not as directly affected by the circadian clock, such as the rhythmic pattern of breathing. The central pattern generator for breathing appears to be located in the pre-Bötzinger complex of the brain stem [6]. There exist other central pattern generators for rhythmic motor behaviors such as mastication, walking or running. The central pattern generators for these rhythms are highly organized and complex, involving coordinated neural activation of multiple muscles. A regularly occurring physiological process that recurs at longer than 24-h is infradian. The infradian rhythms include cycles associated with reproduction, seasonal change and aging. The infradian rhythms are influenced by the circadian clock as well as other factors related to environmental exposure. In addition, there are other rhythmic patterns which are generated outside the CNS, the most obvious being the cardiac rhythm [7]. The cardiac pacemaker cells with complex interaction between conductance for various ions affect membrane excitability and action potential generation. In addition to the heart, there are rhythmic contractions of muscles controlling gastrointestinal motility and uterine contractions.
The Organ Reserve and Redundancy
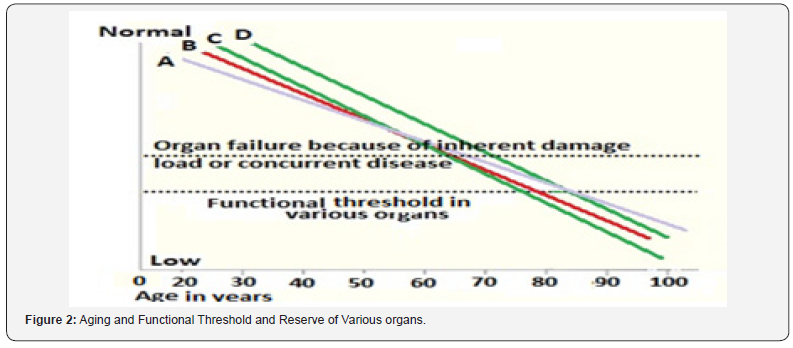
The organ reserve describes the ability of an organ to endure recurring stressful conditions and restore the homeostasis and normal physiological function in following such conditions [8]. A part of the age-related decline in physiological functions is attributable to reduction in organ reserve as present in various body systems and the gradual functional decline in specific tissues or organs (e.g., immune-, musculoskeletal-, nervous systems) is a key characteristic of aging. Although organs vary in the rate of functional decline with age, this gradual linear decline of reserve capacity with age shows values ranging from 0.5% to 1.4% per year [9]. The decline appears to accelerate by the fifth decade of age, which may explain, in part, the age-related increase in vulnerability to disease and disability (Figure 2).
The clinical evidence shows that organ reserve correlates with the ability of older adults to cope with an added workload or stress, suggesting its role in the aging process. Several metabolic pathways exhibit excess metabolic capacities, such as, bioenergetics pathways and antioxidants system. These pathways comprise molecular components having an excess of quantity and/or activity than that required for basic physiological demand. These excess capacities may provide the intermediary metabolism the ability to instantly cope with, or manage, an additional workload or stress. The excess metabolic capacities, thus, can be viewed as an adaptability mechanism that substantiates organ reserve and contributes to the cellular defence systems. When metabolic excess capacities or organ reserves are impaired or exhausted, the ability of the cell to cope with stress is reduced. Under these circumstances, there occurs cell senescence, transformation or apoptosis [10].
The reserve is also pertaining to the ability of an organ to recover from trauma. Organ reserve may, thus, be a verifiable phenomenon. In any given individual, various organs, tissues and cell types are affected by aging in unique ways depending on complex interactions among genetics, nutritional status, medications and environmental and physical as well as emotional stressors. There occurs organ-specific decline in functions with age in the older adults, such as decreases in strength, balance and cognition. The reserve capacity in a healthy young adult is 7 to 11 times greater than the average demand, but, by age 85, organ reserve is reduced to about 50% or less of its original capacity. There lies an individual variability in the organ reserve as well as the pace at which it diminishes with age. The decrease in organ mass, tissue anatomy and physiological function with age have been demonstrated in the human heart, brain, liver, kidney, salivary glands, stomach and muscle tissue. The major storage reservoir of protein in the body is skeletal muscle, which tend to be lost with age manifesting as decrease in muscle mass and strength. By 6th decade of life, voluntary muscle contractile strength decreases by over 20% in both men and women. By the time men and women are in their 7th or 8th decade of life,on average they have lost about 20% to 40% of the contractile strength of voluntary muscles, and over 50% by the 9th decade.
The brain shows a decrease of viable cells with age, amounting in some areas to about 25% to 30% decrease, and an overall decrease in brain tissue of about 9% to 17%. Similarly, renal mass tends to decrease with advancing age. From birth to young adulthood, renal mass increases from about 50 g to 400 g, then decreases to 300 g in the 9th decade. The cardiac output shows a significant average decline of approximately 1% per year after the third decade of life [11]. The aging research supports the notion that rate, and severity of decline varies between individuals and even between specific organs within individuals and may not always correlate with age. Further, the clinical trials have shown that declines in organ mass and function are reversible in some tissues, such as muscle and brain. The organ reserve appears to be, in part, a joint contribution of excess metabolic capacities of several metabolic pathways and biochemical structures. These pathways collectively contribute to organ reserve and enhance the ability of the tissues to cope with stress and recovery from injury. Physiologically, the organs are thought to have a certain capacity to withstand perturbations and return to equilibrium or homeostasis, a concept that has been termed organ redundancy, which in turn depends on the reserve [12]. The concept of redundancy applies to various essential physiological functions frequently in overlapping ways that govern and adapt the organism as a whole to the environments and social circumstances [13]. The decline or exhaustion of the excess capacities diminishes organ reserve.
The Concept of Degeneracy and Plasticity
The degeneracy refers to the physiological concept that different pathways can lead to the same functional output [14]. Thus, degeneracy, is the ability of elements that are structurally different to perform the same function and relates to the structural variation underlying functional plasticity [15]. It is different from redundancy, in which the same function is performed by identical elements. The degeneracy promotes stability in a self-organizing system, but it also allows elements to functionally diverge by an evolutionary process [16].
Degeneracy is a property of complex adaptive systems operating at multiple levels. In biological systems, the loss of a component can be compensated either by redundant elements (the other identical components) or by degenerate elements (structurally different components that perform the same function). Degeneracy is a phenomenon whereby different structural permutations recurrently lead to similar end results. Degeneracy is not limited to the internal structures of an organism but may also occur between internal structures and environmental resources, enabling epigenetic adaptation to the environment. Plasticity of biological systems refers to the ability of living organisms to inculcate a change in their state in response to stimuli for an adaptive response. Plasticity isusually an evolutionary adaptation to environmental variation that is reasonably predictable and occurs within the lifespan of an individual organism, allowing to adapt the phenotype to an external or internal stressor. The plasticity can increase fitness, generate novelty and facilitate evolution. Plasticity of biological systems can occur at various levels such as molecular, cellular, systemic and behavioral [17].
Neuroplasticity, neuroelasticity or neural plasticity is the ability of the brain to change brain activity associated with a particular function transferred to a different area, the proportion of grey matter or strengthen or weaken the synapses over time [18]. The aim of neuroplasticity is to optimize the neural networks during phylogenesis, ontogeny, and physiological learning, as well as after a brain injury [19]. The recent research has shown that many aspects of the brain can be altered even through adulthood [20]. Neuroplasticity can be observed at multiple scales, from microscopic changes in individual neurons to larger-scale changes such as cortical remapping in response to injury, which has significant implications for recovery from brain damage. The modulation of neuroplastic mechanisms by physical and chemical agents is a promising therapeutic tool in restorative neurology [21].
Molecular Basis of Reserve and Redundancy
Reserve Capacity and Intermediary Metabolism
The intermediary metabolism refers to the cellular biochemical reactions and metabolites. It is enriched with biochemical steps or intracellular structures exhibiting excess metabolic capacity than required for normal basic functions [22]. The excess metabolic capacities are present in energy and redox systems such as glycolysis, hexose monophosphate shunt (HMS), tricarboxylic acid (TCA) cycle, oxidative phosphorylation and Na/K ATPase. The energy and redox systems are intrinsically connected to the cellular structural and metabolic integrity. The mitochondrial DNA (mtDNA) found in multi-copies, serves as a structural excess capacity for cellular Energy metabolism and contributes to organ reserve. In addition, the DNA repeats in telomeres serve as structural excess capacity. Thus, there is a built-in robust metabolism and structural mechanisms for stress response and repair. These mechanisms decline with age around the 5th decade as seen in the skeletal muscle which shows decreasing mitochondrial respiratory capacity [23]. The organ reserve and redundancy may require the contribution of excess metabolic capacities from various pathways of intermediary metabolism relating to energy metabolism and genome stability. There occurs molecular stress with the increased metabolic demand, chronic stress or deranged homeostasis leading to hyperfunction and compensatory hypertrophy. The molecular correlates of hyperfunction utilize the excess metabolic capacities for the adaptive response, as seen in the heart response to chronic high blood pressure in form of myocardial hypertrophy [24].
Apart from, the effacement of the excess metabolic capacities, aging at the molecular level is characterized by the progressive accumulation of molecular damage through environmental and metabolically generated free radicals, spontaneous errors in biochemical reactions and nutritional components. The damage to the maintenance and repair pathways comprising homeo-dynamic mechanisms leads to age-related failure of homeo-dynamics, increased molecular heterogeneity, reduced stress tolerance, altered cellular functioning, senescence and apoptosis [25]. It is argued that the cellular life involves inevitable accumulation of damage at cellular level which is diluted when cells divide if the damage is milder and the severe damage is cleared by protective systems. The milder damages at cellular level lead to cell cycle arrest and restriction on cell division necessarily leading to cellular senescence and aging, accompanied by loss of physiological reserve and redundancy at tissue level. In the long run, the processes affecting the damage accumulation are lifespan regulators [26].
Excess Metabolic Capacity of Bioenergetics Pathways
The excess metabolic capacities include enzymatic activities, copy numbers of mtNDA and repeats of telomeres. The excess capacity may also rely on the functional resilience of a biochemical network. Further, the excess metabolic capacities appear to be interconnected and influence each other through synergistic or additive effects. The reduction in excess metabolic and structural capacities results in an increase in cell entropy and oxidation resulting in cell senescence, transformation and apoptosis, and compromised organ reserve. Energy is required for various cellular functions maintaining activity, structural integrity and the homeostasis. The bioenergetics pathways adjust to the functional demand of the biological system. The increased functional demand leads to metabolic and physical stress, and production of excessive heat and free radicals, which may accelerate the cell entropy [27]. The excess capacity of bioenergetics pathway intermediates affects the aging process through altering the cell epigenetic patterns as documented by the increased levels of acetyl-CoA and NAD in aging Drosophila and mice, seem to influence the lifespan by altering the epigenetics balance [28,29]. The glycolytic pathway as such has excess capacity in vivo and its excess metabolic capacity is demonstrated by excess activity its enzymes, such as phosphorglucose isomerase, phosphor-glucomutase, triosephosphate isomerase and glycerol-phosphate dehydrogenase, which are present in considerable excess capacity. NADPH helps the cells resist oxidative stress and protects cellular functions from oxidative damage [30].
Excess Capacity of Mitochondrial DNA
The mtDNA is critical for maintaining oxidative phosphorylation and ATP production. It also maintains an excess respiratory capacity. The mtDNA-encoded subunits provide the catalytic activities in electron transport chain (ETC) complexesI, III, and IV as well as ATP synthase. The coordinated gene expressions of mtDNA and nuclear DNA are necessary for the assembly of the ETC complexes and ATP synthase. Further, the mitochondrial complex IV exists in excess relative to the other three ETC complexes and drives most of the respiratory capacity of the ETC. The excess capacity of complex IV appears to decline with age and in animal models, the decrease in the reserve capacity of complex IV diminishes resilience and shortens lifespan in Drosophila melanogaster [31]. Whereas, function-reducing mutants in the respiratory complexes of the mitochondria extend, rather than shorten the life span in C elegans, which can be interpreted as a trade-off for delayed development and loss of fertility. It appears that the metabolic compensation by an alternative metabolic pathway bypass and the mutated enzyme could explain the effect of the mutations in C. elegans on longevity. The higher organisms, including flies and mammals lack the specific alternative metabolic pathways that bypass and compensate for, the deficient mitochondrial function. Given that the damage to mtDNA increases with age, reduced mtDNA stability negatively impacts the reserve capacity of mitochondrial respiration and thus the organ reserve and redundancy. At a certain threshold level of mtDNA mutant load, there occur cellular dysfunction and the accumulating mtDNA mutations appear to be involved in pathogenesis of various agerelated disorders of skeletal muscle, heart and vascular system, and neurodegenerative diseases [32].
The Telomeres Reserve Capacity
Telomeres are located at the terminals of a linear DNA string of chromosomes and have reserve capacity of the repetitive DNA sequence TTAGGG, which maintains genome integrity and controls cellular senescence and apoptosis. Telomeres excess capacity is essential for maintaining metabolic functions for tolerating stress and recovery from an injury and delaying cell senescence. The constitutive telomerase activity appears to represent an excess metabolic capacity that through oxidative defence and DNA repair mechanisms compensates for the short telomere. Each time a somatic cell divides, there occurs erosion of the telomere sequence. Further, the exposure to oxidizing agents, such as free radicals, radiation and a high oxygen tension, cause telomeric DNA damage and telomeres dysfunction which induces DNA damage response (DDR) and triggers replicative senescence [33]. The mtDNA damage and mutations increase sharply after the fifth decade of age. Major causes for mtDNA susceptibility to mutations include oxidant injury and limited repair of mtDNA. Further, aging reduces the copy number of mtDNA. Ecological factors can also contribute to increasing mtDNA instability and thus influence the aging process. Mitochondrial dysfunction contributes to telomeres shortening and senescence in somatic cells. These factors compromise the bioenergetics reserve capacity, increase cell entropy, trigger cell senescence, and apoptosis and accelerate the process of aging and increase the risk of age-related disorders.
Physiology of Metabolic Reserve and Redundancy
The Bio-Homeo-Dynamic space
The living systems owe their survival and health to a series of complex biochemical pathways of maintenance and repair. Simultaneously, the defence systems create the homeo-dynamic space characterized by stress tolerance, molecular damage control and continuous remodeling. The homeo-dynamics encompasses the ever-dynamic biological systems - cells, tissues, organs and organisms, which are never static. The idea of homeo-dynamic space may be considered as the ‘survival ability’ or the ‘buffering capacity’ of a biological system [34]. It refers to the three main characteristics of the living systems which are the ability to respond to external and internal stress, the ability to control the levels of molecular damage and the ability to constantly repair and adapt in dynamic interactions. The biological systems maintain the biological rhythms, sense and respond to intra- and extra-cellular stressors through innate and adaptive immune responses, scavenge and remove of reactive oxygen and other free radical species and keep thermal regulation and neuro-endocrine balance. Concurrently, there occurs repair of nuclear and mitochondrial DNA, repair and turnover of normal and damaged RNA and proteins, detoxification of chemicals, drugs and nutritional metabolites, removal of damaged membranes and organelles and wound healing, apoptosis and tissue regeneration.
Maintenance and Repair in Biological Systems
The maintenance and repair systems, and bio-homeodynamics depend on reserve and redundancy and may explain the issues related to aging, age-related diseases and longevity. The aging and the age-related diseases can be held as the consequences of a progressive shrinkage of the homeo-dynamic space, due to the failure of maintenance and repair. The cells are the basic building blocks of tissues and with aging, become larger but less able to divide and multiply. There occurs an increase in intracellular pigments and fatty substances. Many cells lose their ability to function or begin to function abnormally. The aging changes occurring in the cells, tissues and organs, in turn affect the functioning of the biological systems [35]. Several metabolic pathways including bioenergetics pathways and antioxidants system, exhibit excess metabolic capacities than required for basic physiological demand in vivo. The excess metabolic capacities can be viewed as an innate mechanism of adaptability that substantiates organ reserve. When the metabolic excess capacities or organ reserves are impaired or exhausted, the ability of the cell to cope with stress is reduced leading to cellular senescence, transformation or apoptosis. Aging is a complex process due to the interaction of many lifelong influences which include heredity, environment, culture, diet, exercise and leisure, past illnesses, and various other factors. All vital systems begin to lose some function with age during adulthood.
The aging organs slowly lose function, which may not be noticeable because the organs are rarely used to their fullest ability and have a reserve ability to function beyond the usual needs. Some body systems begin aging as early as age 30, whereas other aging processes are not common until much later in life. Further, age affects each individual differently from others. With aging, the connective tissue becomes stiffer making the organs, blood vessels and airways more rigid. The cell membrane changes affect diffusion of oxygen and nutrients and removal carbon dioxide and other wastes. Many tissues atrophy. The major changes in organ reserve occur in the heart, lungs, and kidneys, usually appearing slowly and over a long period. The stressors producing an extra workload include illness, significant life changes and sudden increased physical demands on the body as a result of change in activity level. The recovery from illnesses is seldom complete, leaving some residual disability. The loss of reserve also makes it harder to restore homeostasis in the body with robustness.
The Importance of Redundancy
The human body is designed with parts that are supposed to last a lifetime. The redundancy ensures that when function of a part of organ is compromised because of blockage of blood vessels or airways, the other parts can take over to preserve the vital functions. When the severity of the injury or disease is mild to moderate, often the patient remains asymptomatic or subsymptomatic, as the loss of functionality of the diseased areas is compensated by the unaffected regions. With progressing pathology, when the unaffected areas lose the ability to compensate, there occurs a functional decline manifesting with disruption of normal function and symptoms and signs of the disease. The disease-related outcomes or other derivative measures, thus, cannot be predicted on basis of clinical features but the screening of the organ is required to provide a measure of unaffected or healthy anatomical structure remaining to understand the prognostic outcomes. Further, evidence from multiple parameters is needed as one parameter may not provide sufficient information. The majority of research and clinical studies using the primary endpoint approach, thus, may be erroneous because the biological redundancy entails the use composite endpoints to determine the extent of damage to an organ, disease status and best possible treatment intervention in a particular setting [36].
Impact of Senescence of Organs and Systems
There is rise in percentage of the older adult population over the years as the life expectancy is improving and fertility trends are declining. With age, the functional reserve and redundancy of various organs wear out. Most organ functions decline by about one percent per year, the decline starting at about age forty or earlier, continues throughout life often without perceptible loss of function. Further, the age of functional decline is variable for different organs in different individuals. The internal organsincluding brain, heart, lungs, and kidneys show a slow and gradual decline. The older adults often have multiple age-related functional impairments at level of various organs due to loss of organ reserve and redundancy.
Senescence, Debility and Age-related Diseases
The process of aging of organs is universal, progressive and vastly irreversible. With aging, the brain loses its abilities affecting the cognitive function. The peak cognitive function is around age of twenty, plateaus for about ten years and then starts the slow decline. Given the large amount of redundancy in the brain, the decline is not noticeable and becomes apparent initially with highly technical or fast paced activities, in unfamiliar situations, or in emotional or physical stressful situations. Some individuals may age without much deterioration in body organs and systems, whereas others may be ridden with the extreme age-associated changes. Further, the aging process may affect some body systems more severely whereas others are spared from a serious disability. Thus, aging is associated with a progressive but varying decline in numerous physiological functions and significantly affects the body organs and various systems including the brain, heart and vasculature. Similarly, the bone mineral density (BMD) along with a protein-collagen matrix in the bones is highest at age of 20 BMD in men, higher than women. Thereafter, for women as for men, the decline is about one percent per year leading to gradual osteopenia and osteoporosis, and increased fracture risk. Muscle mass and strength decline in a similar fashion resulting in sarcopenia. Most people lose perhaps 30 percent of their muscle mass between ages 50 and 70. The usual loss is about 1 percent per year starting in third decade of life. The typical aspect of aging is the variability of the functional decline affecting different organs.
Concepts of Genome Stability and Aging
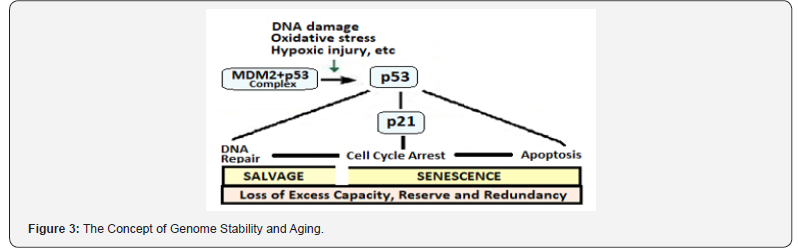
Pathophysiologically, a number of diverse stimuli induce senescence. These factors appear to converge on certain pathways that influence cell cycle regulation, DNA repair and apoptosis, and the process of cellular senescence. At the cellular level, these pathways are regulated by the tumor suppressor proteins p53 and pRb. The p53 is a crucial mediator of the cellular response to damaged DNA and dysfunctional telomeres, and in turn activates the cyclin-dependent inhibitor p21 (Figure 3). It is considered that senescence occurs via the p53 pathway in response to DNA damage and telomere dysfunction, whereas the p16/pRb pathway mediates senescence caused by oncogenic stimuli, chromatin disruption, and other cellular stresses [37]. Many of the genes and their related pathways have been studied in model organisms like Saccharomyces cerevisiae (yeast), the Caenorhabditis elegans (nematode), Drosophila melanogaster (fruit fly) and Mus musculus and other mice. Further, the chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in animal studies as well as in the human endothelial cells. Angiogenesis is another critical mechanismresponsible for repairing tissues after damage caused by daily wear and tear and events such as myocardial ischemia and infarction and cerebrovascular stroke. Processes and pathways having impact on angiogenesis, include cellular senescence, telomere attrition, oxidative damage, NO and hypoxia, and vascular growth factors [38]. The age-related impairment of angiogenesis contributes to increased end-organ damage and affects cardiovascular health.
Alterations in CV Morphology and Physiology
The age-related CV pathophysiological alterations at macro level include altered ventricular systolic and diastolic functions and diminished cardiac reserve, cardiac hypertrophy, increased arterial stiffness, and impaired endothelial function. Simultaneously, important changes are taking place at micro level; at cellular, subcellular and molecular levels. At the subcellular level, mitochondrial oxidative stress and dysfunction play an important role in cardiac aging. There is an increasing evidence to suggest that microRNAs (miRNAs) are important regulators of aging and cardiovascular diseases [39]. The evidence suggests that increased miR-34a expression in the aged heart contributes to cardiac aging [40]. At the cellular and subcellular levels, there are defects in mitochondrial function. The myocardial ATP content falls due to decreased ability to generate ATP by oxidative metabolism and leads to progressively reduced myocardial performance. Myocardial metabolism is altered, with the substrate utilization switching from mostly fatty acids to glucose. The macro- and micro level changes in CV structure and function pose the older adults to the increased risk for CV Diseases, manifesting as various patterns of diseases of heart and vasculature, have impact on quality of life, and affect the individual survival. In a response to increased vascular resistance, there occurs remodelling of myocardium which includes myocyte hypertrophy and alterations in extracellular matrix (ECM). The cardiac hypertrophy results in increased myocardial oxygen demand and decreased coronary perfusion pressure due to compression of the coronary microcirculation causing a mismatch in oxygen/nutrient supply-demand and inducing a state of relative myocardial ischemia. The reduced myocardial performance and functional cardiac reserve and predisposes to the development of heart failure (HF). The HF adversely affects the cardiac pumping capacity, which may be asymptomatic for a variable period. The clinical course of HF isgradually progressive, which affects QOL especially during later years of life.
The research suggests the existence of mitochondrial plasticity. The heart has multiple redundant mechanisms that increases its resiliency [41]. The changes in the metabolic fluxes in the failing heart lead to accumulation of metabolic intermediates that alter signal transduction pathways to promote LV remodeling [42]. In the failing heart, the redundant ATP production/transportation systems, such as CK (creatine kinase), mitochondrial electron transport system, AK (adenylate kinase), glycolytic, and guanine nucleotide phosphor-transfer pathways, may all be impaired leading to exhaustion of the cardiac reserve [43]. There is impaired resiliency of the heart due to blockade of one or multiple metabolic pathways and the redundancy of energy production systems entails that some regulators of mitochondrial metabolism such as calcium may contribute to the maintenance of cellular energy homeostasis in the failing heart. The cardiac muscle can increase ATP turnover rates from resting to heavy exercise, by 10-fold; whereas, the skeletal muscle manifests an energy reserve range of up to 100-fold [44]. The apparent narrower range in the heart relative to skeletal muscle may reflect fundamental differences in the physiological roles of the heart versus skeletal muscle, in which the heart maintains consistently high levels of contractile function, whereas the need for large increases in skeletal muscle performance is intermittent. The data are consistent with the notion that metabolic stress modulates mitochondrial oxidative phosphorylation activity in the heart. The myocardial energy insufficiency has been recognized as a key feature of systolic heart failure [45]. In the failing heart, the mitochondrial bioenergetics dysfunction is brought about by a complex interplay of multiple perturbations that progressively hamper the myocardial function. The activity of complexes I and IV, nicotinamide nucleotide transhydrogenase (NADPHtranshydrogenase, Nnt) and the Krebs cycle enzymes isocitrate dehydrogenase, malate dehydrogenase and aconitase are markedly decreased in end-stage heart failure. The diminished REDOX capacity with lower total glutathione and coenzyme Q10 levels are also a feature of chronic left ventricular failure. In brief, the energy deficiency in end-stage failing human left ventricle predominantly involves concomitantly impaired activities of key electron transport chain and Krebs cycle enzymes. Augmented oxidative modification of these enzyme subunit structures, and the formation of highly reactive secondary metabolites, involves dysfunction due to diminished capacity for management of mitochondrial reactive oxygen species, which contribute further to progressive decreases in bioenergetic capacity and contractile function in human heart failure [46].
Cardiovascular Aging and Disease
In general, aging is associated with a progressive but varying decline in numerous physiological functions, and significantly affects the body organs and various systems including the heart and vasculature. The aging process and its impact, in turn, depend on various factors, from individual genetic constitution to the lifestyle patterns which include the environment and lifestyle influencing the risk factors variously. In fact, age is the most important determinant of cardiovascular (CV) health. With advancing age, there is an increase in incidence and prevalence of atherosclerosis, hypertension, coronary artery disease and cardiac failure, and cerebrovascular and peripheral vascular disease. Further, the cardiovascular system (CVS), which is a nonlinear and nonstationary system, is affected by multiple factors such as systolic arterial pressure, arterial stiffness and systemic vascular resistance and their interactions [47]. During perinatal life striated muscles grow through the acquisition of more contractile cells - myocytes or fibres, followed by their postnatal hypertrophy. With aging process, in the aging heart and skeletal muscle, these events are reversed. There occurs a progressive loss of myocytes that cannot be fully compensated despite the presence of cell renewal systems or reactive myocyte hypertrophy [48]. Hence the functional reserve capacities of the heart and skeletal muscles decline with age. In fact, the reserve capacity of the heart is a major determinant of an individual’s ability to remain active and cope with pathophysiological stressors and cardiovascular diseases. There occurs an exponential rise in both the CV risk and the incidence of CV events in older adults parallel with the advancing age [49]. Statistically, the older adults (>60 years old) account for more than 80 percent of coronary heart disease, more than 75 percent of congestive heart failure, and more than 70 percent of atrial fibrillation patients [50]. It is estimated that by 2030, approximately 20% of the population will be aged 65 or older, and in this age group, CVDs will amount to over 40% of all deaths and rank as the leading cause of disability and suboptimal quality of life during later years [51,52].
Conclusion: Retarding Aging and its Impact
The modalities to prevent, retard or treat the CV changes that accompany aging will reduce the prevalence of CVDs. But, retarding the rate of progression of CVDs at subclinical level need to be considered before the clinical disease becomes manifest. There are strategies aiming to reduce the oxidative stress which exacerbates aging process and leads to debility to human body in general and CVS in particular. The life-style interventions in form of regular physical exercise, smoking cessation and intervention in sleep disorders are established practical ways to prevent and treat atherosclerosis and CVD. There are promising data from experimental biology high-lightening calorie restriction (CR) as a tool for reduction in oxidative stress and increasing longevity. The dietary modification in form of calorie restriction with adequate nutrition (CRAN) and adding antioxidants as nutraceuticals is a practical and simple intervention. Multiple mechanisms have been implicated in the beneficial effects of CR including inhibition of mTOR signaling, normalization of mitochondrial biogenesis, attenuation of mitochondrial ROS production and the subsequent ROS-induced signaling, and increased SIRT1 signaling [53]. There are several synergistic mechanisms at work with CRAN (Fig 4). There occurs an increase in NO with a combination of reduced ROS is both neuro and cardio protective, due to activation of the Nrf2 antioxidant pathway. The CR reduces oxidative stress-induced induction of pro inflammatory markers, like NF-κB-mediated cytokine synthesis, protection from endothelial damage and reversal of the progression to atherosclerosis. In human clinical studies, the CR has been shown to improve diastolic function and rejuvenate the aging heart [54.55]. The use of the CR may appear challenging. But it is possible to mimic the beneficial effects of diet regulation by using certain neutraceuticals or pharmaceuticals, called CR Mimetics (CRMs) [56,57]. The use of CRMs such as resveratrol and metformin, which activate the SIRT1-AMPK system, and rapamycin, which inhibits mTOR, show that it is possible for a rodent to be obese and sedentary while maintaining the physiology of a lean animal [58,59]. The supplementation of CRMs like resveratrol have been shown calorie restriction-like effects on energy metabolism and metabolic profile in obese humans [60].
References
- Hines TM (1998) Comprehensive review of biorhythm theory. Psychol Rep 83(1): 19-64.
- Takahashi JS, Zatz M (1982) Regulation of circadian rhythmicity. Science 217(4565): 1104-1111.
- Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418 (6901): 935-941.
- Bernard S, Gonze D, Cajavec B, Hanspeter Herzel, Achim Kramer (2007) Synchronization-induced rhythmicity of circadian oscillators in the suprachiasmatic nucleus. PLoS Computational Biology 3(4): e68.
- Draijer S, Chaves I, Hoekmana MFM (2018) The circadian clock in adult neural stem cell maintenance. Prog Neurobiol 173: 41-53.
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals Science 254(5032): 726-729.
- Otsuka K, Cornélissen G, Halberg F (2001) Circadian rhythms and clinical chronobiology. Biomed Pharmacother 55(1): 7S-18S.
- Neustadt J, Pieczenik SR (2008) Organ reserve and healthy aging. Integrative Medicine 7(3): 50-52.
- Sehl ME, Yates FE (2001) Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people J Gerontol A Biol Sci Med Sci 56(5): B198-208.
- Atamna H, Tenore A Dhahbi J, Lui F (2018) Organ reserve, excess metabolic capacity, and aging. Biogerontology19(2):171-184.
- Brandfonbrener M, Milton Landowne M, Shock NW (1955) Changes in Cardiac Output with Age. Circulation 12: 557-566.
- Montgomery HD (2000) Cardiac reserve: linking physiology and genetics. Intensive Care Med 26(1): S137-144.
- Michael J Joyner (2013) Editorial: Physiology and Redundancy. Physiology 28(3):136-137.
- Mason PH, Domínguez D JF, Winter B, Grignolio A (2015) Hidden in plain view: degeneracy in complex systems. Biosystems128: 1-8.
- Tononi G, Sporns O, Edelman GM (1999) Measures of degeneracy and redundancy in biological networks. PNAS 96(6): 3257-3262.
- Maleszka R, Mason PH, Barron AB (2014) Epigenomics and the concept of degeneracy in biological systems. Brief Funct Genomics 13(3): 191-202.
- Kelly SA, Panhuis TM, Stoehr AM (2012) Phenotypic Plasticity: Molecular Mechanisms and Adaptive Significance. Compr Physiol pp.1417-1439.
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR (1964) "Chemical and Anatomical Plasticity of the Brain". Science 146 (3644): 610-619.
- Hugues D (2016) Brain Plasticity and Reorganization Before, During, and After Glioma Resection. Glioblastoma pp. 225-236.
- Sasmita AO, Kuruvilla J, Ling APK (2018) Harnessing neuroplasticity: modern approaches and clinical future. Int J Neurosci 128(11) 1061-1077.
- Bergado-Rosado JA, Almaguer-Melian W (2000) Cellular mechanisms of neuroplasticity [Article in Spanish]. Rev Neurol 31(11): 1074-1095.
- Atamna H, Tenore A, Lui F, Dhahbi JM (2018) Organ Reserve, Excess Metabolic Capacity, and Aging. Biogerontology 19(2): 171-184.
- Desler C, Hansen TL, Frederiksen JB (2012) Is There a Link between Mitochondrial Reserve Respiratory Capacity and Aging? Journal of Aging Research pp.9.
- Zimniak P (2012) What is the Proximal Cause of Aging? Front Genet 3: 189.
- Rattan SI (2008) Increased molecular damage and heterogeneity as the basis of aging. Biol Chem 389(3): 267-272.
- Gladyshev VN (2012) On the cause of aging and control of lifespan: heterogeneity leads to inevitable damage accumulation, causing aging; control of damage composition and rate of accumulation define lifespan. Bioessays 34(11): 925-929.
- Hayflick L (2007) Biological aging is no longer an unsolved problem. Ann N Y Acad Sci 1100: 1-13.
- Peleg S, Feller C, Ladurner AG, Imhof A (2016) The Metabolic Impact on Histone Acetylation and Transcription in Ageing. Trends Biochem Sci 41(8): 700-711.
- Miller BF, Hamilton KL. 2017. Overview: the modulation of ageing through altered proteostasis. J Physiol 595(20): 6381-6382.
- Bolaños JP, Almeida A, Moncada S Glycolysis: A bioenergetic or a survival pathway? Trends Biochem Sci 35(3): 145-149.
- Klichko, Chow ES, Kotwica-Rolinska J, Orr WC, Giebultowicz JM et al. (2015) Aging alters circadian regulation of redox in Drosophila. Front Genet pp.6: 83.
- Sallevelt SCEH, de Die-Smulders CEM, Hendrickx ATM, Hellebrekers DM, de Coo IF et al. (2017) De novo mtDNA point mutations are common and have a low recurrence risk. J Med Genet 54(2): 73-83.
- Fumagalli M, Rossiello F, Mondello C, Fabrizio d’Adda di Fagagna (2014) Stable cellular senescence is associated with persistent DDR activation. PLoS One 9(10): e110969.
- Rattan SI (2015) Biology of ageing: principles, challenges and perspectives. Rom J Morphol Embryol 56(4): 1251-1253.
- Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, et al. (2018) The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne)5: 61.
- Atamna H, Tenore A, Forshing Lui F, Dhahbi JM (2018) Organ reserve, excess metabolic capacity, and aging. Biogerontology 9(2): 171-184.
- Moslehi J, DePinho RA, Sahin E (2012) Telomeres and Mitochondria in the Aging Heart Circ Res 110(9): 1226-1237.
- La ̈hteenvuo J, Rosenzweig A David Sinclair, Brian North (2012) Effects of Aging on Angiogenesis, Circulation Research 110(9): 1252-1264.
- Afjeh SSA, Ghaderian SMH (2013) The role of microRNAs in cardiovascular disease. Int J Mol Cell Med 2(2): 50-57.
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, et al. (2013) MicroRNA-34a regulates cardiac ageing and function. Nature 495(7439): 107-110.
- Quiat D, Olson EN (2013) MicroRNAs in cardiovascular disease: From pathogenesis to prevention and treatment. J Clin Invest 123(1): 11-18.
- Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, et al. (2016) The failing heart relies on ketone bodies as a fuel. Circulation 133(8): 698-705.
- Abel ED, Doenst T (2011) Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 90(2): 234-242.
- Jianyi Zhang, E Dale Abel (2018) Effective Metabolic Approaches for the Energy Starved Failing Heart: Bioenergetic Resiliency via Redundancy or Something Else? Circ Res 123(3): 329-331.
- Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113(6): 709-724.
- Sheydina A, Riordon DR, Boheler KR (2011) Molecular mechanisms of cardiomyocyte aging Clin Sci (Lond) 121(8): 315-329.
- Sheeran FL, Pepe S (2017) Mitochondrial Bioenergetics and Dysfunction in Failing Heart. Adv Exp Med Biol 982: 65-80.
- Yeh JR, Lin TY, Chen Y, Abbod MF, Shieh JS (2012) Investigating Properties of the Cardiovascular System Using Innovative Analysis Algorithms Based on Ensemble Empirical Mode Decomposition. Comput Math Methods Med 2012: 943431.
- Goldspink DF (2005) Ageing and activity: their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics 48(11-14): 1334-1351.
- North BJ, Sinclair DA (2012) The Intersection between Aging and Cardiovascular Disease; Circ Res 110(8): 1097-1108.
- Incidence of CVD by age and sex, Incidence and prevalence 2006; Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Nat. Heat, Lung and Blood Inst.
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, (2011) et al. Executive Summary: Heart Disease and Stroke Statistics—2011 Update: A Report from the American Heart Association. Circulation123(4): e18-e209.
- Mahmood SS, Levy D, Vasan RS, Wangb TJ. 2014. The Framingham Heart Study and the Epidemiology of Cardiovascular Diseases: A Historical Perspective. Lancet 383(9921): 999-1008.
- Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, et al. (2011) Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 50(1): 117-127.
- Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, et al. (2014) Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13(3): 5295-539.
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO et al. (2006) Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 47(2): 398-402.
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, et al. (2006) Calorie restriction mimetics: an emerging research field. Aging Cell 5: 97-108.
- Kitada M, Koya D (2013) The Use of Calorie Restriction Mimetics to Study Aging. Methods Mol Biol 1048: 95-107.
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, et al. (2006) Resveratrol improves health and survival of mice on a high-calorie diet Nature 444 (7117): 337-342.
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, et al. (2006) Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol 16(3):296-300.
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T et al. (2011) Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14:612-622.
- Vinod N (2017) Aging Heart: Recent Research and Concepts. Gerontol & Geriatric stud 1(1).






























