Histology and Biologic Characteristics of Breast Cancer in Elderly of Balinese Population
IGP Suka Aryana1*, Putu AT Adiputra2, Pande KA Prayudi3, Yulan Permatasari3, Hendra P Setiawan3 and RA Tuty Kuswardhani1
1Geriatric Division, Internal Medicine Department
2Division of Surgical Oncology, Surgery Department, Sanglah Teaching Hospital
3Medical Student of Medical Faculty of Udayana University, Denpasar, Bali, Indonenesia
Submission: March 26, 2018 Published: April 09, 2018
*Correspondence author: IGP Suka Aryana, Geriatric Division, Internal Medicine Department, Denpasar, Bali, Indonesia, Tel: +62361246663; Email: aryanasuka@yahoo.com
How to cite this article: IGP S A, Putu A A, Pande K P, Yulan P, Hendra P S, et al . Histology and Biologic Characteristics of Breast Cancer in Elderly of Balinese Population. OAJ Gerontol & Geriatric Med. 2018; 3(5): 555625. DOI: 10.19080/OAJGGM.2018.03.555625
Abstract
Objective: The aim of this study is to evaluate the histology and biologic characteristics of breast cancers in older Balinese women (age >65 years).
Materials and Methods: This is a hospital-based analytic cross-sectional study. Data are obtained from the breast cancer registry of admitted patients in Sanglah General Hospital.
Results: A total of 1,045 cases of breast cancer among Balinese women were recorded during the period of 1997-2013, of which 75 (7.2%) were diagnosed in elderly. Infiltrating ductal carcinoma (IDC) was the most common histologic type (75.4%), followed by infiltrating lobular carcinoma (ILC, 4.3%), invasive carcinoma of no special type (4.2%), mucinous carcinoma (1.9%), medullary carcinoma (0.9%), and malignant phylloides tumor (0.8%). There was a significant difference in the rate of IDC (76.2% vs. 65.3%, p = 0.035) and invasive carcinoma of no special type (3.7% vs. 10.7%, p = 0.010), but not for ILC, mucinous carcinoma, medullary carcinoma, and malignant phylloides tumor. The rate for ER- positive, PR-positive, p53-positive, and HER2-positive, as well as the rate of Luminal A, Luminal B, over-expressed HER2, and Triple Negative breast cancer also did not differ significantly between younger and older patients.
Conclusion: The biologic characteristics of breast cancer differ between younger and older Balinese women in terms of histologic type but not for the expression of hormone receptors.
Keywords: Balinese; Breast cancer; Elderly; Histology; Immunohistochemistry
Abbreviations: SEER: Surveillance Epidemiology and End Results; ER: Estrogen Receptor; VEGF: Vascular Endothelial Growth Factor; PR: Progesterone Receptor; HER2: Human Epidermal Growth Factor Receptor-2; VEGF: Vascular Endothelial Growth Factor; IDC: Infiltrating ductal carcinoma
Introduction
Breast cancer is the most common cancer among women in both developed and developing countries. It is also the leading cause of cancer death in females. In the United States, there were more than 200,000 new cases of breast cancer and 40,000 deaths in 2005 [1]. Currently, the incidence of breast cancer in Indonesia is 26 per 100.000 populations [2]. Advance age is the most important risk factor for breast cancer. According to Surveillance Epidemiology and End Results (SEER) databases, 43% of breast cancer patients are aged 65 and older at diagnosis, and 58% aged 65 and older at the time of breast cancer-related death [1].
The general population of Indonesia is now aging. According to the data published by the Indonesian Ministry of Health, with the increase of life expectancy, the number of older population will be continuously increasing over the next decades. In 2000, the life expectancy for Indonesian is 64.5 years with 7.18% of the population are aged 60 years and older. The life expectancy increases to 69.43 years in 2010 (elderly population being 7.56% of total population) and to 69.55 years in 2011 (elderly population being 7.58% of total population) [3]. Therefore, elderly will represent an increasing cohort of patients with breast cancer in the future.
Although the number of elderly patients with breast cancer is increasing, knowledge about possible differences in the biology and clinical outcomes of breast cancer according to age is limited. The relative under-enrollment of elderly patients in clinical trials is an important factor contributing to this limited knowledge [4]. In spite of the paucity of data, age is considered to be an important determinant of therapy, and the pattern of care of breast cancer patients differs depending on age [5-7]. More information about the biology and clinical features of breast cancer is needed to support the different approaches to therapy in elderly patients. Several large reviews have reported that the biology of breast cancer differs according to age, with the elderly tend to have more favorable tumor biology [8,9]. However, little is known about the biology of breast cancer among older Indonesian women, particularly of Balinese ethnicity. The aim of this study is to evaluate the biologic characteristics of breast cancer among older Balinese women.
Materials and Methods
We use the breast cancer registry of Sanglah General Hospital as the source of our data. The registry is the largest hospital registry in Bali covering Balinese population in nine districts, including Denpasar, Badung, Tabanan, Negara, Gianyar, Bangli, Klungkung, Karangasem, and Buleleng. The registry also includes data from patients that reside outside the island of Bali since Sanglah General Hospital is the center of referral for East and West Nusa Tenggara region. Thus, we exclude patients who reside outside Bali. We got 1,045 cases of primary breast cancer diagnosed between the year of 1997 and 2013. Only 160 cases presented with hormone receptor status (ER, PR, HER2) and 128 cases presented with p53 staining since immunohistochemistry analysis was applied since 2004 and it was costly and not covered in insurance, so not every patient could afford the examination. Only tumor with histologic type of infiltrating ductal carcinoma present with grading (n=711). We defined elderly as those who are 65 years and older. Then, we describe the characteristics for histology and grading, hormone receptor (ER, PR, HER2, p53), and IHC classification (Luminal A, B, HER2-type, Triple negative) according to the age group (elderly vs. non-elderly breast cancer). We compare the rate for breast cancer characteristics among the elderly and non-elderly or younger women. We use Statistical Packages for Social Science (SPSS ver.16) to aid the statistical analysis. The rates for each characteristic within each group of age were presented within percentage. Chi-square (x2) or Fischer-Exact test was used to test for significance between 2 proportions.
Results
A total of 1,045 cases of breast cancer among Balinese women of various ages were recorded during the period of 1997-2013, of which 75 (7.2%) cases were attributed to elderly (age >65 years). The mean age was 45.35 ± 8.74 years among the younger women and 70.03±4.49 years among the older women (mean age ± SD). The biologic characteristics of all study population were presented in Table 1. Infiltrating ductal carcinoma (IDC) was the most common histologic type, followed by infiltrating lobular carcinoma, invasive carcinoma of no special type, mucinous carcinoma, medullary carcinoma, and malignant phylloides tumor. Luminal A was the most common type of breast cancer in this study population.
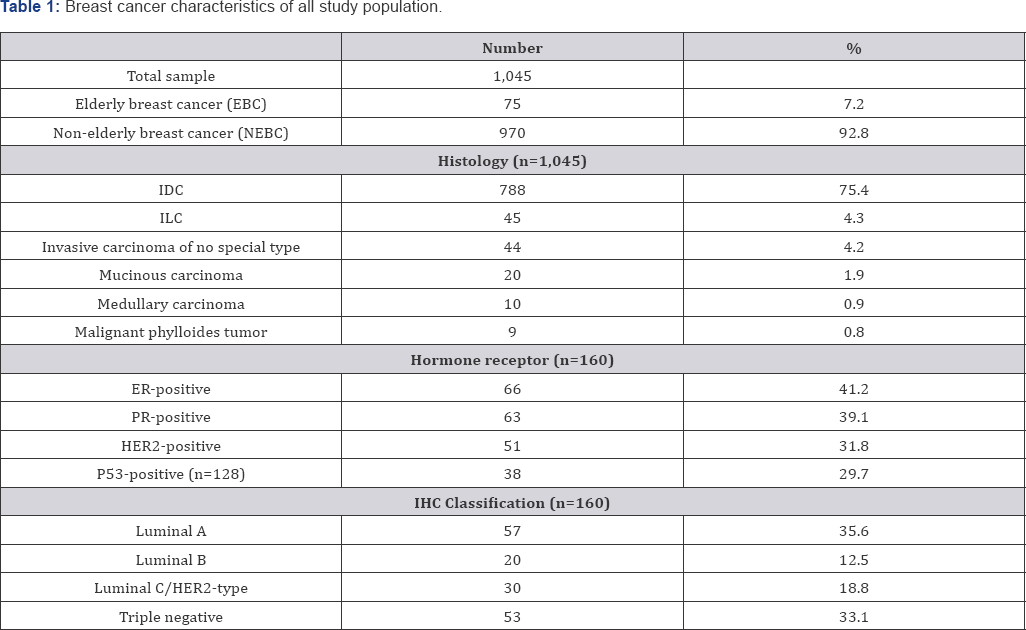
Elderly had a significantly lower rate of IDC but a higher rate of invasive carcinoma of no special type compared to younger patients. However, there was no significant difference in the rate of ILC, mucinous carcinoma, medullary carcinoma, and malignant phylloides tumor between older and younger patients. The rate of high grade IDC also did not differ significantly (Table 2). Although there was a tendency of the younger patients to had Table 2: Histologic type according to age group.a higher rate of positivity for the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2), the results did not statistically significant. The rate of Luminal A, Luminal B, over-expressed HER2, and Triple Negative breast cancer also did not differ significantly between older and younger patients (Table 3).
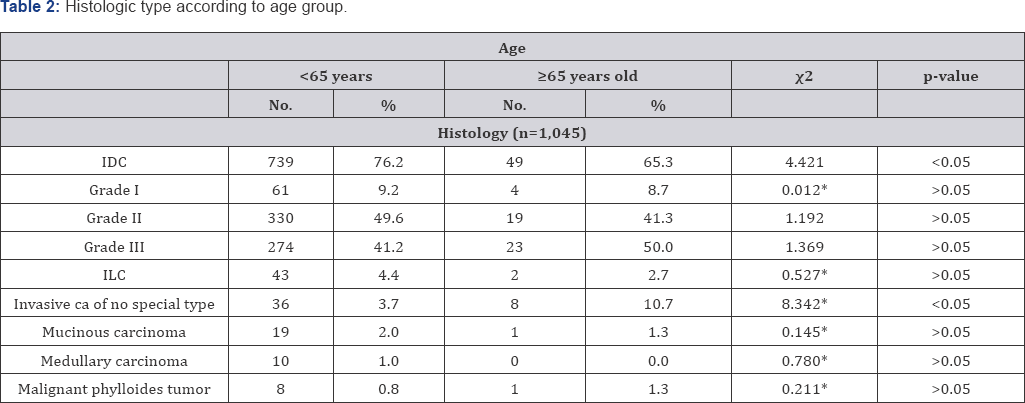
*Fisher-Exact test.
Number of sample that presented with grading was 711, 665 in the younger group and 46 in the older group.
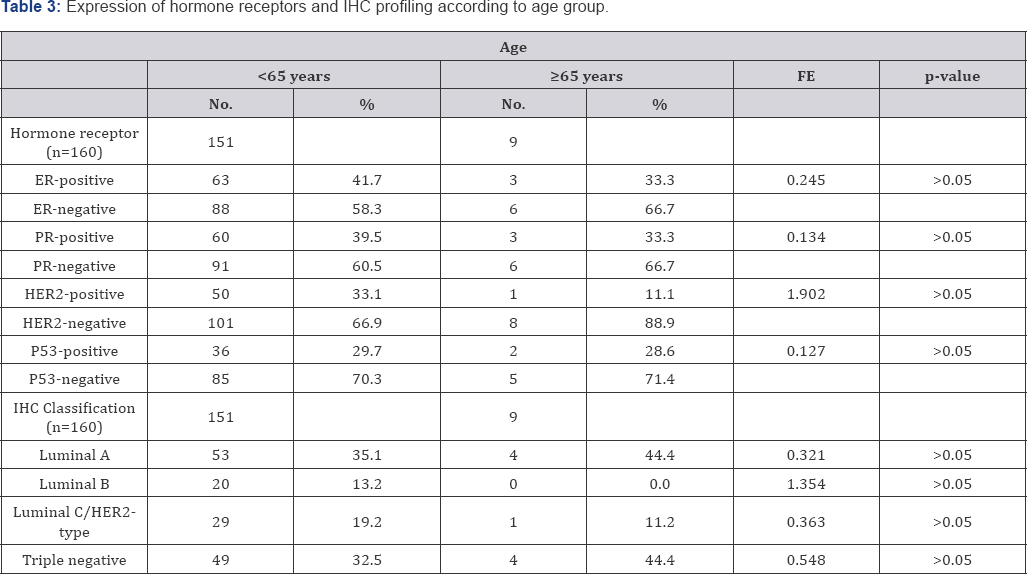
FE: Fischer-Exact test
Number of sample that presented with p53 staining was 128, 121 in the younger group and 7in the older group.
Discussion
Our results suggest that biologic characteristics of breast cancer in older Balinese women are different compared with the younger women. We found a statistically significant difference in the rate of certain histologic subtypes. Older women in our study had a lower rate of IDC but higher rate for invasive carcinoma of no special type. Although there was a tendency for higher rate of mucinous and lobular carcinoma (histologic subtype which are associated with more indolent growth and progression) among older women, these results were not statistically significant. For the expression of certain protein markers (ER, PR, HER2, p53) as well as immunohistochemistry profile, we found no statistically significant difference between older and younger women.
Several large epidemiologic studies involving thousands of patients in North America and Europe confirmed that the biology of breast cancer in elderly patients appears to be different than in young women, with different molecular markers and a slower, more-indolent pattern of growth and progression. In a study involving more than 3,200 patients in Europe and 800 patients from the Massachusetts General Hospital, various prognostic markers were compared according to age [8]. They found no significant association between age and histology (ductal vs. lobular) or tumor stage at diagnosis, although there were significant correlations between age and expression of hormone receptors. They found that estrogen receptor (ER) expression, a known biomarker of improved prognosis, correlated positively with age. ER positivity was observed in 40% of patients aged 40, in 60% of patients aged 60, and in more than 70% of patients aged 80 (p-value <0.001). HER-2 overexpression, a known poor prognostic indicator, correlated inversely with age, with 29% positivity in those younger than 40, 17% in those aged 40-60, 14% in those aged 60-70, and only 9% in those aged >70 years (p-value <0.001). No significant association between age and markers of angiogenesis or invasion, i.e. vascular endothelial growth factor (VEGF), cathepsin D, and uroplasminogen activator.
In a study involving 50,828 and 256,287 women with invasive breast cancer in the San Antonio Breast Cancer and SEER (Surveillance, Epidemiology, and End Results) databases in United States, biologic characteristics of breast cancer are more favorable in older women compared with the younger women[9]. In San Antonio database, the rate of positivity for estrogen receptors increased from 83% in patients 55-64 years old to 87% in patients 65-74 years old to 90% in patients 75-84 years old to 91% in patients ≥85 years old (p<.001). Older women had higher rate of tumors that were diploid, had a low S-phase fraction and normal p53, and were negative for epidermal growth factor receptor and c-erbB2 compared with the younger women. The SEER database also supported similar correlations between age and biologic characteristics [9]. However, in both San Antonio and SEER databases, they observed no statistically significant differences in histological subtype, although there was a trend toward more-lobular and mucinous types (which are associated with more-indolent disease).
However, many of the large studies investigating the biologic characteristics among older women use younger women (age < 60 years) as a control group. In our study, the mean age for the younger group was 45.35±8.74 years (mean±SD). Only few that investigates the correlation between increasing age and the tumor characteristics among the very old patients. In a study involving 49,616 women from SEER-Medicare data set, the investigator examine the tumor characteristics of women age 80 years or older (80-84, 85-89, and ≥90 years) with stage I/II breast cancer compared with younger women (age 67-79 years). They reported that tumor characteristics (i.e. histology, grade, hormone receptivity) were similar across age groups [10]. However, the youngest women in this study were older than most women included in other studies. This study suggested that it is possible that tumors present with more favorable characteristics with older age but beyond age 67 years, these differences are negligible[10]. Other studies have also demonstrated that there were no correlations between increasing age and hormone receptor positivity among women older than 70 years [11,12].
Our study population was of Balinese ethnicity, and therefore are Asians. Asians may have a different tumor characteristics compared to Caucasians or Africans. One study has reported that breast cancer characteristics indeed differ according to race and ethnicity [13]. They reported that relative to non-Hispanic white women, Asians women (Filipinos, Chinese, Koreans, Vietnamese, Indian/Pakistanis) who live in the United States have a greater risk of presenting with biologic characteristics that associated with a poorer prognosis. Asian women had 1.2- to 2.6-fold elevation in the risk of having either ER-negative or PR-negative tumor. Asian women also had 20-70% reductions in the risk of having lobular and/or ductal carcinoma (except for Indians/ Pakistanis). Among Filipino and Chinese women, there were even 1.3- to 3.4-fold increases in risk of mucinous carcinoma[13] .In a study involving 280 Chinese women with invasive breast cancer, it was reported that the immunohistochemical typing characteristics of the elderly and youths were different [14] .
Race/ethnicity may play a significant role to the expression of certain breast cancer phenotype. The role may be attributed to the difference in genetics and lifestyle among the races. We have known that nulliparity/late age at first live birth, early age at menarche, and higher body mass index have been associated with an increased risk of developing an ER-positive tumor, but a decreased risk of developing an ER-negative tumor [15-18]. There was a tendency for Balinese women to be married young and being multipara. Unfortunately, data about parity, age at menarche, body mass index, or other factors associated with the hormonal status are still lacking in our registry.
However, there are several limitations to our study. Our study is a hospital-based cross-sectional study that used data from a hospital registry. The number of elderly breast cancer in our registry is far smaller than the number of non-elderly breast cancer. The number of patients that present with result from immunohistochemistry analysis is even smaller since not every patient can afford the examination. On the other hand, we are not sure of how many percent of Balinese population are covered in our registry since our registry is a hospital registry that rely its data source solely from the admitted patients. Thus, Balinese women with breast cancer that are not admitted to Sanglah General Hospital will not be included in our registry. Our study population may be less representative to the general population of women with breast cancer. Further efforts to increase the number of sample are needed in the future study.
Conclusions
The biologic characteristics of breast cancer differ between younger and older Balinese women in terms of histologic type but not for the expression of hormone receptors. Future study involving a larger number of elderly diagnosed with breast cancer is needed to further understand the exact correlation between age and breast cancer characteristics among the elderly of Balinese population.
Acknowledgement
The authors present their thanks to Made Utari Rimayanti who kindly helped in spell-checking and grammar checking of the manuscript.
References
- (2014) Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review 1975-2002, based on SEER data submission.
- (2007) Dinas Kesehatan Nasional. Data penderita kanker payudara di Indonesia.
- (2013) Data and Information Center Indonesian Ministry of Health. Gambaran Kesehatan Lanjut Usia di Indonesia. Buletin Jendela Data dan Informasi Kesehatan. Jakarta: Bakti Usadha, pp.1-18.
- Goodwin JS, Hunt WC, Humble CG, Key CR, Samet JM (1988) Cancer treatment protocols. Who gets chosen? Arch Intern Med 148: 22582260.
- Bergman L, Kluck HM, Van Leeuwen FE, Crommelin MA, Dekker G, et al. (1992) The influence of age on treatment choice and survival of elderly breast cancer patients in south-eastern Netherlands: a population- based study. Eur J Cancer 28(9): 1475-1480.
- Rijke JM, Schouten LJ, Schouten HC, Jager JJ, Koppejan AG, et al. (1996) Age-specific differences in the diagnostics and treatment of cancer patients aged 50 years and older in the province of Limburg, The Netherlands. Ann Oncol 7(7): 677-685.
- Goodwin JS, Hunt WC, Samet JM (1993) Determinants of cancer therapy in elderly patients. Cancer 72(2): 594-601.
- Eppenberger Castori S, Moore DH Jr, Thor AD (2002) Age associated biomarker profiles of human breast cancer. Int J Biochem Cell Biol 34(11): 1318-1330.
- Diab SG, Elledge RM, Clark GM (2000) Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 92(7): 550-556.
- Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, et al. (2010) Breast Cancer Among the Oldest Old: Tumor Characteristics, Treatment Choices, and Survival. J Clin Oncol 28(12): 2038-2045.
- Gazetas P, Estabrook A, O Neil J (1992) Importance of adequate staging and of hormone receptors in women older than age 70 with breast cancer. Ann Surg 216: 22-26.
- Ferno M, Borg A, Johansson U (1990) Estrogen and progesterone receptor analyses in more than 4,000 human breast cancer samples: A study with special reference to age at diagnosis and stability of analyses-Southern Swedish Breast Cancer Study Group. Acta Oncol 29(2): 129-135.
- Li CI, Malone KE, Daling JR (2002) Differences in Breast Cancer Hormone Receptor Status and Histology by Race and Ethnicity among Women 50 Years of Age and Older. Cancer Epidemiol Biomarkers Prev11(7): 601-607.
- Ji H, Ai N, Li Q, Zhang K, Di W (2014) Clinical pathologies of breast cancer in the elderly and youths and their prognosis. Park J Med Sci 30(3): 535-538.
- McGuire WL, Clark GM (1992) Prognostic factors and treatment decisions in axillary-node-negative breast cancer. N Engl J Med 326: 1756-1761.
- Potter JD, Cerhan JR, Sellers TA, McGovern, PG, Drinkard C, et al. (1995) Progesterone and estrogen receptors and mammary neoplasia in the Iowa Women's Health Study: how many kinds of breast cancer are there? Cancer Epidemiol Biomark Prev 4(4): 319-26.
- Enger SM, Ross RK, Paganini-Hill A, Carpenter CL, Bernstein L. (2000) Body size, physical activity, and breast cancer hormone receptor status: results from two case-control studies. Cancer Epidemiol Biomark 9(7): 681-87.
- Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, et al. (2000) Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol 151(7): 703-714.






























