The Altered Metabolic Homeostasis in Middle Aged and Elderly: Aging and Other Determinants of Insulin Secretion, Action and Resistance
Vinod Nikhra*
Senior Chief Medical Officer and Consultant, Department of Medicine, Hindu Rao Hospital and NDMC Medical College, India
Submission: January 25, 2018; Published: January 31, 2018
*Corresponding author: Vinod Nikhra, Department of Medicine, Hindu Rao Hospital and NDMC Medical College, New Delhi, India, Email: drvinodnikhra@gmail.com
How to cite this article: Vinod Nikhra. The Altered Metabolic Homeostasis in Middle Aged and Elderly: Aging and Other Determinants of Insulin Secretion, Action and Resistance. OAJ Gerontol & Geriatric Med. 2018; 3(2): 555608. DOI: 10.19080/OAJGGM.2017.03.555608
Abstract
Metabolic Homeostasis and Aging: There is a close relationship between homeostasis and metabolism. The inability to maintain homeostasis leads homeostatic imbalance, resulting in various metabolic disorders and disease states including diabetes. Aging is an important risk factor for metabolic disorders including obesity, impaired glucose tolerance, and Type 2 Diabetes Mellitus (T2DM). It affects carbohydrate homeostasis by altering pancreatic β cell function, β cell mass, insulin secretion and insulin sensitivity, and predisposes for insulin resistance (IR), impaired glucose tolerance and diabetes.
Islets β Cells and Insulin Secretion: Insulin is the major hormone for regulating glucose homeostasis. Its secretion from β cells is a complex process and involves the integration of various stimuli. Glucose is transported into the β cells, undergoes oxidation to build-up ATP required for insulin secretion through exocytosis. The aging affects various aspects of β-cell mass and function decline with age, there is decreased GLUT expression and defects in insulin secretion, sensitivity and action. There is impaired glucose tolerance and insulin resistance with age.
Visceral Fat, Aging and Insulin Action: The adiposity with aging leads to increased visceral fat (VF). The excess VF contributes to impaired insulin action and various defects in β-cell mass/function. The insulin deficiency and IR lead to increased rate of lipolysis in adipose tissue which increases circulating FFA levels and exacerbates IR, and activation of hepatic gluconeogenesis, further adding to hyperglycemia and its consequences.
Conclusion-Risk Factors for Altered Glucose Homeostasis: A multitude of factors lead to the somatic carbohydrate homeostatic and metabolic imbalance. The leptin deficiency and resistance leads to obesity and IR, and also associated with aging. The changes in body composition due to decreased lean body mass and contractile strength, and the aging-associated sedentary life style and diminished physical activity are important factors for age-related changes of glucose homeostasis.
Keywords: Aging; Metabolic homeostasis; Insulin resistance; Type 2 diabetes mellitus; metabolic syndrome; GLUT; β-cells; Obesity; Adiposity; Visceral fat; Leptin; Lifestyle factors; Sirtuins; Calorie restriction
Abbreviations: IR: Insulin Resistance; VF: Visceral Fat; T2DM: Type 2 Diabetes Mellitus; GSIS: Glucose-Stimulated Insulin Secretion; CVD: Cardiovascular Disease; AD: Alzheimer disease; GPDH: Glycerol-Phosphate Dehydrogenase; LDCV: Large Dense Core Vesicles; IAPP: Islet Amyloid Polypeptide; CR: Calorie Restriction; ER: Endoplasmic Reticulum; HGP: Hepatic Glucose Production; CRAN: Calorie Restriction With Adequate Nutrition; NAD-: Nicotinamide Adenine Dinucleotide-
Introduction
Homeostasis and Metabolism
Homeostasis refers to the body's ability to physiologically regulate its inner milieu and ensure its stability in response to fluctuations in the outside environment. There is a close relationship between homeostasis and metabolism. An optimal metabolic efficiency is maintained by the negative feedback loops by which homeostasis operates, and in event of any disruption in homeostasis, metabolism will be negatively affected. The inability to maintain homeostasis leads homeostatic imbalance, resulting in various disorders and disease states including diabetes.
Metabolic Alterations with Aging
The aging is can be defined as the accumulation of alterations over time at cellular, tissue and organ level in an organism. At the cellular level such changes entail cessation of DNA replication and cell division; the cells become senescent and undergo apoptosis. Further, the aging cells are characterized by alterations in the rate and accuracy of protein synthesis as compared to young ones [1]. The aging also affects carbohydrate homeostasis by altering pancreatic β cell function, β cell mass, insulin secretion and insulin sensitivity, and predisposes for insulin resistance (IR), impaired glucose tolerance and diabetes.
Aging is an important risk factor for metabolic disorders including obesity, impaired glucose tolerance, and type 2 diabetes mellitus (T2DM). The prevalence of T2DM increases with age and peaks at 60-74 and diabetes and its complications are a major cause of morbidity and mortality world-wide [2]. About one third of the elderly have diabetes and three quarters have diabetes or prediabetes. The diabetes in itself increases the risk for multiple other age-related diseases such as atherosclerosis, cardiovascular disease (CVD), stroke, Alzheimer disease (AD), Parkinson's disease and cancer [3]. The salient features of T2DM with aging are peripheral insulin resistance and impaired insulin secretion from β cells [4]. The aging predisposes to diabetes and impaired glucose tolerance through effects on insulin secretion and insulin action.
Physiology of Insulin Secretion and Aging
Insulin is the major hormone for regulating glucose homeostasis. It binds to its receptors located on various tissues including muscle, liver, and adipose tissue and initiates signalling effects. The metabolic effects resulting from insulin signalling include regulation of glucose homeostasis through a decrease in hepatic glucose output (via decreased gluconeogenesis and glycogenolysis) and increase in glucose uptake primarily into striated muscle and adipose tissue, and increase in lipid synthesis and attenuation of release of free fatty acid from triglycerides from adipose tissue. Insulin secretion from β cells is a complex process and involves the integration of various stimuli, such as nutrients mainly glucose and amino acid, hormones and neurotransmitters [5].
Aging and Glucose Metabolism
Insulin Secretion and IR
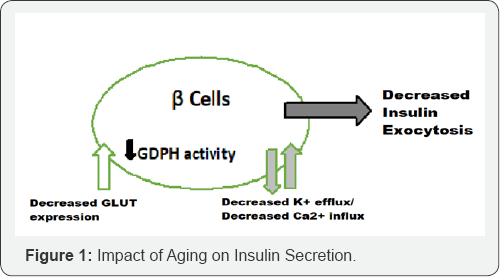
With age, there is decreased insulin secretion following stimulus (Figure 1), disorderly insulin release, decreased insulin pulse amplitudes, and decreased response to glucose oscillations as well as alterations in insulin clearance [6]. Many factors lead to decreased insulin secretion with age and include the age-associated loss of Sirtl-mediated GSIS, decreased β-cell sensitivity to circulating incretins, age-associated decrease in mitochondrial function, as well as increased oxidative stress [7,8]. Insulin resistance results when normal circulating concentrations of the hormone are insufficient to regulate the glucose metabolic processes appropriately.
The insulin secretory defects are superimposed on an increased insulin demand because of increased insulin resistance with age results in impaired glucose homeostasis, glucose intolerance and diabetes. Further, aging is associated with a decline of insulin action, which contributes to the high prevalence of impaired glucose tolerance and T2DM among the elderly.
Glucose Transporters in β cells
The initiation of the glucose transport is the first step that links glucose metabolism to insulin release in the β cell. GLUT2 is the main transporter, but GLUT1 and GLUT3 are also important. The glucose transporters expressed in β cells ensure a bidirectional flux of glucose and other dietary sugars, such as fructose and galactose. The GLUT2 is essential for glucose- stimulated insulin secretion (GSIS) and deficiency of GLUT2 causes hyperglycemia [9]. The loss of pancreatic β-cell GLUT2 expression is, thus, associated with hyperglycemia and impaired GSIS [10]. The age-associated decrease in GLUT2 expression has been documented in aged rodent models [11]. The human β cells, as compared to rodents β cells, express all three glucose transporters, GLUT1, 2, and 3, and the levels of GLUT1 and GLUT3 are also important apart from GLUT2 in in humans [12].
Aging and β-cell Glucose Oxidation
After transport into the β cells, glucose undergoes oxidation, initiated by its phosphorylation by glucokinase (GCK), a hexokinase, and generates ATP in cytosol and mitochondria via the tricarboxylic acid (TCA) cycle, resulting in build-up in the ATP which is required for insulin secretion. The metabolism of glucose in β cells from T2DM patients exhibit lower ATP content and a blunted GSIS, and appear to be responsible for β cell dysfunction [13].
GCK mRNA level has shown to be decreased in diabetic compared to the normal rats and the gene expression for GCK is significantly increased with age in healthy rats, suggesting a potential mechanism by which β cells attempt to overcome age-associated glucose intolerance and insulin resistance [14]. The pancreatic islet has about 40-70 times the activity of mitochondrial glycerol-phosphate dehydrogenase (GPDH) compared to other tissues. The decrease in GDPH activity leads to decrease in glycolysis and GSIS. This has been supported by animal studies, older rats showing approximately 50% reduction in the GDPH activity in islets compared with young rats [15].
Aging and Calcium and Potassium Channels
The increased ATP/ADP ratio following glucose oxidation reduces the whole cell K+ permeability, leads to cell membrane depolarization and extracellular Ca2+ influx into the β cell. Following Ca2+ influx, there occurs elevation of cytosolic free Ca2+ concentration which initiates the insulin exocytosis. The animal studies suggest that the decreased insulin secretory response to glucose in aged rats is due, at least in part, to inadequate inhibition of K+ efflux and diminished net Ca2+ uptake [16].
Effects of Age on Insulin Granule Exocytosis
The final step of insulin secretion is the exocytosis of insulin granules. Insulin is stored in large dense core vesicles (LDCVs), also called insulin granule, and released by exocytosis, a multistage process involving vesicle trafficking, docking, and eventually fusion with the plasma membrane. Calcium constitutes the major stimulus for exocytosis and regulates several steps in exocytosis, such as the size of vesicle pools, the fusion event, and the size of the fusion pore, and may act on distinct protein targets. Since the net uptake of Ca2+ is decreased with age, it is reasonable to speculate that insulin granule exocytosis is also inhibited by age.
Aging and β-Cell Mass and Visceral Fat
The β-cell mass Turnover
The islets β-cell mass is regulated by the balance of cell proliferation and apoptosis. The β-cell mass normally grows well into adulthood to provide increased insulin secretion to match the greater insulin requirements with growth. The β-cell mass has also been shown to expand in adult rodents during pregnancy. Thereafter, the rate of β-cell proliferation gradually declines with aging (Figure 2). In addition, the young mice respond to high- fat diet by increasing β-cell mass by proliferation to maintain normal blood glucose levels. The older mice, in contrast, do not display increase in β-cell mass or β-cell proliferation in response to high-fat diet and become diabetic. Further, various aspects of β-cell mass and function decline with age, thus contributing to the age-associated defects in insulin secretion. This defect when superimposed on an increased demand for insulin, contributes to impaired glucose homeostasis, glucose intolerance and diabetes.
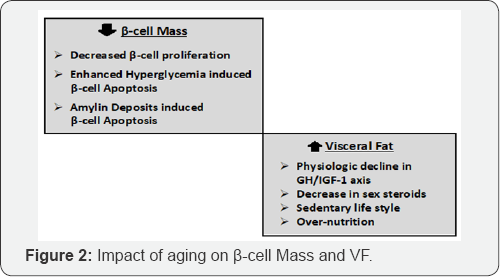
In fact, the age correlates with a decreased proliferative activity and an enhanced sensitivity to glucose-induced β-cell apoptosis [17]. There is an increased β-cell apoptosis in diabetic patients, varying from 3-10 folds, as compared to nondiabetic individuals [18]. The high glucose level induces apoptosis in β cells through inducing the expression of Fas and activation of caspase 8 and 3 [19]. Simultaneously, there is age-associated decrease in activities of various antioxidant enzymes and an increased oxidative stress which contributes to increased β cells apoptosis. Reers et al, on the other hand, have reported that the relative β-cell volume in human pancreatic islets remains constant with aging and β-cell apoptosis does not impact significantly, but the β-cell replication decreases with age [20].
Amylin or islet amyloid polypeptide (IAPP) is also cosecreted along with insulin from β cells. Amylin suppresses the glucagon secretion and helps to regulate glucose homeostasis. In insulin-resistant states, the increased secretion of insulin results in hypersecretion of amylin. With age there is an increased deposition of amylin in the islets of diabetic patients, which aggregates into amyloid plaques that induce β-cell apoptosis leading to reduced islet volume and β-cell mass [21].
The islet neogenesis is the differentiation of progenitor cell or trans-differentiation of pancreatic non-p cells to β cells. Neogenesis of islets occurs during normal embryonic development and early postnatal life leading to β-cell mass expansion. In addition, β cell neogenesis has been shown to occur in the adults during periods of increased insulin demand such as pregnancy and obesity. The potential for β-cell replication declines significantly with age, but the rate of islet neogenesis (expressed as percentage of insulin positive duct cells) does not appear to be affected by aging in humans.
Visceral Fat, Aging and Insulin Action
The visceral fat (VF) located in and around the abdominal viscera, and excess VF has been related to various pathological states including metabolic syndrome and CVD, IR and T2DM [22]. The VF has been related to aging, and as determinant of insulin action [23]. The adiposity with aging appears to be an important association [24]. In fact, the VF increases throughout the lifespan in men and women of all ages and race, independent of increases in body weight [25]. Various factors contribute to the increased VF seen with aging such as physiologic decline in GH/IGF-1 axis, decrease in sex steroids as well as sedentary life style. The removal of VF prevents the progressive decline in insulin action and improves or delays the onset of diabetes [26]. Calorie restriction (CR) extends life span and retards age-related chronic diseases in a variety of species, including Saccharomyces, C elegans, Drosophila, rodents and rhesus monkeys [27]. Various studies have shown that the reduction in fat mass, specifically VF, may be one of the possible underlying mechanisms of the antiaging effect of CR [28].
The adipose tissue is an active metabolic-endocrine organ [29]. The obesity is a low-grade inflammatory condition and has been related to adverse metabolic outcome [30]. Further, the adiposity increases with age and increased VF induces inflammatory state, which increases the risk of IR, glucose intolerance, T2DM, CVD, and hypertension, and accelerates the process of aging. IR and/or insulin deficiency lead to two metabolic alterations, namely, increased rate of lipolysis in adipose tissue which increases circulating FFA levels and exacerbates IR, and activation of hepatic gluconeogenesis in spite of high plasma glucose levels. The elevated plasma FFA exacerbates IR [31]. The elevated levels of FFA decrease glucose uptake and glycogen synthesis in human, and inhibit PI3 kinase activity in skeletal muscle by impairment of insulin signalling. In animal models, the circulating FFA levels are significant higher in older rats as compared to younger ones. The high FFA levels, thus, may be the unifying mechanism that accounts for the insulin resistance in obesity and T2DM, and with aging [32]. The increased VF and high circulating FFA both contribute to impaired insulin action and various defects in β-cell mass/ function.
The Concept Central Insulin Resistance
The recent studies suggest that IR exists in CNS and central insulin action plays an important role in regulating somatic glucose metabolism [33]. The hypothalamic insulin signaling is an important determinant of the response to insulin in the management of uncontrolled diabetes [34]. Interestingly, evidence from recent research documents a link between diabetes and AD, a neurodegenerative disorder and the commonest form of dementia [35]. Further, the patients with T2DM have increased prevalence of AD by 2-3 folds, and insulin levels and insulin activity in the central nervous system are reduced in AD [36]. This is endorsed by various studies in human subjects showing that administration of insulin improves memory in AD patients [37].
This brings an interesting concept that aging is associated with central insulin resistance [38]. In this context, it has been shown that the protein levels of elements in the insulin signalling pathway such as IRs and SRC homology adaptor protein (SHC) do not vary significantly in the forebrain cortex and cerebellum of older and young rats [39]. Thus, the aging-associated increase in central and peripheral insulin resistance could contribute to both diabetes and AD.
Aging and Whole Body Glucose Homeostasis
Impaired Glucose Tolerance
The risk for impaired glucose tolerance and diabetes increase with age. There are various factors which play role in somatic glucose homeostasis. Leptin, a hormone secreted from adipose tissue, plays a key role in energy intake and expenditure. Deficiency of leptin and its receptor leads to severe obesity, IR, and diabetes in rodents and humans. Resistant to the effects of leptin, termed leptin resistance, is seen in obesity. Leptin resistance is also associated with aging [40]. The endoplasmic reticulum (ER) stress plays a role in the pathogenesis of diabetes by contributing to both pancreatic β-cell dysfunction and peripheral insulin resistance [41].
Whole body glucose homeostasis is a complex balance of glucose production and utilization by different tissues. Food intake and hepatic glucose production (HGP) are the two sources of glucose production, while skeletal muscle contributes to the majority of the glucose uptake and utilization. HGP plays crucial roles in glucose homeostasis, both in the fasting and postprandial states. The hepatic glucose production does not increase with age, when adjusted for lean body mass [42]. Furthermore, hepatic glucose output has not been shown to be increased in elderly patients with T2DM. Thus, hepatic insulin resistance does not seem play a significant role in decreased glucose tolerance of elderly people.
Risk Factors for Glucose Homeostasis
The aging-associated sedentary life style and diminished physical activity are important factors for age-related changes of glucose homeostasis. Research has shown that healthy elderly with greater degrees of physical fitness have better glucose tolerance and lower level of insulin resistance than less active old people. The skeletal muscles are the major source of glucose utilization. Glucose is transported into the myocytes by glucose transporters, GLUT4 being the major glucose transporter in skeletal muscle for insulin-stimulated glucose uptake. The muscle GLUT4 protein level is not altered in obesity and T2DM, but its expression levels decline with age [43]. The changes in body composition due to decreased lean body mass and contractile strength with age contribute to the reduction in insulin stimulated glucose uptake.
Finally, the lifestyle modalities, calorie restriction (CR) and calorie restriction with adequate nutrition (CRAN) appear to work through reduction in adipose cell mass specifically VF, oxidative stress and by activating sirtuins, a family of nicotinamide adenine dinucleotide- (NAD-) dependent protein deacetylases [44]. Interestingly, over expression of sirtuins or treatment with activators of sirtuins, such as resveratrol protects against metabolic decline with aging, increases insulin sensitivity and insulin secretion, improves quality of life and longevity.
References
- Rattan S (2010) Synthesis, Modification and Turnover of Proteins during Aging. Advances in Experimental Medicine and Biology 694: 1-13.
- Gunasekaran U, Gannon M (2011) Type 2 diabetes and the aging pancreatic beta cell. Aging 3(6): 565-575.
- Reaven GM (1988) Role of insulin resistance in human disease. Diabetes 37(12): 1595-1607.
- Kadowaki T (2000) Insights into insulin resistance and Type 2 diabetes from knockout mouse models. J Clin Invest 106(4): 459-465.
- Gong Z, Muzumdar RH (2012) Pancreatic Function, Type 2 Diabetes, and Metabolism in Aging. International Journal of Endocrinology 1-13.
- Chang AM, Halter JB (2003) Aging and insulin secretion. The Journal of Clinical Endocrinology & Metabolism 284(1): E7-E12.
- Ramsey KM, Mills KF, Satoh A, Imai SI (2008) Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7(1): 78-88.
- Cooksey RC, Jouihan HA, Ajioka RS (2004) Oxidative stress, β-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 145(11): 5305-5312.
- Ohneda M, Johnson JH, Inman LR (1993) GLUT2 expression and function in β-cells of GK rats with NIDDM: dissociation between reductions in glucose transport and glucose-stimulated insulin secretion. Diabetes, 42(7): 1065-1072.
- Ohtsubo K, Takamatsu S, Minowa MT (2005) Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123(7): 1307-1321.
- Ihm SH, Moon HJ, Kang JG (2007) Effect of aging on insulin secretory function and expression of beta cell function-related genes of islets. Diabetes Research and Clinical Practice 77(3): S150-S154.
- Mc Culloch LJ, van de Bunt M, Braun M (2011) GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Molecular Genetics and Metabolism 104(4): 648-653.
- Anello M, Lupi R, Spampinato D (2005) Functional and morphological alterations of mitochondria in pancreatic beta cells from Type 2 diabetic patients. Diabetologia 48(2): 282-289.
- Frese T, Bazwinsky I, Muhlbauer E, Peschke E (2007) Circadian and age-dependent expression patterns of GLUT2 and glucokinase in the pancreatic beta-cell of diabetic and nondiabetic rats. Hormone and Metabolic Research 39(8): 567-574.
- Azhar S, Ho H, Reaven EP, Reaven GM (1983) Evidence of an age- related decline in mitochondrial glycerol-phosphate dehydrogenase activity of isolated rat islets. Metabolism 32(11): 1019-1021.
- Ammon HPT, Fahmy A, M Mark (1987) The effect of glucose on insulin release and ion movements in isolated pancreatic islets of rats in old age. J Physiol 384: 347-354.
- Maedler K, Schumann DM, Schulthess F (2006) Aging correlates with decreased β-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes 55(9): 2455-2462.
- Butler AE, Janson J, Bonner-Weir S (2003) β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52(1): 102110.
- Maedler K, Spinas GA, Lehmann R (2001) Glucose induces β-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes 50(8): 1683-1690.
- Reers C, Erbel S, Esposito I (2009) Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol 160(2): 185-191.
- Law E, Lu S, Kieffer TJ (2010) Differences between amyloid toxicity in alpha and beta cells in human and mouse islets and the role of caspase-3. Diabetologia 53(7): 1415-1427.
- Carr DB, Utzschneider KM, Hull RL (2004) Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 53(8): 2087-2094.
- Bryhni B, Jenssen TG, Olafsen K, Eikrem JH (2003) Age or waist as determinant of insulin action?. Metabolism 52(7): 850-857.
- Catalano KJ, Bergman RN, Ader M (2005) Increased susceptibility to insulin resistance associated with abdominal obesity in aging rats. Obes Res 13(1): 11-20.
- Huffman DM, Barzilai N (2009) Role of visceral adipose tissue in aging. Biochim Biophys Acta 1790(10): 1117-1123.
- Gabriely I, Ma XH, Yang XM (2002) Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine- mediated process?. Diabetes 51(10): 2951-2958.
- Heilbronn Lk, Ravussin E (2003) Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 78(3): 361-369.
- Muzumdar R, Allison DB, Huffman DM 2008. Visceral adipose tissue modulates mammalian longevity. Aging Cell 7(3): 438-440.
- Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6): 2548-2556.
- Alvehus M, Buren J, Sjostrom M (2010) The human visceral fat depot has a unique inflammatory profile. Obesity 18(5): 879-883.
- Samuel VT, Petersen KF, Shulman GI (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375(9733): 22672277.
- Koch L, Wunderlich FT, Seibler J (2008) Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 118(6): 2132-2147.
- Gelling RW, Morton GJ, Morrison CD (2006) Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab 3(1): 67-73.
- Zemva J, Schubert M (2011) Central insulin and insulin-like growth factor-1 signaling: implications for diabetes associated dementia. Curr Diabetes Rev 7(5): 356-366.
- Steen E, Terry BM, Rivera EJ (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease-is this type 3 diabetes?. J Alzheimer's dis 7(1): 63-80.
- Craft S, Baker LD, Montine TJ (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69(1): 29-38.
- Mc Call AL (2002) Diabetes mellitus and the central nervous system. International Rev Neurobiol 51: 415-453.
- Fernandes MA, Saad MJA, Velloso LA (2001) Effects of age on elements of insulin-signaling pathway in central nervous system of rats. Endocrine 16(3): 227-234.
- Wang ZW, Pan WT, Lee Y (2001) The role of leptin resistance in the lipid abnormalities of aging. FASEB J 15(1): 108-114.
- Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29(1): 42-61.
- Ferrannini E, Vichi S, Beck-Nielsen H (1996) Insulin action and age, European Group for the Study of Insulin Resistance (EGIR). Diabetes 45(7): 947-953.
- Houmard JA, Weidner MD, Dolan PL (1995) Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes 44(5): 555-560.
- Guarente L, Picard F (2005) Calorie restriction--The SIR2 connection. Cell 120(4): 473-482.
- Imai S, Guarente L (2014) NAD+ and sirtuins in aging and disease. Trends Cell Biol 24(8): 464-471.






























