Diagnostic Value of Real-Time Polymerase Chain Reaction (RT-PCR) in Smear Negative Viral Keratitis
Radhika Natarajan*
Department of Cornea & Refractive Surgery, Sankara Nethralaya, India
Submission: January 14, 2018; Published: January 25, 2018
*Corresponding author: Radhika Natarajan, Department of Cornea & Refractive Surgery, Sankara Nethralaya, Chennai, India; Email: 100radsam@gmail.com
How to cite this article: Radhika Natarajan. Diagnostic Value of Real-Time Polymerase Chain Reaction (RT-PCR) in Smear Negative Viral Keratitis. OAJ Gerontol & Geriatric Med. 2018; 3(2): 555607. DOI: 10.19080/OAJGGM.2017.03.555607
Abstract
Aim: To study value of RT-PCR in diagnosis of doubtful viral keratitis.
Settings and Design: Retrospective, Interventional study.
Methods and Materials: Clinical features, indications for RT-PCR and management outcomes of patients with RT-PCR positive for virus were studied.
Statistical analysis used: Microsoft Excel version 2013.
Results: Of 322 patients of viral keratitis, 94 (29.19%) needed RT-PCR for diagnosis due to clinical dilemma and/or non-response to therapy, and it was positive in 25(26.5%) patients (4 non-dendritic epithelial keratitis, 2 stromal,14 both, and 5 epithelial, stromal and endothelial keratitis). Giemsa stain was negative for virus in all corneal scrapings. Herpes Simplex Virus-1 (HSV-1) was isolated in 22, HSV-2 in 1 and Varicella Zoster Virus (VZV) in 2. 16 were managed medically and 9 surgically. Infection resolved in all medically treated patients and there was no recurrence in surgical grafts.
Conclusion: In viral keratitis, where clinical diagnosis is doubtful and response to therapy is delayed, RT-PCR can be a valuable tool for diagnosis.
Keywords: PCR; Viral keratitis; Diagnostic dilemma
Abbreviations: PCR: Polymerase Chain Reaction; RT-PCR: Real-Time Polymerase Chain Reaction; HSV-1: Herpes Simplex Virus-1; TPK: Therapeutic Penetrating Keratoplasty; IFA: Immuno Fluorescence assay; VZV: Varicella Zoster Virus
Introduction
Viral keratitis is one of the major causes of visual impairment in India. Therefore, rapid and accurate diagnosis and detection of virus is important, particularly in atypical cases [1]. Several laboratory tests have been introduced, such as virus culture for virus isolation, Immuno Fluorescence and Polymerase Chain Reaction (PCR) assay to detect HSV. Recently, herpes PCR has been reported to have the advantages of higher sensitivity and shorter processing time than direct virus isolation as a standard procedure [2-5]. So, our purpose was to study the value of Real-Time Polymerase Chain Reaction (RT-PCR) in the diagnosis of clinically doubtful viral keratitis.
Subjects and Methods
This was a retrospective, interventional case study done with the approval from Institutional Review Board and the protocol complied with the tenets of the Declaration of Helsinki. It was done during the period of April 2016 to March 2017. The medical records of total 94 patients, who were clinically diagnosed as infectious keratitis and whose RT-PCR was done for the diagnosis of viral etiology, were retrospectively reviewed. The patients who had combined bacterial infections or other corneal degenerative or immune-related keratitis and who has less than three months of follow-up were excluded. Risk factors and epidemiological information, as well as the duration of disease and its clinical evolution, were recorded for each patient. All patients underwent a clinical examination that included slit lamp bio microscopy and digital photography. Diseased corneas were scraped and specimens were sent for direct smear examination (Gram, Potassium Hydroxide - KOH and Giemsa staining techniques) and microbiological culture on blood agar, chocolate agar, Sabouraud's dextrose agar and Brain Heart Infusion Broth and RT-PCR for virus. Corneal scrapings were obtained from the edge of the ulcer after application of topical anaesthesia - 0.5% proparacaine. A portion of the sample was rapidly sent for molecular diagnostic techniques. Total DNA extraction was performed using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA), according to the manufacturer's protocol.
Molecular detection was carried out using Taq Man-based technology, and reactions for Herpes Simplex Virus - HSV-1 and -2 and Varicella Zoster Virus - VZV were tested for analytical specificity using positive (known viral DNA from HSV-1 and -2, and VZV) and negative (molecular grade water) controls [6]. Oligonucleotide primers for HSV and VZV were designed to bracket a well-conserved region in the DNA polymerase gene of herpes simplex and varicella zoster viruses. Primer pair HSV-P1 (5'-CGACTTTGCCAGCCTGTACC-3') and P2 (5'-AGTCCGTGTCCCCGTA-GATG-3') was used to amplify the locus of the DNA polymerase gene of HSV-1 and HSV-2, and primer 5’-TGTCTTTCACGGAGGCAAACACGT-3’ was used for VZV amplification. The reaction was standardized with primers and probes for HSV-1/2 in a duplex format and a VZV target in a separate reaction. Briefly, 5 ml of DNA was added to a final reaction volume of 25.0 ml (12.5 ml of the 2X Taq Man TM universal PCR master mix, 0.3 mm of each primer, 0.3 mm of each probe, and 5.7 ml of molecular grade sterile water), and 40 PCR cycles were run: denaturation step (20 sec at 95°C), primer annealing and extension (1 min at 60°C).
The beta-globin gene was used as the endogenous control for HSV-1 and 2 (10.0 ml of 2X Taq Ma TM universal PCR mix, 0.4 mm of each primer, 0.2 mm of the probe, 7.0 ml molecular grade sterile water, and 2.0 ml of DNA sample to a final volume of 20.0 ml) and ORF29 protein was used for VZV and the same PCR conditions described above were used [7]. The clinical samples were then analysed for the presence of HSV-1 and -2 and VZV DNA.
Results
This was a retrospective interventional study done from the period of April 2016 to March 2017 with total 94 patients' medical records being reviewed. Total 322 patients were diagnosed to have viral keratitis in that duration of which 94 patients (29.19%) required RT-PCR for the diagnosis because of diagnostic dilemma or non-response to therapy. Mean age of the sample in study was 44 years (Range 1 to 74 years). Males were 68 and females were 26. Right eye was involved in 35 patients, left eye was involved in 53 patients and 6 patients had involvement of both eyes. History of prior episode of viral keratitis was present in 34 patients and they had been treated with anti-viral drugs orally or topically. Giemsa stain was negative for virus in all corneal scrapings. Out of 94 patients, RT-PCR was positive in 25 patients (26.5%).
Morphology of lesions in patients with positive and negative PCR have been shown in Table 1. Herpes Simplex Virus-1 (HSV- 1) was isolated in 22, HSV-2 in 1 and Varicella Zoster Virus (VZV) in 2. 16 were managed medically and 9 needed surgical intervention. PCR was positive in 8 out of 34 patients (23.5%) who had prior history of viral keratitis episodes and treatment with antiviral drugs topically or orally, whereas it was positive in 17 out of 60 remaining patients (28.33%). Treatment details of patient needing medical treatment are shown in Table 2. Out of 9 patients who were managed surgically, Therapeutic Penetrating Keratoplasty (TPK) was needed in 5 patients, 2 had application of cyanoacrylate glue with bandage contact lens, 1 required conjunctival hooding followed by optical penetrating keratoplasty and 1 required lateral tarsorrhaphy. Those requiring TPK had extensive stromal involvement, 2 with glue application had impending perforation, 1 requiring hooding and 1 requiring lateral tarsorrhaphy had neurotrophic epithelial keratitis with persistent epithelial defect. Infection resolved in all medically treated patients and there was no recurrence in surgical grafts in follow up duration of 3 months.
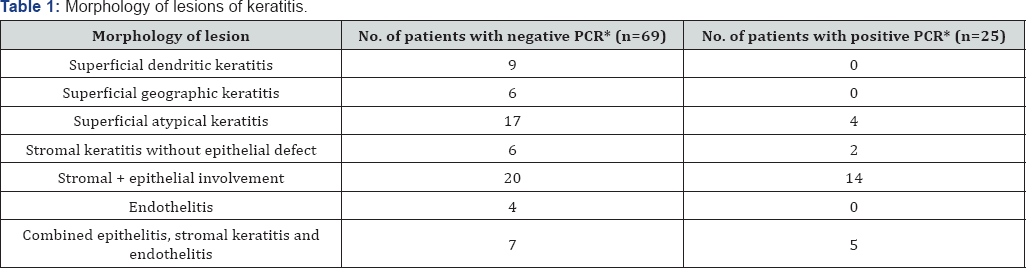
*PCR: Polymerase Chain Reaction
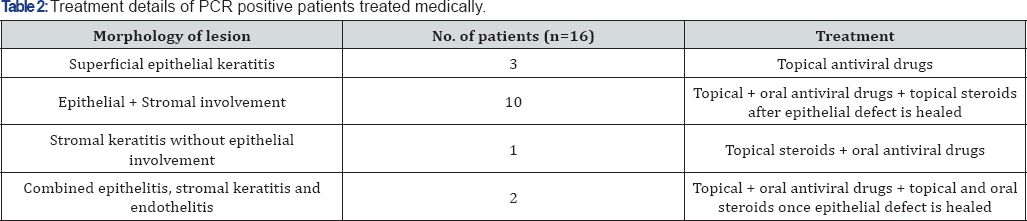
Discussion
Herpes simplex virus 1 (HSV) keratitis has been one of the major causes of keratoplasty in developing countries. A rapid, accurate diagnosis and immediate treatment using antiviral medication are critical to preventing corneal blindness. However, the clinical manifestation of herpes keratitis is too varied to be diagnosed solely based on its clinical findings. Up until now, laboratory diagnostic tools have not been good enough to detect HSV definitively in patients with herpes keratitis. Several laboratory tests have been introduced, such as virus culture for virus isolation, Immuno Fluorescence assay (IFA) and polymerase chain reaction (PCR) assay to detect HSV. Viral culture is considered the gold standard for identifying HSV. When compared to viral culture, clinical diagnosis is only 55%-65% accurate [8]. Unfortunately, culturing HSV is time-consuming and can take a week or longer when few infectious viruses are in the sample, and typically underestimates the number of patients whose disease is due to HSV [9,10].
IFA has also been used to diagnose HSV and detects 33.3% more positive cases than viral culture. It also had sensitivity of 80%, specificity of 71.4%, positive predictive value of 63.6%, and negative predictive value of 81.8%. However, sample size and false-positive and false-negative results can unfavourably influence IFA Satpathy et al. [9]. Recently, herpes PCR has been reported to have the advantages of higher sensitivity and shorter processing time than direct virus isolation as a standard procedure [2-5]. Nevertheless, PCR has some shortcomings that include altered results depending on the primer composition for the target DNA, the proficiency of the clinical laboratory worker and the risk of contamination. Quantitative real-time PCR has advantages over conventional methods because it is quicker and more sensitive [11]. It can also detect genes that are expressed only in the replication state and can relatively quantify the DNA sample [12,13]. Our study showed positive rate of PCR as 26.5% (25 out of 94 patients) which is comparable to study done by [9]. which showed PCR positive in 56 out of 153 corneal scrapings (36.66%).
Clinical treatment appeared to decrease the detection rate of HSV DNA in PCR. Systemic and topical antiviral agents, such as acyclovir or valaciclovir have been commonly used to treat and prevent the HSV keratitis. They could effectively suppress the replication of HSV by inhibiting viral DNA polymerase in the ocular tissue. Similar to a study by Heo JY et al. [14]. Stating that HSV DNA had a lower detection rate in samples of the HSV keratitis with pre-antiviral medication (3 out of 18, 16.66%) than in those without pre-antiviral medication (3 out of 3, 100%), this study also says that HSV DNA has lower detection rate in those patients having history of previous treatment with antiviral drugs (8 out of 34, 23.5%) than in those without pre-antiviral medications (17 out of 60, 28.33%) though the difference in this study is not significant. So, in conclusion, though RT-PCR cannot be considered as a gold standard for the diagnosis of viral keratitis, but wherever clinical diagnosis is doubtful and response to therapy is delayed, RT-PCR can be a valuable tool for diagnosis.
References
- Yasuko Mori, Yoshitsugu Inoue, Yoshikazu Shimomura, Tetsuo Kase, Yasuo Tano (1998) Detection of HSV mRNA using reverse transcription polymerase chain reaction for diagnosid in murine keratitis model. Jpn J Ophthalmol 42(1): 8-11.
- Kaye SB, Baker K, Bonshek R, Hart C, Tullo A (2000) Human herpesviruses in the cornea. Br J Ophthalmol 84(6): 563-571.
- Khodadoost MA, Sabahi F, Behroz MJ (2004) Study of a polymerase chain reaction-based method for detection of herpes simplex virus type 1 DNA among Iranian patients with ocular herpetic keratitis infection. Jpn J Ophthalmol 48(4): 328-332.
- Kowalski RP, Thompson PP, Cronin TH (2010) Cell culture isolation can miss the laboratory diagnosis of HSV ocular infection. Int J Ophthalmol 3(2): 164-167.
- El-Aal AM, El Sayed M, Mohammed E (2006) Evaluation of herpes simplex detection in corneal scrapings by three molecular methods. Curr Microbiol 52(5): 379-382.
- Sugita S, Shimizu N, Watanabe K, Mizukami M, Morio T, et al. (2008) Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 92(7): 928-932.
- Grinde B (2013) Herpes viruses: latency and reactivation - viral strategies and host response. J Oral Microbiol 5.
- Kowalski RP, Gordon YJ, Romanowski EG, Araullo-Cruz T, Kinchington PR (1993) A comparison of enzyme immunoassay and polymerase chain reaction with the clinical examination for diagnosing ocular herpetic disease. Ophthalmology 100(4): 530-533.
- Satpathy G, Mishra AK, Tandon R, Sharma N, Nayak N, et al. (2011) Evaluation of tear samples for herpes simplex virus 1 (HSV) detection in suspected cases of viral keratitis using PCR assay and conventional laboratory diagnostic tools. Br J Ophthalmol 95(3): 415-418.
- Madhavan HN, Priya K, Anand AR, Therese KL (1999) Detection of herpes simplex (HSV) genome using polymerase chain reaction (PCR) in clinical samples comparison of PCR with standard laboratory methods for detection of HSV. J Clin Virol 14(2): 145-151.
- Kennedy DP, Clement C, Arceneaux RL, Bhattacharjee PS, Huq TS, et al. (2011) Ocular HSV-1: Is the Cornea a Reservoir for Viral Latency or a Fast Pit Stop? Cornea 30(3): 251-259.
- Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, et al. (2005) HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci 46(1): 241-247.
- Kakimaru-Hasegawa A, Kuo CH, Komatsu N, Komatsu K, Miyazaki D, et al. (2008) Clinical application of real-time polymerase chain reaction for diagnosis of herpetic diseases of the anterior segment of the eye. Jpn J Ophthalmol 52(1): 24-31.
- Heo JY, Kim SJ, Kim JC, Hahn TW (2001) The early diagnosis of herpetic keratitis by polymerase chain reaction. J Korean Ophthalmol Soc 42: 36-42.






























