Motor Recovery and Milestone Achievement in the Acute Phase of Stroke: Evaluation Based on Lesion Location
Hsiao Ching Yen1*, Jiann Shing Jeng2, Wen shiang Chen3 and Ting Teng1
1Division of Physical Therapy, Department of Physical Medicine and Rehabilitation, National Taiwan University Hospital, Taiwan
2Stroke Center & Department of Neurology, National Taiwan University Hospital, Taiwan
3Department of Physical Medicine and Rehabilitation, National Taiwan University Hospital, Taiwan
Submission: November 3, 2017; Published: November 30, 2017
*Corresponding author: Hsiao Ching Yen, Division of Physical Therapy, Department of Physical Medicine and Rehabilitation, National Taiwan University Hospital, No.7, Chung Shan South Road, Taipei 100, Taiwan, Tel: 886-2-23123456-53034;Email: jassicayen@yahoo.com. tw
How to cite this article: Hsiao Ching Yen, Jiann Shing Jeng, Wen shiang Chen, Ting Teng. Motor Recovery and Milestone Achievement in the Acute Phase of Stroke: Evaluation Based on Lesion Location. OAJ Gerontol & Geriatric Med. 2017; 3(1): 555604. DOI: 10.19080/OAJGGM.2017.03.555604
Abstract
Purpose: This study aimed to investigate the influence of lesion sites on recovery patterns and the time to reach mobility milestones in patients of acute stroke.
Methods: Sixty-nine patients were assessed using the lower extremity subscales of Fugl-Meyer assessment, the functional independence measure (FIM), and the postural assessment stroke scale (PASS). Three mobility milestones were identified from the results of FIM and PASS. The stroke was categorized into five groups: anterior cerebral artery (ACA) infarct, middle cerebral artery (MCA) infarct, posterior cerebral artery (PCA) infarct, putamen hemorrhage, and thalamus hemorrhage. The motor recovery speed was compared among the 5 groups of patients. To analyze factors related to the achievement or non-achievement of the three motor milestones within 14 days from onset, binary outcomes were analyzed with logistic regression analyses.
Results: There was significant difference of functional states at discharge, motor recovery rates and the days of achieving mobility milestones during the hospitalization among the 5 subgroups.
Conclusion: The hemorrhage groups showed better recovery rates than other infarct groups. Combination of NIHSS score at admission and the present of PCA infarction or putamen hemorrhage could help predict the milestone achievement of ambulation independence > 50 m at acute stage.
Keywords: Outcome; Milestone; Acute stroke; Mobility; Location
Abbreviations: DRG: Diagnosis-Related Group; LOS: Length Of Hospital Stay; MRI: magnetic Resonance Imaging; CT: Computed Tomography; MRA: Magnetic Resonance Angiography; NIHSS: National Institute of Health Stroke Scale; ACA: Anterior Cerebral Artery; MCA: Middle Cerebral Artery; PCA : Posterior Cerebral Artery; FIM: Functional Independence Measure; PASS: Postural Assessment Scale For Stroke Patients; IQR: Interquartile Range
Introduction
Stroke commonly causes chronic functional disabilities, especially in the elderly [1]. Inpatient care accounted for approximately 32.9% of Taiwan's national health insurance expenditures in 2010, including 10.3% of total costs spent in caring for patients with cerebrovascular diseases [2]. Since November, 2011, the diagnosis-related group (DRG) has been implemented to classify hospital cases into groups, control medical capital expenditures, and correlate expenditures to functional outcomes in Taiwan [3]. Under the DRG system, costs that are not related to a single service are entered in a day-component, and they are dependent on patients' length of hospital stay (LOS). It may lead to high costs for longer LOS of stroke patients. Prognostic knowledge is crucial for optimizing acute to subacute stroke management. The extent of physical impairment, rate of motor recovery, and functional outcomes post-stroke vary with the lesion site [46]. Thus, it is better for understanding the influence of various lesion sites on recovery for planning rehabilitation to ensure the effectiveness of limited medical resources.
Stroke recovery begins early. Studies have shown that the majority of improvement occurs during the first month [7-13]. The rate of recovery for walking is more prominent during the first 2 weeks after stroke [14-16]. Initial stroke severity and functional status may influence the recovery and functional status at discharge. However, there is little available information concerning recovery during the first few weeks post-stroke as previous studies often focused on mid-term outcomes (3-6 months) or longer period (>6 months) [4-6,17-20]. Although the extent of disability during acute stage and at discharge is an important factor for discharge deposition, there is little information regarding patients' motor function status from acute stage. Early and accurate prediction of motor function outcomes in stroke patients is important for setting realistic and attainable therapeutic goals; facilitating proper discharge planning/ allocation; and anticipating the need for home adjustment and community support.
Lesion location is an important determinant in the extent of motor recovery post-stroke [4-6,19-23]. Therefore, outcome observations for stroke patients' must be classified by stroke subtypes according to location. Although previous studies have been conducted on functional outcomes and stroke patients' recovery, this research was limited because most stroke types were heterogeneous [7-11,13,24-27]. Therefore, the present study aimed to investigate the motor recovery patterns and mobility outcome milestones by the lesion location in acute stroke patients.
Method
Study subjects
Patients who were recruited if they fulfilled the following criteria: (1) admission within 5 days after stroke onset, (2) independent ADL before stroke event, (3) age 50 to 80 years, (4) stroke with unilateral hemiparesis, and (5) cortical or subcortical infarction or hemorrhage with conservative treatment confirmed by magnetic resonance imaging (MRI) or computed tomography (CT), with vascular lesions verified by magnetic resonance angiography (MRA). Patients were excluded if they had peripheral or other central nervous system dysfunction, active inflammation or pathologic changes in the joints. The study was approved by the Institutional Review Board and informed consents were signed by each patient.
All patients received neuro-facilitation and motor relearning physiotherapy treatment for 30 to 50 minutes a day, and 5 days a week throughout our study. Medical, nursing, occupational therapy and speech therapy cares were provided to all patients as indicated. The National Institute of Health Stroke Scale (NIHSS) score at admission was assessed. We categorized the stroke lesion into 5 groups: anterior cerebral artery (ACA) infarct, middle cerebral artery (MCA) infarct, posterior cerebral artery (PCA) infarct, putamen hemorrhage, and thalamus hemorrhage. The performance-based motor recovery data were collected bi-weekly until hospital discharge. The motor recovery data included the lower extremity subscales of the Fugl-Meyer (FM-LE) assessment, the functional independence measure (FIM), and the postural assessment scale for stroke patients (PASS) [28-33]. A FIM score of 107 was considered to be indicative of dependence in ADL [34]. The number of days required to achieve motor milestones was identified from the bi-weekly FIM and PASS scores. Three motor milestones were selected from the FIM or PASS assessments as our primary outcome measures due to their importance in characterizing motor function recovery after stroke [30,32].
The first milestone was the ability to sit on the edge of a 50-cm high examination table with the feet touching the floor without support (PASS subtest: maintaining posture, sitting without support for > 5 min, grade 3). The second milestone was the ability to stand without support for longer than 1 minute while simultaneously performing arm movements above shoulder level without other constraints (PASS subtest: maintaining posture, standing without support for > 1 min, grade 3). The third milestone involved walking on a level surface for a minimum of 50 m with an assistive device for safety (FIM subtest: locomotion; walk, wheelchair item, level 6, ambulation independence >50 m). All physiotherapy staff involved in the assessments were trained in the use and recording of assessments and milestones.
Statistical analysis
Descriptive analysis of all collected data was first observed for any skewness of distribution. Data related to patient characteristics followed a normal distribution, and mean data are reported as well. However, data related to duration of stay, assessment scores, and milestones were found to be skewed and platykurtic. Therefore, median values were used in order to enable comparisons between subgroups for each milestone. The scores from the FM-LM, FIM, and PASS subtests were plotted over time to graph post-stroke motor recovery. Furthermore, the rates of change for all recoveries were analyzed and normalized to 100 points for the duration of hospitalization. Then the coefficients of a third-degree polynomial that fits the data for all the curves were calculated. All mathematical analyses were done using MEDLAB 16.0 (The MathWorlds, Inc.).
The time taken to achieve each milestone is reported for the 25th and 75th percentiles to illustrate the range associated with each milestone; this range is reported as the interquartile range (IQR). Because of the small sample size, all parametric analyses were confirmed using the appropriate nonparametric methods. For statistical analyses, the patients were subdivided into the five groups noted above according to their lesion locations. Group medians were compared with a nonparametric Kruskal-Wallis or Mann-Whitney test. To analyze factors related to the achievement or non-achievement of the three motor milestones within 14 days from onset, binary outcomes were analyzed with logistic regression analyses and were reported as odds ratios with 95% confidence intervals The lesion locations, which constituted the explanatory variable, were entered into the multivariable logistic regression analyses, together with age, gender and NIHSS score at admission. A P value of <0.05 was considered significant for all tests. All statistical analyses were conducted using SPSS 13.0 (SPSS Inc, Chicago, Ill).
Result
A total of 69 stroke patients (40 men, 29 women; mean age, 61.4+13.9 years) participated in this study. The median LOS was 28.5 days (interquartile range, 13 to 52.5 days). Table 1 presents the patients' characteristics and NIHSS at admission, as well as FIM, PASS, and FM-LE scores at both admission and discharge among the 5 groups of patients. With respect to the FM-LE and PASS values, there were no significant differences between the groups at admission and discharge. The FIM values at admission were significantly higher in the PCA group than the other groups (all p<0.05). The FIM values at discharge were significantly higher in the PCA group than the MCA, putamen, and thalamus groups (all p<0.05). In addition, the FIM values at discharge were significantly higher in the putamen group than the MCA group (p=0.042).
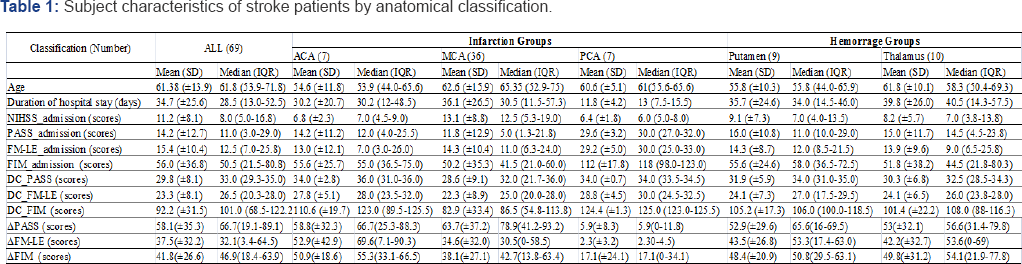
ACA: Anterior Cerebral Artery; MCA: Middle Cerebral Artery; PCA: Posterior Cerebral Artery; DC: the scores at discharge
Graphical patterns of recovery
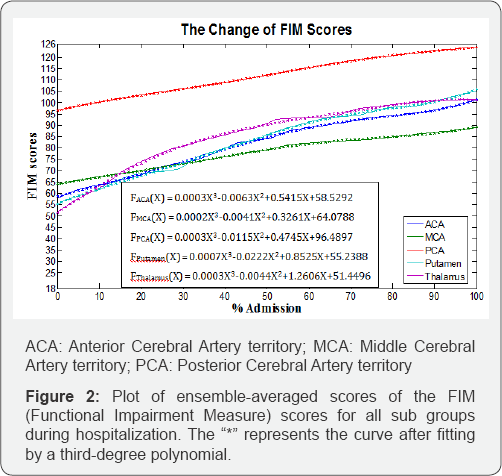
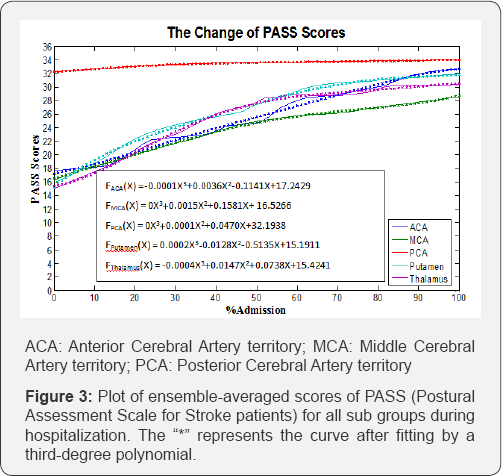
Figure 1 shows the ensemble-averaged scores of the FM-LE assessment by classification, plotted over the time, and Figure 2 illustrates the plots of the FIM scores for all groups. The FIM scores plotted over the time demonstrate similar patterns for the ACA, putamen, and thalamus groups. The MCA group showed a lower ensemble-averaged FIM score at discharge than the other groups. Figure 3 illustrates the plots for the PASS scores, and all graphs were defined by a third-degree polynomial function with one argument.
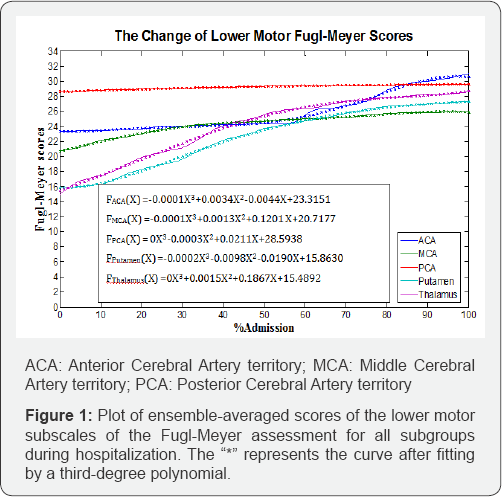
Figure 1 illustrates the recovery slopes of all of the groups. The thalamus group had the highest recovery slope (0.1867) for motor recovery, while the MCA group tended to evince a steeper slope in the recovery of lower motor functions (0.1201).
In Figure 2, both hemorrhage groups demonstrate steeper slopes of functional recovery than the slopes for the infarction groups. In the infarction groups, the MCA group demonstrates more gradual slope of functional recovery (0.3261). According to the graphs for postural-control recovery (Figure 3), the putamen group demonstrated the most rapid recovery rate (0.5135), while the MCA group demonstrated the slowest recovery rate (0.1581).
Achievement of mobility milestones
The following hierarchical pattern of achievement was evident for the three milestones investigated: 91% of the cohort achieved 5-min sitting balance (median = 7 days), 80% achieved l-min standing balance (median = 8 days), and 59% achieved the 50-m independent walk (median = 13 days). Table 2 presents further analysis based on sub-classification. All the ACA, PCA, and putamen groups (100%) achieved the 5-min sitting milestone, while 90% of the thalamus group achieved the sitting milestone hospitalization, and only 78% of the MCA group achieved the milestone prior to discharge. The achievement rates for the l-min standing balance and 50-m independent walk milestones for the ACA, PCA, putamen, and thalamus groups were 100% and 57%; 100% and 57%; 89% and 89%; and 80% and 70%, respectively before discharge. In the MCA group, 67% achieved the l-min standing balance milestone, and 47% were successful in the 50-m independent walk at discharge.
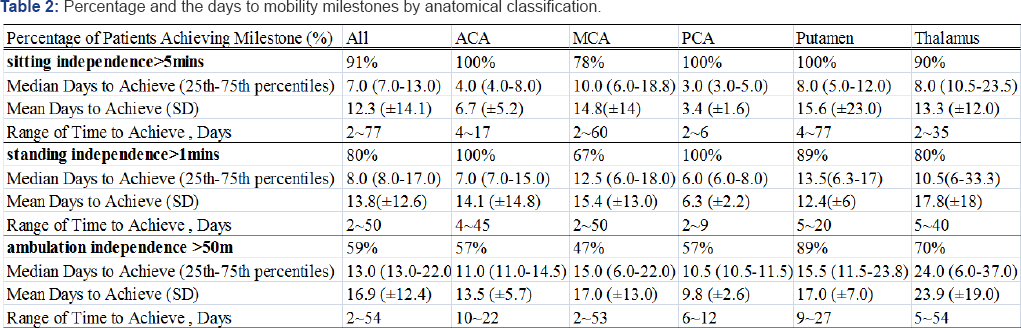
ACA: Anterior Cerebral Artery; MCA: Middle Cerebral Artery; PCA: Posterior Anterior Cerebral Artery
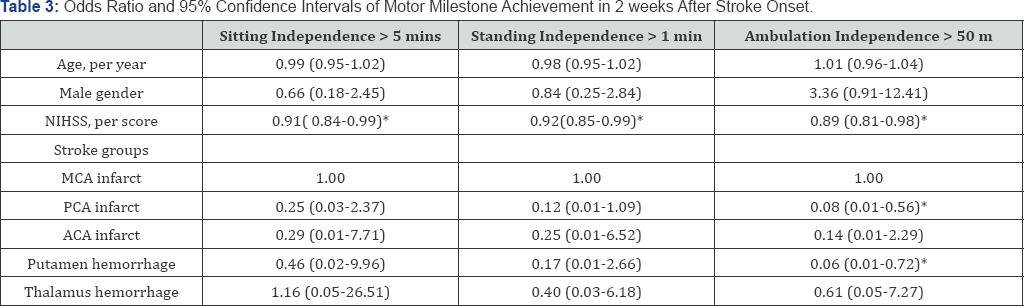
NIHSS: National Institute of Health Stroke Scale; ACA: Anterior Cerebral Artery; MCA: Middle Cerebral Artery; PCA: Posterior Anterior Cerebral Artery
In the multivariable logistic regression analysis (Table 3), only the NIHSS score at admission was shown to be an independent predictor for achieving the motor milestone ofsitting independence >5 min (odds ratio [OR] 0.91; 95% confidence interval [CI] 0.84- 0.99; P <0.05) and achieving standing independence >1 min (OR, 0.92; 95%CI, 0.85-1.00; P <0.05) within 14 days of onset. The PCA- infarct group (OR, 0.08; 95%CI, 0.01-0.56; P <0.05; compared with a reference group of MCA-infarct) or putamen-hemorrhage group (OR, 0.06; 95%CI, 0.01—0.72; P <0.05 ;compared with a reference group of MCA-infarct) and the NIHSS score at admission (OR, 0.89; 95%CI, 0.81-0.98; P <0.05) were associated with achieving ambulation independence >50 m within 14 days of onset after adjustment for confounders. The lesion location was shown to be an independent predictor for achieving ambulation independence >50 m within 14 days of onset.
Discussion
Our study showed that the motor-function and posturalcontrol recovery graphs, spontaneous recovery in the acute stroke patients were rapid during the first month which was consistent with the previous ones [7-13]. Our study also showed that the putamen and thalamus hemorrhage groups showed better recovery rates than other infarct groups, although these groups still did not achieve full independence in terms of ADL at discharge [33,34]. Although the PCA group did not show the steepest slope in its functional curve, according to the statistical results, the PCA group evidenced the best functional status at admission and discharge, reflecting from its relatively milder severity and shorter LOS. Although the motor recovery in the MCA group improved well, this group evidenced less significant rates of functional and posture recovery during hospitalization. Despite the MCA groups had lower functional status at discharge, it actually made less progress in functional recovery during hospitalization. This could imply that the MCA group required more functionality and postural-control training (including ADL training) instead of the neuro-facilitation only they received during an acute period after stroke.
Ninety-one percent of our cohort achieved the 5-min independent sitting balance milestone, and the median time to achieve this goal was 7 days post-stroke. In one report by Smith et al., 93% patients can achieve the l-min independent sitting milestone [35]. The difference may arise from the duration of 5-min vs. 1-min independent sitting milestone and early intervention starting within 5 days of onset after stroke in our study. Regarding the groups in the present study, all ACA, PCA, and putamen groups achieved the goal; however, only 78% of the MCA group attained this milestone. Patients in the MCA group sometimes required 10 days post-stroke to achieve this goal. The study conducted by Smith et al. also showed only 77% of the anterior circulatory infarct patients, with a median time of 11 days, achieved independent sitting balance [35].
Only 59% patients in our cohort achieved an independent 50-m walk, and the majority of patients achieved the goal within 2-3 weeks. Not all investigations were consistent, however, which may be due to differences in the definitions of independent walking, the duration of the assessments, and the population selection [8,25]. In comparison to our study, 89% of the patients who achieved improved walking ability in the putamen group achieved this milestone within a median time of 15.5-days. The MCA group was least likely to achieve independent ambulation. While they may have the potential to recover limited mobility, additional assistance in mobility at the time of discharge was required.
In the present study, achievement of the motor milestones of sitting independence > 5 min and standing independence > 1 min within 14 days of onset was not associated with the lesion locations, whereas achievement of the two milestones was associated with the NIHSS scores at admission. It is well known that different lesion locations result in different impacts to the brain. However, the results of this study support the view that NIHSS scores at admission provide a more efficient means of predicting whether stroke patients will be able to achieve the motor milestones of sitting independence > 5 min or standing independence > 1 min during the acute stage. A lower baseline NIHSS score could indicate a lower lesion burden and, therefore, a higher probability of the patient regaining sitting or standing mobility. More studies will be necessary to confirm this association. However, not only NIHSS score at admission but also the lesion location, the PCA or putamen groups, was independently associated to achievement of the motor milestone of ambulation independence > 50 m within 14 days of onset. The PCA and putamen group had increased odds of achieving ambulation independence > 50 m within 14 days of onset compared to MCA group. The results implied that a combination of NIHSS score at admission and the present of PCA infarction or putamen hemorrhage could help predict the milestone achievement of ambulation independence > 50 m in patients with stroke at acute stage.
The restoration of an independent gait is a major goal of poststroke rehabilitation. Due to the bilateral innervation of the trunk and girdle muscles, the sitting posture is among the first postures to be restored post-stroke [32]. The routine measurements of recovery in our cohort allowed for the accumulation of data for the standard measures of assessment used. Therefore, we chose the three milestones of sitting, standing, and walking independently because of their importance for FIM and PASS assessments in terms of developing a standard criterion for measurement and reliability [30,32]. The mobility milestone definitions used in our study, namely, standing independently for 5 min, standing independently for 1 min, and walking independently for 50 m, reveal more information about functional capacities such as dressing, transferring from bed to chair, and indoor ambulation. As such, they may provide more information for optimizing stroke management as well as for estimating the feasibility of short-term goals and the possibility of ADL independence at discharge. Furthermore, the results also demonstrate that the average number of days required to reach the three milestones differs among the subgroups, in addition to providing references regarding the number of days required for milestone achievement for different lesion sites. Therefore, the three mobility milestones, which provide a quick, simple, and standardized outcome measure, may be an effective evaluation tool for detecting the mobility and functional improvement of stroke patients from the acute stage.
Some noteworthy limitations to this study should be mentioned. All our patients received physiotherapy as well as some occupational or speech therapy during study. Thus the current study did not attempt to investigate the natural recovery curve after stroke. We also did not attempt to record all potentially influencing factors, such as patient age, lesion size, or comobidities [36]. Since both MRI and MRA (including diffusion weighted imaging) were performed, the classification of stroke groups with respect to anatomic structure was reliable. The accuracy of the prognostic estimates based on lesion location was confirmed. Furthermore, patients in this cohort were relatively homogenous in that patients with multiple stroke episodes were not included in the study Lastly, subjects who failed to reach the mobility milestone at the end of the observation period was not included in the data analysis hence our results may demonstrate a more optimistic picture of the motor recovery for patients after their first episode of stroke.
Conclusion
Hemorrhagic stroke has faster motor recovery than ischemic stroke, and the motor recovery may continue after discharge. Bedsides, a combination of NIHSS score at admission and the present of PCA infarction or putamen hemorrhage could help predict the milestone achievement of ambulation independence > 50 m in patients with stroke at acute stage. The information on lesion location allows the rehabilitation team to set realistic prognostic goals on the recovery of mobility. Thus, this study provides pilot information for establishment of clinical pathways to allocate time for rehabilitation services in reaching each milestone from stroke onset.
References
- AR F-M (1976) The effect of rehabilitation in hemiplegia as reflected in relation between motor recovery and ADL function, in Proceedings of the International Rehabilitation Association. Mexico City.
- Fugl Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S (1975) The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7(1): 13-31.
- Dickson HG, Kohler F (1995) Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med 27(4): 253-256.
- Hamilton BB, Laughlin JA, Fiedler RC, Granger CV (1994) Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med 26(3): 115-119.
- Benaim C, Perennou DA, Villy J, Rousseaux M, Pelissier JY (1999) Validation of a standardized assessment of postural control in stroke patients: the Postural Assessment Scale for Stroke Patients (PASS). Stroke 30(9): 1862-1868.
- Hofgren C, Bjorkdahl A, Esbjornsson E, Sunnerhagen KS (2007) Recovery after stroke: cognition, ADL function and return to work. Acta Neurol Scand 115(2): 73-80.
- Bonita R, Beaglehole R (1988) Recovery of motor function after stroke. Stroke 19(12):1497-1500.
- Wade DT, Wood VA, Hewer RL (1985) Recovery after stroke-the first 3 months. J Neurol Neurosurg Psychiatry 48(1): 7-13.
- Newman M (1972) The process of recovery after hemiplegia. Stroke 3(6): 702-710.
- Skilbeck CE, Wade DT, Hewer RL, Wood VA (1983) Recovery after stroke. J Neurol Neurosurg Psychiatry 46(1): 5-8.
- Prescott RJ, Garraway WM, Akhtar AJ (1982) Predicting functional outcome following acute stroke using a standard clinical examination. Stroke 13(5): 641-647.
- Steinberg BA, Augustine JR (1997) Behavioral, anatomical, and physiological aspects of recovery of motor function following stroke. Brain Res Brain Res Rev 25(1): 125-132.
- Mayo NE, Korner Bitensky NA, Becker R (1991) Recovery time of independent function post-stroke. Am J Phys Med Rehabil 70(1): 5-12.
- Smith MT, Baer GD (1999) Achievement of simple mobility milestones after stroke. Arch Phys Med Rehabil 80(4): 442-447.
- Reding MJ, Potes E (1988) Rehabilitation outcome following initial unilateral hemispheric stroke. Life table analysis approach. Stroke 19(11): 1354-1358.
- Jongbloed L (1986) Prediction of function after stroke: a critical review. Stroke 17(4): 765-776.
- Yamamoto L, Magalong E (2003) Outcome measures in stroke. Crit Care Nurs Q 26(4): 283-293.
- Department of Health (2012) Detailed Inpatient Expenses by Contracted Category (Pilot Project).
- Lotter O, Jaminet P, Amr A, Chiarello P, Schaller HE, et al. (2011) Reimbursement of burns by DRG in four European countries: an analysis. Burns 37(7): 1109-1116.
- Beloosesky Y, Streifler JY, Burstin A, Grinblat J (1995) The importance of brain infarct size and location in predicting outcome after stroke. Age Ageing 24(6): 515-518.
- Miller LS, Miyamoto AT (1979) Computed tomography: its potential as a predictor of functional recovery following stroke. Arch Phys Med Rehabil 60(3): 108-109.
- Saeki S, Ogata H, Hachisuka K, Okubo T, Takahashi K, et al. (1994) Association between location of the lesion and discharge status of ADL in first stroke patients. Arch Phys Med Rehabil 75(8): 858-860.
- Partridge CJ, Johnston M, Edwards S (1987) Recovery from physical disability after stroke: normal patterns as a basis for evaluation. Lancet 1(8529): 373-375.
- Ward NS, Cohen LG (2004) Mechanisms underlying recovery of motor function after stroke. Arch Neurol 61(12): 1844-1848.
- Wade D, Langton Hewer R, Skilbeck C DR, editors. (1985) Stroke: a critical approach to diagnosis treatment and management. London: Chapman & Hall Medical.
- Mori S, Sadoshima S, Ibayashi S, Fujishima M, Iino K (1995) Impact of thalamic hematoma on six-month mortality and motor and cognitive functional outcome. Stroke 26(4): 620-626.
- Nazzal ME, Saadah MA, Saadah LM, Trebinjac SM (2009) Acute ischemic stroke: relationship of brain lesion location and functional outcome. Disabil Rehabil 31(18): 1501-1506.
- Chen CL, Tang FT, Chen HC, Chung CY, Wong MK (2000) Brain lesion size and location: effects on motor recovery and functional outcome in stroke patients. Arch Phys Med Rehabil 81(4): 447-452.
- Pan SL, Wu SC, Wu TH, Lee TK, Chen TH (2006) Location and size of infarct on functional outcome of noncardioembolic ischemic stroke. Disabil Rehabil 28(16): 977-983.
- Chaudhuri G, Harvey RF, Sulton LD, WLambert R (1988) Computerized tomography head scans as predictors of functional outcome of stroke patients. Arch Phys Med Rehabil 69(7): 496-498.
- Pantano P, Formisano R, Ricci M, Piero VD, Sabatini U, et al. (1996) Motor recovery after stroke: morphological and functional brain alterations. Brain 119: 1849-1857.
- Loewen SC, Anderson BA (1990) Predictors of stroke outcome using objective measurement scales. Stroke 21(1): 78-81.
- Shah S, Vanclay F, Cooper B (1989) Predicting discharge status at commencement of stroke rehabilitation. Stroke 20(6): 766-769.
- Wade DT, Skilbeck CE, Hewer RL (1983) Predicting Barthel ADL score at 6 months after an acute stroke. Arch Phys Med Rehabil 64(1): 24-28.
- Granger CV, Greer DS, Liset E, Coulombe J, O Brien E (1975) Measurement of outcomes of care for stroke patients. Stroke 6(1): 3441.
- Gladstone DJ, Danells CJ, Black SE (2002) The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 16(3): 232-240.






























