The Influence of Sarcopenia on Walking in Frail Elderly People
Kota Kato12*, Yasuhiko Hatanaka2 and Koichi Saito2
1 Shutaikai Hospital: 8-1 Shirokita-cho, Yokkaichi-city, Mie 510-0823, Japan. Tel: +81-059-354-1771 /Fax: 059-354-0755 E-mail address: HYPERLINK "mailto:kota.kato26@gmaiI.com" kota.kato26@gmail.com
2 Suzuka University of Medical Science: 1001-1 Kishioka-cho, Suzuka-city, Mie 510-0293, Japan. Tel: +81-059-383-8991 / Fax: 059-383-9666
Submission: July 07, 2017;Published: July 21, 2017
*Corresponding author: Kota Kato, Shutaikai Hospital, Japan Tel: +81-059-354-1771; Fax: 059-354-0755; Email: kota.kato26@gmail.com, riha@syutaikai.com
How to cite this article: Kota K, Yasuhiko H, Koichi S. The Influence of Sarcopenia on Walking in Frail Elderly People. OAJ Gerontol & Geriatric Med. 2017; 2(1): 555577 DOI: 10.19080/OAJGGM.2017.02.555577
Abstract
The purpose of this study was to clarify the influence of sarcopenia on walking in frail elderly people. The subjects were 28 elderly people aged 65 years or over who could walk independently, attend community day-care centers. Assessment and measurement items were: history of falls during the past year, the presence or absence of sarcopenia, characteristics of walking, lower limb muscle strength, cognitive function, ability to perform activities of daily living (ADL), and general information. The subjects were divided into two groups depending on the presence or absence of sarcopenia, and a comparison was made between the two groups. Five people (severe sarcopenia group) fulfilled all evaluation items of sarcopenia and seven people (control group) did not fulfill any evaluation items. The remaining 16 fulfilled some items but they were not included in this comparative study. The ankle joint maximum plantar flexion moment and walking velocity of the severe sarcopenia group showed significantly lower values than the control group. This study suggested that reduced lower limb muscle strength is not the only cause of the decrease in walking velocity in frail elderly people with sarcopenia.
Keywords: Frail elderly people, Sarcopenia, Walking velocity
Abbreviations: ADL: Activities of Daily Living; MMSE: Mini-Mental State Examination; FIM: Functional Independence Measure
Introduction
In Japan, the population of elderly people aged 65 and over has been increasing. At the end of fiscal year 2000 when the long-term care insurance system was enforced, the population aged 65 and over was 22.42 million [1]. As of November 1, 2016, the elderly population aged 65 and over has increased to 34.63 million [2]. Among elderly people aged 65 and over, the number of people who are in a condition requiring some kind of support or nursing care is also increasing year by year. The number of people certified as requiring support and care was 2.47 million people at the end of fiscal year 2000, 5.92 million people at the end of fiscal year 2014 [1,3]. Furthermore, it is estimated that in 2025 when the "baby-boomer generation" is over 75 years old, it is predicted that the elderly population aged 65 and over will reach 36.57 million people and will also further increase thereafter [4]. As a result, there is a possibility that the number of people who qualify for support and care may increase, so the field of care prevention has attracted attention.
When an elderly person suffers a fall it is liable to result in injury such as a fracture, and this acts as a trigger to subsequent deteriorating health [5]. According to a survey conducted in fiscal year 2013 [6], falls and fractures are the fourth leading cause of the need for support and long-term care, accounting for 11.8% of the total. The rate of falls in one year in elderly people in Japan is about 10 to 20% for community-dwelling elderly and about 20 to 40% for elderly people in care facilities [7-10]. According to a survey estimate in 2009 [11], it is reported that medical expenses and nursing care expenses associated with falls exceed 900 billion yen annually. In other words, in Japan, which has a very aging society, it can be said that prevention of falls in elderly people is an extremely important task. Falls by elderly people occur in a variety of scenarios, but it is reported that approximately 60% of them occur during walking [7]. Therefore, efforts to prevent falls are necessary, focusing on walking of elderly people.
Fall-related factors affecting the elderly fall into two main categories: internal factors such as physical factors and external factors such as living environment factors [12]. Among internal factors, falls due to sarcopenia have been drawing attention in recent years. Sarcopenia was defined as "the age-dependent loss of skeletal muscle mass" by Rosenberg in 1989 [13]. The prevalence of sarcopenia in elderly people over 65 in Japan is approximately 20% [14]. According to a survey on the relationship between sarcopenia and falls [14-15], sarcopenic elderly people are reported to have an incidence of falls approximately two to three times higher than those in elderly people without sarcopenia. It is also necessary to consider not only the one-way relationship; that sarcopenia induces a fall, but also the opposite relationship; that the fall and the resulting reduction in activity lead to sarcopenia. It is reported that muscle mass differs by approximately 5% between falling and non-falling people [16]. That is, prevention of falls and sarcopenia is considered to be an important issue in extending healthy life-span and reducing medical and nursing care expenses.
However, the specific relationship between falls and sarcopenia has not yet been clarified, and concrete preventive measures have not been established. Therefore, by focusing on sarcopenia, one of the factors causing falls, in this study, we will clarify its relationship with walking and clarify the mechanism of falling occurrence. By doing so, we believe that a more effective method of intervention for fall prevention could be established which would help avoid the need for elderly care.
Materials and Methods
Subjects
The subjects were 28 elderly people (11 males and 17 females, 82.9 ± 5.2 years) aged 65 years and over who could walk independently and who were attending community daycare centers. People who were unable to undergo bioelectrical impedance analysis due to pacemakers or other medical electronic devices, those who had a history of cerebrovascular disorder, those who were undergoing injury treatment, and those who had a Mini-Mental State Examination (MMSE) score of 23 or less were excluded from the study. This study was conducted with the approval of the medical corporation Shutaikai ethics committee (2015.10.19), written informed consent was obtained and the rights of the subjects were protected.
Assessments and Measurements
The history of falls over the past year was ascertained by interview. If there was a history of falls, the number of falls, the date, place, situation, direction and the result of falls (injury or fracture) were recorded.
The presence or absence of sarcopenia was judged based on the diagnostic criteria of the Asian Working Group for Sarcopenia (AWGS) [17]. For measurement of muscular mass (skeletal muscle mass), a body composition meter (InBody 230,InBody Japan Inc., Tokyo, Japan) was used. Then, the value (kg/ m2) obtained by dividing the limb muscle mass (kg) obtained by Bioelectrical Impedance Analysis (BIA) by the square of the height (m) was used for evaluation. Measurement of muscular strength (grip strength) was performed using a grip strength meter (TTM Smedlay's Dynamo Meter 100 kg Yo II, Tsutsumi Seisakusho Co., Ltd., Tokyo, Japan), measuring twice each on the left and right side, with the best value recorded. Measurement of physical function (10 m usual walking velocity) was performed using a stopwatch, measurement was performed twice, and the average value was recorded.
To measure the characteristics of walking, one force plate (Accu Gait, AMTI Inc., Watertown, MA, USA) and four digital high vision video cameras (GZ-G5-B, JVC KENWOOD Inc., Kanagawa, Japan) were used. We installed digital high vision video cameras around the 4 m walking path where the force plate was installed, and photographed walking motion from front to back and from left and right. The sampling frequency was set to 60 Hz, and an optical-synchronization signal generator (PH-145, DKH Inc., Tokyo, Japan) was used for synchronization. The walking velocity was taken as the comfortable walking velocity of each subject and the number of times measurement was performed was set to three on each side. Markers (diameter 15 mm) were applied at 10 points: on both sides of each of the acromion, the greater trochanter, the lateral joint space, the lateral malleolus, and the head of the fifth metatarsal bone. The captured motion picture was imported into a 3D motion analysis system (Frame- DIAS V, DKH Inc., Tokyo, Japan), and the 3D spatial coordinates of each marker were calculated. In addition, the data obtained from the 3D spatial coordinates and the force plate were converted into DIFF (Date Interface File Format) and then converted into Excel format using DIFF gait and Wave Eyes, which are software provided by the clinical gait analysis group [18]. The joint angle and joint moment of the hip joint, knee joint, and ankle joint during one gait cycle were calculated from the respective data in Excel format.
Measurement of lower limb muscle strength used a muscle strength meter (μ Tas F-1, ANIMA Inc., Tokyo, Japan). Isometric hip joint flexion muscle strength, isometric hip joint extension muscle strength, isometric knee joint extension muscle strength, and isometric ankle joint plantar flexion muscle strength, which are the major muscular strengths affecting walking, were measured. Measurement of each muscular strength was performed during maximum isometric contraction of about 3 seconds. Measurements were carried out twice on each side at intervals of 30 seconds or more. Then, each maximum value was adopted, and the value obtained by dividing the left and right average value (kgf) by the body weight (kg) was expressed as the muscle force value (kgf/kg). For each measurement, the procedure was fully explained to the subjects, the subjects practiced beforehand, and performed the test, and when the posture was lost, the measurement was performed again.
For evaluation of cognitive function, the Mini-Mental State Examination (MMSE) was used.
To evaluate activities of daily living (ADL), the Functional Independence Measure (FIM) was used.
General information on each subject was collected from their medical records.
Statistical analysis
The subjects were divided into two groups: those with severe sarcopenia (positive for all evaluation criteria) and control group (negative for all criteria) according to the corresponding number of criteria of sarcopenia. Attributes (gender, age, degree of care) and physical characteristics (height, weight) of the subjects, history of falls during the past year, each joint angle and joint moment in one walking cycle, parameters of walking (walking velocity, stride, walking rate), and lower limb muscle strength were compared between the two groups. Fisher's direct stochastic method, chi-squared independence test, and Mann-Whitney U test were used for comparison between the two groups. JSTAT statistical analysis software was used for statistical analysis, and the significance level was set at P < 0.05.
Results
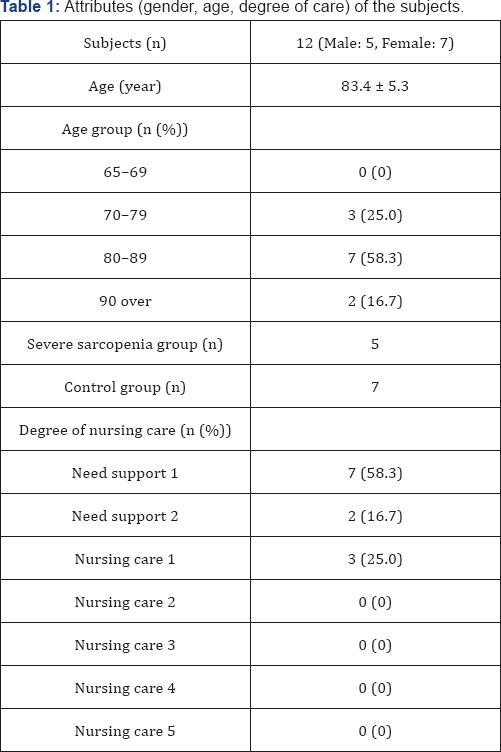
Of the 28 subjects, there were 5 people (1 male and 4 females, 85.8 ± 4.5 years) who fulfilled all the criteria of sarcopenia (severe sarcopenia group) and 7 people (4 males and 3 females, 81.7 ± 5.5 years) who did not fall under any judgment items of sarcopenia (control group) (Table1).
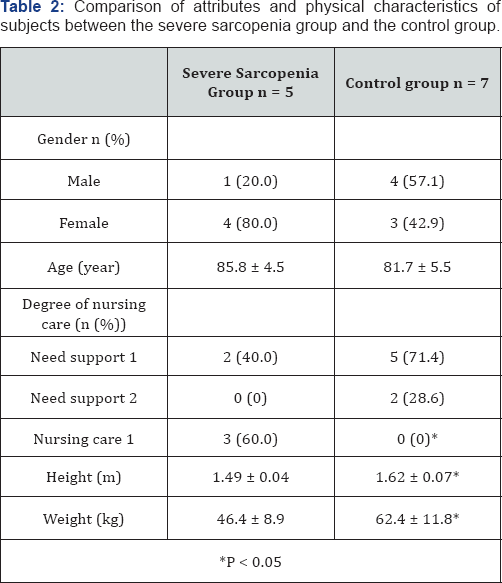
Table 2 shows the results of comparing the attributes and physical characteristics of subjects between the severe sarcopenia group and the control group. There was no significant difference in gender or age between the two groups. However, with regard to the level of nursing care, three people (60.0%) in the severe sarcopenia group required nursing care level 1 which was significantly higher than the control group of whom none (0%) required nursing care (P < 0.05). With regard to physical characteristics of the subjects, both height (severe sarcopenia group: 1.49 ± 0.04 m, control group: 1.62 ± 0.07 m, P < 0.05) and weight (severe sarcopenia group: 46.4 ± 8.9 kg, control group: 62.4 ± 11.8 kg, P < 0.05) were significantly lower in the severe sarcopenia group compared to the control group.
The results of comparing each evaluation and measurement item between the severe sarcopenia group and the control group are shown in Table 3. There was no significant difference in the fall rate of the past year or in lower limb muscle strength between the two groups. Although the fall rate did not show a significant difference between the groups, the rate was 40.0% in the severe sarcopenia group and 28.6% in the control group, showing a higher tendency in the severe sarcopenia group. Regarding the parameter of walking, walking velocity (severe sarcopenia group: 0.67 ± 0.11 m/sec, control group: 1.08 ± 0.16 m/sec, P < 0.01) and walking rate (severe sarcopenia group: 1.79 ± 0.19 steps/sec, control group: 2.04 ± 0.08 steps/sec, P < 0.05) were significantly lower in the severe sarcopenia group than in the control group. In each joint angle and joint moment during one walking cycle, only the hip joint flexion angle at the initial contact (severe sarcopenia group: 22.5 ± 4.5°, control group: 29.4 ± 3.8°, P < 0.05) and the ankle joint maximum flexion moment (severe sarcopenia group: 0.84 ± 0.22 Nm/kg, control group: 1.22 ± 0.08 Nm/kg, P < 0.01) were significantly lower in the severe sarcopenia group than in the control group. The knee joint extension angle at the terminal stance (severe sarcopenia group: -11.9 ± 8.8°, control group: -23.0 ± 7.7°, P < 0.05) was significantly higher in the severe sarcopenia group than in the control group.

Discussion
When the fall rate of the past year was compared between the severe sarcopenia group and the control group, the fall rate was 40.0% in the severe sarcopenia group and 28.6% in the control group, showing a high tendency in the severe sarcopenia group. This seems to be because the severe sarcopenia group has a lower ADL ability than the control group and the risk of a fall is higher. This was in agreement with a previous study [14-15]. In addition, among walking parameters, no significant difference was observed in the stride, but walking velocity and walking rate were both significantly lower in the severe sarcopenia group than in the control group. Generally, it is reported that walking parameters such as walking velocity, stride and walking rate decrease with age [19-21]. However, in this study, the walking velocity and the walking rate of the control group were about the average value for the general elderly, whereas in the severe sarcopenia group it was lower than the average value for the general elderly. Therefore, we considered that both these parameters are affected not only by age but also by sarcopenia, and that the state of sarcopenia can be reflected in the comparison between the severe sarcopenia group and the control group.
As a result of comparing walking characteristics between the severe sarcopenia group and the control group, we found that the maximum plantar flexion moment of the ankle joint at the terminal stance in the severe sarcopenia group was significantly lower than that in the control group. That is, the severe sarcopenia group had weak kicking out of the lower limb required to move the body forward in walking, indicating that the walking velocity was lowered. In general, one of the causes of the decrease in walking velocity is considered to be muscle weakness accompanying muscle atrophy due to aging [22]. In the severe sarcopenia group of this study, the lower limb muscle strength was also lower than in the general elderly. In other words, it seems that there is a possibility that reduced muscle strength of the lower limb may influence the decrease in walking velocity in the severe sarcopenia group. However, when the lower limb muscle strength of the severe sarcopenia group was compared with the control group, no significant difference was found between the two groups. This is probably due to the fact that the number of subjects in this study was small and it was difficult to obtain statistically-significant differences, or that the items for direct evaluation and measurement of lower limb muscle strength were not included in the criteria of sarcopenia. In other words, not only the lower limb muscle strength but also other factors may be influenced by the state of sarcopenia. Therefore, from the results of this study, we considered that the cause of the decrease in the walking velocity of the severe sarcopenia group suggests that the reduction of muscle strength of the lower limb muscle may not be the only pertinent factor.
Other causes ofthe decrease in walking velocity are a decrease in muscle weakness of the trunk muscles, etc. Unlike the upper limbs and lower limbs, trunk muscles are greatly involved in maintenance of postures such as sitting and standing. Therefore, the trunk muscles are considered to be less susceptible to the influence of sarcopenia, which is mainly responsible for the decrease of type II fibers, because the proportion of type I fibers is greater in the trunk muscles than that of the limb muscles [23-26]. However, according to a report by Ikezoe et al. [27] which investigated muscle atrophy of the trunk muscle accompanying aging, in the elderly with independent walking, the rate of decrease of the internal abdominal oblique muscle and external abdominal oblique muscle among the trunk muscles was as large as 47.6% and 40.2% respectively. In frail elderly people who need assistance in walking, the rate of decrease of the transverses abdominis muscle, the multifidus muscle, and the thoracic erector spinae muscle among the trunk muscles was about 3 to 4 times greater than that in the elderly with independent walking. In other words, the trunk muscle may also be affected by sarcopenia. Since the back muscles such as the transverses abdominis muscle, the multifidus muscle, and the erector spinae muscle function in maintaining posture and protecting the spinal column, so, as their muscle strength decreases, postural changes such as kyphosis occur more easily [28-29]. In addition, muscular weakness of the trunk muscle is thought to be one of the causes of lower back pain [30]. Because low back pain presents walking disturbance, it is considered that walking velocity may also be affected. Therefore, one of the causes of the decrease in walking velocity in sarcopenia of elderly people who require support and care may be that the trunk muscles are influential.
In the future, in order to prevent the severity of nursing care due to sarcopenia and falls, it is necessary to clarify the relationship between sarcopenia and walking. Therefore, we will focus on the trunk muscles considered as one of the causes of walking velocity decline in sarcopenia of elderly people who required support and care, which we aim to further compare and examine.
Conclusion
In the severe sarcopenia group, despite no clear difference in the lower limb muscle strength compared to the control group, a decrease in walking velocity was observed. Therefore, this study suggested that reduced lower limb muscle strength is not the only cause of the decrease in walking velocity in frail elderly people with sarcopenia.
References
- Ministry of Health, Labour and Welfare: Long-term care insurance business status report (FY 2000 Annual report).
- Statistic Bureau, Ministry of Internal Affairs and Communications: Outline of results of population estimation (April, 2017 report).?
- Ministry of Health, Labour and Welfare: Long-term care insurance business status report (FY 2014 Annual report).
- Cabinet Office: Aged Society White Paper (2016).
- Suzuki M, Yamada K, Takahashi H, Tsuchiya S (1993) A Study of Falls Among Elderly Living in the Community. Journal of Japan Academy of Nursing Science 13(2): 10-19.
- Ministry of Health, Labor and Welfare: Overview of the National Life Basic Survey (2013).
- Shibata H (1997) A Comprehensive Study on Falls and Fractures in Community Elderly. Grant-in-Aid for Scientific Research Result Report.
- Suzuki T, Sugiura M, Furuna T, Nishizawa S, Yoshida H, et al. (1999) Association of Physical Performance and Falls Among the Community Elderly in Japan in a Five Year Follow-up Study. Japanese Journal of Geriatrics 36(7): 472-478.
- Yasumura S (1999) Frequency of Falls and Fractures in the Elderly. The Journal of the Japan Medical Association 122(13): 1945-1949.
- Niino N, Nakamura K (1996) Circumstances and Factors Related to Falls in the Institutionalized Elderly. Japanese Journal of Geriatrics 33(1) 12-16.
- Hayashi Y (2009) The Influence of the Falls Over the Medical Economy The Bone 23(2) 181-184.
- Suzuki T (2003) Epidemiology of Falls. Japanese Journal of Geriatrics 40(2): 85-94.
- Rosenberg I (1989) Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr 50: 1231-1233.
- Yamada M, Nishiguchi S, Fukutani N, Tanigawa T, Yukutake T, et al. (2013) Prevalence of sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc 14(12): 911-915.
- Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, et al. (2012) Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 31(5): 652-658.
- Yamada M (2014) Sarcopenia and the Risk for Falls in Older Adults. Japanese Journal of Fall Prevention 1(1): 5-9.
- Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, et al. (2014) Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15(2): 95-101.
- The Clinical Gait Analysis Forum of Japan (1997) Walk Analysis by Joint Moment. Ishiyaku Publishers Inc, Tokyo.
- Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH (1988) Age- related changes in speed of walking. Med Sci Sports Exerc 20(2): 161166.
- Kinugasa T, Nagasaki H, Ito H, Hashizume K, Furuna T, et al. (1994) Effect of Aging on Motor Ability in Men Aged 18 to 83 Years. Japanese Society of Physical Fitness and Sport Medicine 43(5): 343-351.
- Maruyama H (1999) Motor Function and Gait in the Aged. Rigakuryoho Kagaku 14(3): 101-105.
- Kim JD, Kuno S, Soma R, Masuda K, Adachi K, et al. (2000) Relationship Between Reduction of Hip Joint and Thigh Muscle and Walking Ability in Elderly People. Japanese Society of Physical Fitness and Sport Medicine 49(5): 589-596.
- Tanimoto Y, Watanabe M, Kono R, Hirota C, Takasaki K, Kono K (2010) Aging changes in muscle mass of Japanese. Japanese Journal of Geriatrics 47(1): 52-57.
- Lexell J, Taylor CC, Sjöström M (1988) What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. J Neurol Sci 84 (2-3): 275-294.
- Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18(1): 111-129.
- Häggmark T, Thorstensson A (1979) Fibre types in human abdominal muscles. Acta Physiol Scand 107 (4): 319-325.
- Ikezoe T, Mori N, Nakamura M, Ichihashi N (2012) Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur J Appl Physiol 112(1): 43-48.
- Mika A, Unnithan VB, Mika P (2005) Differences in thoracic kyphosis and in back muscle strength in women with bone loss due to osteoporosis. Spine 30 (2): 241-246.
- Birnbaum K, Siebert CH, Hinkelmann J, Prescher A, Niethard FU (2001) Correction of kyphotic deformity before and after transection of the anterior longitudinal ligament a cadaver study. Arch Orthop Trauma Surg 121(3): 142-147.
- Ito S (1998) Pain and Muscle Strengthening: About Low Back Pain and Trunk Muscle Strength. The Japanese Journal of Physical Therapy 32(11): 847-854.






























