The Effects of Seroquel on Agitation and Cognition in Alzheimer’s Patients: A Limited Integrative Literature Review
Samantha Lafuente1 and Ladda Thiamwong*2
1Registered Nurse and Graduate Student, College of Nursing, University of Central Florida, USA
2College of Nursing, University of Central Florida, USA
Submission: May 26, 2017; Published: July 17, 2017
*Corresponding author: Ladda Thiamwong, College of Nursing, University of Central Florida, USA, Tel: 1-407-823-5091; Email: ladda.thiamwong@ucf.edu
How to cite this article: Samantha L, Ladda T. The Effects of Seroquel on Agitation and Cognition in Alzheimer’s Patients: A Limited Integrative Literature Review. OAJ Gerontol & Geriatric Med. 2017; 1(5): 555574 DOI: 10.19080/OAJGGM.2017.01.555574
Abstract
Background: Alzheimer's disease is a progressive, irreversible disease that effects over 5 million Americans and costs the United States over $200 billion a year, with no known cure. Side effects can be experienced throughout the disease process, to include agitation, delusions, hallucinations, aggression, memory loss, and many more. This can severely influence both the family's and the patient's quality of life.
Objectives: This limited integrative literature review explores the use of Seroquel (quetiapine), an atypical antipsychotic medication, on Alzheimer's patients with cognitive and/or behavioral disturbances.
Methodology: A comprehensive search strategy was used to search the current literature on the effects of Seroquel (quetiapine) on this patient population. Seven articles were reviewed and chosen for use in this literature review, and their evidence is presented in Table 1 at the conclusion of this paper
Results: Varying results were found, with the majority of patients tolerating the Seroquel (quetiapine) very well. A reduction in extrapyramidal symptoms were also seen with the use of this particular medication, as compared with other atypical antipsychotics. The main limitation of these studies is a small sample size
Conclusion and Recommendation: Future research is needed on this subject, preferably using larger sample sizes, in order to find supporting evidence for the use of Seroquel (quetiapine) with Alzheimer's patients.
Keywords: Agitation; Alzheimer's; Cognitive disturbances; Literature review; Quetiapine, Seroquel
Introduction
Alzheimer's disease is a progressive, irreversible type of dementia that effects approximately 5.4 million Americans and is the fifth leading cause of death for people over the age of 65 [1]. The total payment for 2016 for dementia care was estimated to be $236 billion [1]. Unfortunately, there are currently no known preventions or cures for Alzheimer's dementia. This disease can detrimentally affect both the patient and the family or caregivers caring for the patient.
The cognitive and behavioral changes that occur throughout the disease process can be very stressful to all who are involved. In the beginning stages, the patient may exhibit symptoms such as irritability, anxiety, and depression [2]. In the later stages, symptoms such as agitation, aggression, hallucinations, and delusions can occur. This literature review focuses on the later stages of Alzheimer's, including cognitive decline (i.e. memory loss, word recall, and delusions) and agitation, as they appear to be the most prevalent and difficult symptoms to manage as the disease destroys brain function [1]. Although there are many interventions that may temporarily subside these symptoms, Alzheimer's patients often times need pharmacological interventions when symptoms become severe and/or persist despite non-pharmacological attempts [2].
Atypical antipsychotics, such as Seroquel (quetiapine), have not been approved by the U.S. Food and Drug Administration (FDA) for use in Alzheimer's patients with cognitive and behavioral disturbances, because they can potentially worsen those same cognitive and behavioral disturbances [2]. The Alzheimer's Association [2] urges caution when trying to choose the proper medication for an off-label use, such as Seroquel (quetiapine). The two types of medications that have been approved by the FDA to treat cognitive symptoms of Alzheimer's disease are cholinesterase inhibitors, such as Aricept, Exelon and Razadyne, and Namenda (memantine) [2]. More research is needed to either support or refute the off-label use of these medications. The purpose of this paper is to evaluate how Seroquel (quetiapine) effects agitation and cognition in Alzheimer's patients.
Methods
An ultimate database search of CINAHL, Cochrane Database of Systematic Reviews, and MEDLINE was performed. Search terms included "Alzheimer", "Seroquel/quetiapine", and "agitat*". The use of the asterisk for the term agitation allowed for the search to include every word beginning with that prefix. These search terms also resulted in articles about both agitation and cognition of Alzheimer's patients taking Seroquel.
Articles were included if the majority of the research subjects were diagnosed with Alzheimer’s disease. The subjects were being administered Seroquel/quetiapine, and the outcomes being measured included both/either symptoms of agitation and/or cognitive effects, such as psychosis and/or altered mental status/confusion. Exclusion criteria included articles addressing other medications and articles discussing dementia without specifying Alzheimer’s disease. Articles included both inpatient and outpatient participants to prevent substantial limitation of data. Level and quality of evidence was determined by using the strength of evidence pyramid [3]. All articles used were quantitative studies of randomized controlled trials and/ or evidence summaries.
Results
Search Results
Two hundred and ninety-six articles resulted in the initial Medline search using "Alz*" and "Agita*" as search terms, followed by one hundred and sixty-six with the addition of the search term "Med*". Upon reviewing the titles and abstracts, many of the studies addressed patients with dementia, but not specifically patients with Alzheimer's disease. There were also many studies comparing the use of antipsychotics other than Seroquel (quetiapine). Due to the majority of these articles not fully addressing every aspect of the research question, this initial search was discarded.
A subsequent search was performed, comparing Seroquel (quetiapine) to Haldol (haloperidol), resulting in thirty-three, fifty-eight, and sixty-seven articles from Medline, Cochrane database of systematic reviews, and CINAHL respectively. Five of these articles were initially chosen, since they addressed patients with Alzheimer’s disease with the two anti-psychotic medications. However, the dependent variables of both agitation and cognition were not sufficiently addressed, resulting in a final revision of the research question to solely include Seroquel (quetiapine) as the independent variable.
A final search resulted in twenty-eight articles from Medline, Cochrane database of systematic reviews, and CINAHL using the search terms "Alz*", "Seroquel" OR "quetiapine", and "agit*". After careful review of all twenty-eight articles’ titles and abstracts, fifteen articles were selected for full-text review. The remaining thirteen articles were discarded, because they did not fully address the research question. Ultimately, seven articles were chosen for use in this limited integrative literature review addressing the use of Seroquel (quetiapine) for both agitation and cognition signs and symptoms in Alzheimer's patients. Four of the seven studies were level 2 randomized controlled trials, with one retrospective naturalistic study, and one level 4 and level 6 study. Given the limited nature of this integrated literature review, it was found to be educational in nature, despite the overwhelming evidence against the use of Seroquel (quetiapine) in Alzheimer’s patients.
A summary of the seven included studies’ design/method/ level of evidence, sample/setting, major variables studied and their definitions, interventions, measurements, data analysis and results, and an appraisal of worth to practice can be found in Table 1 in the appendix. The articles are organized alphabetically by the first author's last name. The data analysis and results sections are color-coded to assist in the finding of the two dependent variables being studied: agitation and cognition.
Effects of Seroquel (quetiapine) on Agitation
Six of the seven studies addressed the effects of Seroquel (quetiapine) on agitation with Alzheimer's patients (Table 1) [49]. They all assessed agitation levels using the Cohen-Mansfield agitation inventory (CMAI), the Neuropsychiatric Inventory (NPI), and/or the Behavioral Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD). Overall, Seroquel (quetiapine) showed to be well-tolerated in this patient population and decreased clinical signs and symptoms of agitation, such as irritability and aggression. This is evidenced by lower CMAI, NPI and BEHAVE-AD scores when compared to baseline. Ballard et al. [4] presented one exception to these findings; there were no significant differences seen in agitation levels between groups. All participants in the Ballard et al. [4] study showed improved scores from baseline, regardless of being in the control or intervention group.
Deyn, Eriksson, and Svensson [5], Fujikawa et al. [6], Onor, Saina, and Aguglia [7], Rocca et al. [8], and Savaskan et al. [9] all discussed Alzheimer's patients as having cognitive stability while taking Seroquel (quetiapine) and that the study participants tolerated the medication well. Fujikawa et al. [6] specifically discussed the decrease in occurrence of extrapyramidal symptoms with the use of Seroquel (quetiapine) compared to other antipsychotics. Onor, Saina, and Aguglia [7] showed decent efficacy of Seroquel (quetiapine) in reducing psychotic symptoms and decreasing the risk of iatrogenic parkinsonism and tardive dyskinesia in elderly patients. Savaskan et al. [9] recommended that Seroquel (quetiapine) be started at the lowest possible dose and increased slowly in order to avoid side effects, even though it is generally well tolerated.
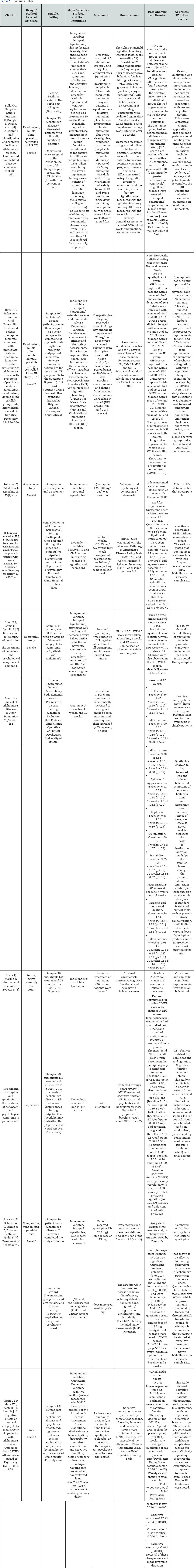
Seroquel (quetiapine) is currently not approved by the Food and Drug Administration (FDA) for use in Alzheimer's patients. In 2005, the FDA issued a black box warning on atypical antipsychotics, like Seroquel (quetiapine), because they were shown in some studies to increase the risk of death in elderly patients with dementia [5]. The increased level of mortality has created a great debate as a result of conflicting data from studies concerning the use of atypical antipsychotics. Ballard et al. [4] and Vigen et al. [10] showed evidence of adverse reactions to Seroquel (quetiapine) with Alzheimer's patients, along with other atypical antipsychotics like risperidone and olanzapine. It is important to note, though, that this evidence has come from studies with schizophrenia and other psychotic disorders, rather than patients with Alzheimer’s dementia. Thus far, Seroquel (quetiapine) has shown to be favorable for Alzheimer’s patients with agitation. Although the majority of articles discussed thus far contain small sample sizes, they were still able to achieve statistical and clinical significance levels (Table 1).
Effects of Seroquel (quetiapine) on Cognition
All seven studies addressed the effects of Seroquel (quetiapine) on cognition in patients with Alzheimer’s disease [4-10]. The results of these studies are displayed in Table 1. The cognitive changes of study participants were measured using the Severe Impairment Battery (SIB), the Mini-Mental State Examination (MMSE), the Clinical Global Impression- Severity of Illness scale (CGI-S), and portions of the BEHAVE-AD scale. As previously mentioned, Ballard et al. [4] and Vigen et al. [10] showed a cognitive decline in patients taking atypical antipsychotics like Seroquel (quetiapine), whereas the other five studies showed stable cognitive function [5-9].
The variable of cognition was broken down into delusions, hallucinations, word recall, word list memory, euphoria, and disinhibition. Vigen et al. [10] summarized their findings as showing a cognitive decline in patients taking atypical antipsychotics, but they also commented that these results are consistent with results of meta-analyses with larger sample sizes. Without more specific and supporting results pertaining to the cognitive decline in patients, it is difficult to analyze if these statistics are truly accurate or if the sample size was so large that it created significant results. Overall, a decrease in delusions and hallucinations and an increase in word recall and word list memory can be seen in these studies [4-10]. A positive change in cognition can be seen with decreased MMSE and SIB scores. Although Rocca et al. [8] reported no significant changes in MMSE scores, they did report that MMSE scores were significantly correlated with a decreased NPI score for agitation and delusions.
Limitations of the Evidence
One of the major limitations for this review is the lack of evidence supporting the use or disuse of Seroquel (quetiapine) for the treatment of agitation and/or cognitive function in Alzheimer's patients. There are many studies reviewing the effects on patients experiencing psychosis, but not patients with Alzheimer’s disease. This subject has only recently become more studied, despite not being approved by the FDA for use in this patient population. It would be beneficial to have more clinical trials evaluating the use of Seroquel (quetiapine) on patients with Alzheimer's disease.
Six of the seven studies stated a limitation of a small sample size. Ballard et al. [4] reported a sample size of 93; Deyn, Eriksson, and Svensson [5] reported 100; Fujikawa et al. [6] reported 16; Onor, Saina, and Aguglia [7] reported 41; Rocca et al. [8] reported 58; Savaskan et al. [9] reported 30. Vigen et al. [10] did not report any study limitations and had the largest sample size of 421 participants, yet reported cognitive decline with the use of Seroquel (quetiapine). It is important to note that these moderate to large sample sizes are a potential limitation of valid study results. Without more research to support these findings, it is difficult to make that judgement call at this time.
Rocca et al. [8] reported limitations relative to the nature of observational studies, as there were no control groups, no randomization, and no blinding involved. The researchers were unable to control for other influencing variables, such as patients taking other medications that could create a combined effect with Seroquel (quetiapine). It is important to note, though, that even observational studies can add a lot of clinical importance, and this study produced significant results that were in line with other studies and randomized controlled trials.
Two of the studies reported a limitation of having multiple comparisons or evaluations [4,7]. Onor and colleagues [7] compared different dosages of Seroquel (quetiapine), whereas Ballard and colleagues [4] compared multiple anti-psychotics and a placebo group. The greatest limitation for these studies that were not level 2 evidence or randomized controlled trials is that they lacked randomization, a control group, and the blinded fashion that eliminates certain biases.
Discussion
Two of the seven included studies showed a decline in cognitive function with the use of Seroquel (quetiapine) in Alzheimer's patients [4,10]. The other five included studies either showed a stable cognitive function or some improvement in scores for cognitive function and/or agitation [5-9]. Based on the majority of the included studies having small sample sizes and the variability in obtained results, there is not enough consistent data to support the use of Seroquel (quetiapine) in Alzheimer's patients for cognitive and/or behavioral problems. However, it is worth noting the clinical importance of the off-label use of Seroquel (quetiapine) within this patient population. Despite their limitations, many of the studies showed improvement in symptoms of psychosis and agitation without any changes in cognition [5-9].
Alzheimer’s disease is a progressive disease that worsens over time as the brain slowly dies [1]. Given this definition, it is expected to see cognitive decline in patients with Alzheimer's as their disease progresses. Ballard et al. [4] and Vigen et al. [10] discuss this steady deterioration in mental status over time, and they believe Seroquel (quetiapine) hastens that process. On the other hand, there have been many other studies that have investigated the off-label use of atypical antipsychotics, such as Seroquel (quetiapine), that have shown time and again the efficacy of this drug for use in reducing the debilitating symptoms of dementia [6]. Research has shown that typical antipsychotics can cause severe side effects, such as iatrogenic parkinsonism and tardive dyskinesia, but Seroquel (quetiapine) does not have these effects [7].
Recommendations
Practice
The FDA has placed a black box warning label on atypical antipsychotics, such as Seroquel (quetiapine), for the use in Alzheimer’s patients, as there is an increased risk for death [5]. However, Seroquel (quetiapine) has shown to be tolerated very well within this patient population, and is currently being used to manage cognitive and behavioral symptoms in Alzheimer's patients as an off-label use [5-9]. Based on the evidence provided in Table 1, it is noted that Seroquel (quetiapine) is well tolerated but should be started at the lowest dose possible and then adjusted thereafter according to the patient's response to treatment.
Education
Nurses, physicians, and other healthcare professionals should be aware of the risks and possible side effects that Seroquel (quetiapine) could have on patients with Alzheimer's disease. They should also educate themselves on the disease process itself and try as many non-pharmacological interventions as possible before resorting to the use of atypical antipsychotics in this medically vulnerable population. The Alzheimer's Association [2] is a great resource that provides education to both the health profession and the general public in ways that are best understood.
Healthcare Policy
Alzheimer’s disease is a global issue with no currently known causes or cures [1]. It is a progressive and irreversible disease. It costs the United States over $200 billion a year, which is expected to climb as the incidence rises over time. Further research needs to be done to find and prevent the causes of Alzheimer's disease. Although Seroquel (quetiapine) does not treat the disease itself, it allows for the more psychotic disturbances to be at bay, ultimately improving the patients' quality of life, as evidenced by the research within this study (Table 1).
Future Research
Given what is currently known about atypical antipsychotics, such as Seroquel (quetiapine), for the use in Alzheimer’s patients with behavioral and cognitive disturbances, more research needs to be done. Many of the other atypical antipsychotics, excluding Seroquel (quetiapine), have extrapyramidal effects on patients [6-7]. Most of the presented studies are single studies with small sample sizes. More randomized controlled trials need to be done on larger sample sizes in order to get accurate and generalizable results that are clinically and statistically significant. This patient population is extremely vulnerable to the side effects of medication due to polypharmacy. The FDA needs more supportive evidence in order to approve such medications for what is currently being used as off-label.
References
- Centers for Disease Control and Prevention (2016) Alzheimer's disease: Promoting health and independence for an aging population.
- Alzheimer's Association (2017) Treatments for behavior.
- Melnyk B M, Fineout-Overholt E (2015) Evidence based practice in nursing & healthcare: A guide to best practice(3rd ed.). Philadelphia, PA: Wolters Kluwer.
- Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, et al. (2005) Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: Randomised double blind placebo controlled trial. BMJ 1-5.
- Deyn P P, Eriksson H, Svensson H (2012) Tolerability of extended- release quetiapine fumarate compared with immediate-release quetiapine fumarate in older patients with Alzheimer's disease with symptoms of psychosis and/or agitation: A randomized, double-blind, parallel-group study. IntJ Geriatr Psychiatry 27: 296-304.
- Fujikawa T, Takahashi T, Kinoshita A, Kajiyama H, Kurata A, et al. (2004) Quetiapine treatment for behavioral and psychological symptoms in patients with senile dementia of Alzheimer type. Neuropsychobiology 49: 201-204.
- Onor ML, Saina M, Aguglia E (2007) Efficacy and tolerability of quetiapine in the treatment of behavioral and psychological symptoms of dementia. Am J of Alzheimer's Dis Other Demen 21(6): 448-453.
- Rocca P, Marino F, Montemagni C, Perrone D, Bogetto F (2007) Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer's disease: Preliminary findings from a naturalistic, retrospective study. Psychiatry ClinNeurosci 61: 622-629.
- Savaskan E, Schnitzler C, Schroder C, Cajochen C, Muller-Spahn F, et al. (2006) Treatment of behavioural, cognitive and circadian rest-activity cycle disturbances in Alzheimer's disease: Haloperidol vs. quetiapine. Int J Neuropsychopharmacol 9: 507-516.
- Vigen C L P, Mack W J, Keefe R S E, Sano M, Sultzer D L, et al. (2011) Cognitive effects of atypical antipsychotic medications in patients with Alzheimer's disease: Outcomes from CATIE-AD. Am J Psychiatry 168(8): 831-839.






























