Improved Structure and Development of Bone using Biofield Energy Enriched Vitamin D3 in Human Bone Osteosarcoma Cells (MG-63)
Maria Isabel A Tiraboschi1*, Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1, Gopal Nayak1 Mayank Gangwar2, Snehasis Jana2
1Trivedi Global, Inc., Henderson, USA
2Trivedi Science Research Laboratory Pvt. Ltd., India
Submission: May 10, 2018;Published: June 27, 2018
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., Bhopal, India, Email: publicaion@Trivedisri.com
How to cite this article: Maria I A T, Mahendra K T, Alice B, Dahryn T, Gopal N, et al. Improved Structure and Development of Bone using Biofield Energy Enriched Vitamin D3 in Human Bone Osteosarcoma Cells (MG-63). Nutri Food Sci Int J. 2018; 7(1): 555702. DOI:10.19080/NFSIJ.2018.07.555702.
Abstract
The present study aimed to investigate the effect of Consciousness Energy Healing based vitamin D3 and DMEM medium on various bone health parameters in MG-63 cells such as alkaline phosphatase enzyme (ALP) activity, collagen levels and bone mineralization. The test items (TI) i.e. vitamin D3 and DMEM medium were divided into two parts. Both the samples received Consciousness Energy Healing Treatment by Maria Isabel Aguilar Tiraboschi and samples were defined as the Biofield Energy Treated (BT) samples, while the other parts of each sample were denoted as the untreated test items (UT). Cell viability using MTT assay showed that cell viability was more than 70% with safe and nontoxic profile on MG-63 cell line. The level of ALP was significantly increased by 191.7% (0.1μg/mL), 127.8% (at 1μg/mL), and 136.1% (at 10μg/mL) in BT-DMEM+UT-TI, BT-DMEM+BT-TI, and UT-DMEM+BT-TI groups respectively as compared with the untreated test item and DMEM group. Collagen level was significantly increased by 267.9%, 128.9%, and 91.8% at 1, 10, and 50μg/mL, respectively in UT-DMEM+BT-TI group, while 169.6% at 1μg/mL in BT-DMEM+UT-TI group respectively as compared with the untreated group. Besides, BT-DMEM+BT-TI group showed an increased collagen value by 155.4% and 100% at 1 and 10 μg/mL respectively as compared with the untreated test item and DMEM group. The percent of bone mineralization was significantly increased by 139.8%, 91%, and 44.7% at 0.1, 10, and 100 μg/mL, respectively in UT-DMEM+BT-TI group, while 126.7%, 79.3%, and 67.5% at 0.1, 10, and 100 μg/mL, respectively in BT-DMEM+UT-TI group as compared with the untreated group. In addition, BT-DMEM+BT-TI group showed a significant increased bone mineralization by 31.3%, 33.2%, and 152.5% at 0.1, 10, and 100μg/mL, respectively as compared with the untreated group. Overall, the experimental data suggested that the Biofield Energy Treated vitamin D3 and DMEM would play an important role in the promotion and maintenance of strong and healthy bones, which improve quality of life. Biofield Energy Treatment might be vital in maintaining the various clinical safety and quality of life by assisting them in maintaining optimal vitamin D levels. It regulates the osteoblast function, improves bone mineralization, and calcium absorption in wide range of bone disorders along with wide range of adverse health conditions, comprising cancer and certain autoimmune diseases.
Keywords: Biofield energy; Bone mass; Bone strength; Osteosarcoma cells; Vitamin D; Bone mineralization
Abbreviations: CAM: Complementary and Alternative Medicine, NCCAM: National Center for Complementary and Alternative Medicine; MG-63: Human Bone Osteosarcoma Cells, ALP: Alkaline phosphatase, DMEM: Dulbecco's Modified Eagle's Medium, FBS: Fetal Bovine Serum, FBS: Fetal bovine serum; EDTA: Ethylene Diamine Tetra Acetic Acid, UT: Untreated, BT: Biofield Energy Treated, TI: Test Item
Introduction
Vitamin D has multiple effects which regulate the functions in different organs such as brain, lungs, liver, kidneys, and heart, immune, skeletal, and reproductive systems. Moreover, it has significant anti-inflammatory, anti-arthritic, anti-osteoporosis, anti-stress, anti-aging and anti-apoptotic, wound healing, anti-cancer, anti-psychotic, and anti-fibrotic roles. Vitamin D receptors (VDRs) are widely present in most of the body organs like brain, heart, lungs, kidney, liver, pancreas, large and small intestines, muscles, reproductive, nervous system, etc. [1]. VDRs influence cell-to-cell communication, normal cell growth, cell differentiation, cell cycling and proliferation, hormonal balance, neurotransmission, skin health, immune and cardiovascular functions. Bone-related health issues become a major problem among the population from village to the cities. Vitamin D plays a vital role in preserving a healthy mineralized skeleton of most of the vertebrates including humans. Cod liver oil, irradiation of other foods including plants, sunlight, etc. are found to be effective against bone related disorders, which lead to discovering the active principle- vitamin D [1]. The role of vitamin D has been well defined not only for improving the bone mineralization but also with increased bone resorption, aging, inflammation and overall quality of life. Vitamin D3 is synthesized in the skin by sunlight and once formed it sequentially metabolized in the liver and kidney to 1,25-dihydroxyvitamin D (calcitriol, the vitamin D hormone) [2]. Calcitriol play an important role in maintaining the normal level of calcium and phosphorus, promotes bone mineralization, induce or repress the genes responsible for conserving the mineral homeostasis and skeletal integrity, and inhibit hypertension, kidney damage, cardiovascular and immune disorders (such as Lupus, Addison Disease, Graves’ Disease, Hashimoto Thyroiditis, Multiple Sclerosis, Myasthenia Gravis, Anemia, Sjogren Syndrome, Systemic Lupus Erythematosus, Diabetes, Alopecia Areata, Fibromyalgia, Vitiligo, Psoriasis, Scleroderma, Chronic Fatigue Syndrome and Vasculitis), and the secondary hyperparathyroidism [3]. Vitamin D insufficiency and deficiency is the major health problem, which causes metabolic bone disease in the young and elderly populations [4]. Fortified foods have a variable amount of vitamin D and most of the foods do not contain vitamin D, which can be fulfilled using some supplements. In order to avoid the bone related disorders such as osteomalacia, exacerbate osteoporosis, hyperparathyroidism, immune disorders, etc. calcium 1000- 1500mg/day along with vitamin D supplement around 400IU/ day is very important for maintaining the good bone health [5].
Various in vitro studies have readily demonstrated the role of bone health using cell lines and its resorbing effects using three important key biomarkers, such as alkaline phosphatase (ALP), collagen and calcium. MG-63 cell line derived from juxtacortical osteosarcoma, which represents an immature osteoblast phenotype and undergoes temporal development in long term culture. The response of MG-63 cells to 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) administration has been studied to be similar to normal human osteoblast cells [6]. Hence, MG-63 cell line is widely used for studying the potential of any test compounds to improve the bone health [7]. The formation of new bone involves a complex series of events including the proliferation and differentiation of osteoblasts, and eventually the formation of a mineralized extracellular matrix. ALP is a phenotypic marker for the early differentiation and maturation of osteoblasts. ALP increases the local concentration of inorganic phosphate for bone mineralization and hence is an important marker for osteogenic activity [8]. Similarly, active osteoblasts synthesize and extrude collagen, which plays an important role in the formation of bone extracellular matrix by providing strength and flexibility. Collagen fibrils formed an array of an organic matrix known as Osteoid [9]. Likewise, calcium phosphate is deposited in the Osteoid and gets mineralized (combination of calcium phosphate and hydroxyapatite) and provides rigidity to the bone [10]. Thus, these parameters are very essential in order to study the bone health in cell lines. Authors evaluated the in vitro effect of the Biofield Energy Treated vitamin D3 as a test item, a Complementary and Alternative Medicine (CAM) on bone health using MG-63 cell line for major biomarkers.
Within the burgeoning ground of CAM therapies, Biofield Energy Treatment or energy medicine is emerging with significant benefits in various scientific fields. The effects of the CAM therapies have great potential, which include external qigong, Johrei, Reiki, therapeutic touch, yoga, Qi Gong, polarity therapy, Tai Chi, pranic healing, deep breathing, chiropractic/ osteopathic manipulation, guided imagery, meditation, massage, homeopathy, hypnotherapy, progressive relaxation, acupressure, acupuncture, special diets, relaxation techniques, Rolfing structural integration, healing touch, movement therapy, pilates, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems both in vitro and in vivo [11]. Biofield Energy Healing Treatment (The Trivedi Effect®) contains a putative bio energy, which is channeled by a renowned practitioner from a distance. Biofield Energy Healing as a CAM showed a significant results in biological studies [12]. However, the National Center for Complementary and Alternative Medicine (NCCAM), well-defined Biofield therapies in the subcategory of Energy Therapies [13]. The Trivedi Effect®- Consciousness Energy Healing Treatment has been reported with significant revolution in the physicochemical properties of metals, chemicals, ceramics and polymers [14- 16], improved agricultural crop yield, productivity, and quality [17,18], transformed antimicrobial characteristics [19-21], biotechnology [22-23], improved bioavailability [24-26], skin health [27,28], nutraceuticals [29,30], cancer research [31,32], and human health and wellness.
Based on the significant outcomes of Biofield Energy Treatment and vital role of vitamin D3 on bone health, authors sought to evaluate the impact of the Biofield Energy Treatment (The Trivedi Effect®) on vitamin D3 as test sample for bone health activity with respect to the assessment of different bone health parameters like ALP, collagen content, and bone mineralization using standard in vitro assays in MG-63 cells.
Materials and Methods
Chemicals and Reagents
Rutin hydrate was purchased from TCI, Japan, while vitamin D3 (denoted as test item) and L-ascorbic acid were obtained from Sigma-Aldrich, USA. Fetal bovine serum (FBS) and Dulbecco's Modified Eagle's Medium (DMEM) were purchased from Life Technology, USA. Antibiotics solution (penicillinstreptomycin) was procured from HiMedia, India, while 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium) (MTT), Direct Red 80, and ethylene diamine tetra acetic acid (EDTA) were purchased from Sigma, USA. All the other chemicals used in this experiment were analytical grade procured from India.
Cell culture
Human bone osteosarcoma cell line -MG-63 was used as test system in the present study. The MG-63 cell line was maintained in DMEM growth medium for routine culture supplemented with 10% FBS. Growth conditions were maintained at 37°C, 5%CO2 and 95% humidity and sub cultured by trypsinisation followed by splitting the cell suspension into fresh flasks and supplementing with fresh cell growth medium. Three days before the start of the experiment (i.e., day -3), the growth medium of near-confluent cells was replaced with fresh phenol-free DMEM, supplemented with 10% charcoal dextran stripped FBS (CD-FBS) and 1% penicillin-streptomycin [33].
Experimental design
The experimental groups consisted of cells in baseline control, vehicle control groups (0.05% DMSO with Biofield Energy Treated and untreated DMEM), positive control group (rutin hydrate) and experimental test groups. The experimental groups included the combination of the Biofield Energy Treated and untreated vitamin D3/DMEM. It consisted of four major treatment groups on specified cells with Untreated-DMEM + Untreated-Test item (UT-TI), UT-DMEM + Biofield Energy Treated test item (BT-TI), BT-DMEM + UT-TI, and BT-DMEM + BT-TI.
Consciousness energy healing treatment strategies
The test item and DMEM were divided into two parts. One part each of the test item and DMEM was treated with the Biofield Energy by a renowned Biofield Energy Healer (also known as The Trivedi Effect®) and coded as the Biofield Energy Treated item, while the second part did not receive any sort of treatment. This Biofield Energy Healing Treatment was provided by Maria Isabel Aguilar Tiraboschi remotely for ~5 minutes. Biofield Energy Healer was remotely located in the Uruguay, while the test samples were located in the research laboratory of Dabur Research Foundation, New Delhi, India. This Biofield Energy Treatment was administered for 5minutes through the Healer’s unique Energy Transmission process remotely to the test samples under laboratory conditions. Maria Isabel Aguilar Tiraboschi in this study never visited the laboratory in person, nor had any contact with the test item and medium. Further, the control group was treated with a sham healer for comparative purposes. The sham healer did not have any knowledge about the Biofield Energy Treatment. After that, the Biofield Energy Treated and untreated samples were kept in similar sealed conditions for experimental study.
Determination of non-cytotoxic concentration
The cell viability was performed by MTT assay in human bone osteosarcoma cell line (MG-63). The cells were counted and plated in 96-well plates at the density corresponding to 5 x 103 to 10 x 103 cells/well/180μL of cell growth medium. The above cells were incubated overnight under growth conditions and allowed the cell recovery and exponential growth, which were subjected to serum stripping or starvation. The cells were treated with the test item, DMEM, and positive control at various low to high concentrations. The untreated cells were served as baseline control. The cells in the above plate(s) were incubated for a time point ranging from 24 to 72 hours in CO2 i ncubator a t 3 7°C, 5 % C O2, a nd 9 5% h umidity. Following incubation, the plates were taken out and 20μL of 5mg/mL of MTT solution were added to all the wells followed by additional incubation for 3 hours at 37°C. The supernatant was aspirated and 150μL of DMSO was added to each well to dissolve formazan crystals. The absorbance of each well was read at 540nm using Synergy HT microplate reader, BioTek, USA [34]. The percentage cytotoxicity at each tested concentrations of the test substance were calculated using the following equation (1):
% Cytotoxicity = (1-X/R)*100 ----------------------- (1)
Where, X = Absorbance of treated cells; R = Absorbance of untreated cells
The percentage cell viability corresponding to each treatment was obtained using the following equation (2):
% Cell Viability = 100 - % Cytotoxicity ----------------------- (2)
The concentrations exhibiting ≥70% cell viability was considered as non-cytotoxic.
Assessment of alkaline phosphatase (ALP) activity
The cells were counted using an hemocytometer and plated in a 24-well plate at the density corresponding 1 x 104cells/well in phenol-free DMEM supplemented with 10% CD-FBS. Following respective treatments, the cells in the above plate were incubated for 48 hours in CO2 incubator at 37°C, 5% CO2, and 95% humidity. After 48 hours of incubation, the plate was taken out and processed for the measurement of ALP enzyme activity. The cells were washed with 1X PBS and lysed by freeze-thaw method i.e., incubation at -80°C for 20minutes followed by incubation at 37°C for 10 minutes. To the lysed cells, 50μL of substrate solution i.e., 5mM of p-nitrophenyl phosphate (pNPP) in 1M diethanolamine and 0.24mM magnesium chloride (MgCl2) solution (pH 10.4) was added to all the wells followed by incubation for 1 hour at 37°C. The absorbance of the above solution was read at 405nm using Synergy HT micro plate reader (Biotek, USA). The absorbance values obtained were normalized with substrate blank (pNPP solution alone) absorbance values [33]. The percentage increase in ALP enzyme activity with respect to the untreated cells (baseline group) was calculated using equation (3):
% Increase = [(X-R)/R)]*100 ----------------------- (3)
Where, X = Absorbance of cells corresponding to positive control and test groups
R = Absorbance of cells corresponding to baseline group (untreated cells)
Assessment of collagen synthesis
The MG-63 cells were counted using an hemocytometer and plated in 24-well plate at the density corresponding to 10 x 103 cells/well in phenol-free DMEM supplemented with 10% CD-FBS. Following respective treatments, the cells in the above plate were incubated for 48 hours in CO2 incubator at 37°C, 5%CO2, and 95% humidity. After 48 hours of incubation, the plate was taken out and the amount of collagen accumulated in MG-63 cells corresponding to each treatment was measured by Direct Sirius red dye binding assay. In brief, the cell layers were washed with PBS and fixed in Bouin’s solution (5% acetic acid, 9% formaldehyde and 0.9% picric acid) for 1hours at room temperature (RT). After 1 hour of incubation, the above wells were washed with milliQ water and air dried. The cells were then stained with Sirius red dye solution for 1 hour at RT followed by washing in 0.01 N HCl to remove unbound dye. The collagen dye complex obtained in the above step was dissolved in 0.1 N NaOH and absorbance was read at 540nm using Biotek Synergy HT microplate reader. The level of collagen was extrapolated using standard curve obtained from purified Calf Collagen Bornstein and Traub Type I (Sigma Type III) [33]. The percentage increase in collagen level with respect to the untreated cells (baseline group) was calculated using equation (4):
% Increase = [(X-R)/R]*100 ----------------------- (4)
Where, X = Collagen levels in cells corresponding to positive control and test groups
R = Collagen levels in cells corresponding to baseline group (untreated cells)
Assessment of bone mineralization by alizarin red S staining
The MG-63 cells were counted using an hemocytometer and plated in 24-well plate at the density corresponding to 10 x 103 cells/well in phenol free DMEM supplemented with 10% CD-FBS. Following respective treatments, the cells in the above plate were incubated for 48 hours in CO2 incubator at 37°C, 5% CO2, and 95% humidity to allow cell recovery and exponential growth. Following overnight incubation, the above cells will be subjected to serum stripping for 24 hours. The cells will be then be treated with non-cytotoxic concentrations of the test samples and positive control. After 3-7 days of incubation with the test samples and positive control, the plates were taken out cell layers and processed further for staining with Alizarin Red S dye. The cells were fixed in 70% ethanol for 1 hour, after which Alizarin Red solution (40μm; pH 4.2) was added to the samples for 20 minutes with shaking. The cells were washed with distilled water to remove unbound dye. For quantitative analysis by absorbance evaluation, nodules were solubilized with 10% cetylpyridinium chloride for 15 minutes with shaking. Absorbance was measured at 562 nm using Biotek Synergy HT microplate reader [33]. The percentage increase in bone mineralization with respect to the untreated cells (baseline group) was calculated using the following equation (5):
% Increase = [(X-R)/R]*100 ----------------------- (5)
Where, X = Absorbance in cells corresponding to positive control or test groups;
R = Absorbance in cells corresponding to baseline (untreated) group.
Statistical analysis
All the values in terms of absorbance (n=3) were calculated and respective parameters were represented as percentage of respective parameters. For multiple group comparison, oneway analysis of variance (ANOVA) was used followed by posthoc analysis by Dunnett’s test. Statistically significant values were set at the level of p≤0.05.
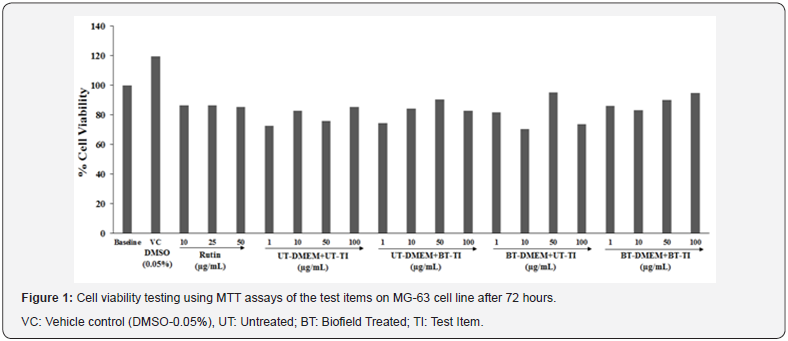
Results and Discussion
Cell viability study using MTT
The cell viability data was presented in terms of percentage using MTT assay to test the cell viability of the Biofield Energy Treated test samples (vitamin D3 and DMEM medium) in MG- 63 cells. The data in term of percentage values are presented in Figure 1. The percentage of cell viability in different tested groups showed significant improved cell viability. The results showed that both the test samples in combination at tested concentration ranges were found to have significant cell viability with more than 70%. Overall, the experimental data of MTT showed that the test items were found safe up to maximum of 100μg/mL against the tested MG-63cells. Thus, different concentrations i.e. safe concentrations are used to study the bone health parameters such as on the levels of alkaline phosphatase (ALP) activity, collagen synthesis, and bone mineralization in MG-63 cells.
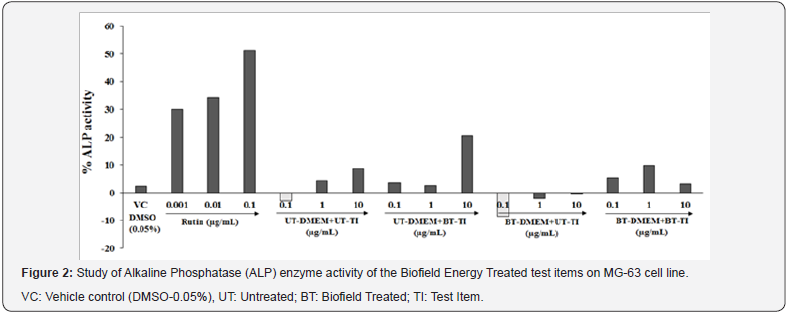
Alkaline phosphatase (ALP) enzyme activity
The level of ALP was calculated in various test systems. The experimental data of ALP activity showed significant alterations in the Biofield Energy Treated test item and DMEM at various concentrations on MG-63 cell line were presented in Figure 2. The percentage of ALP was calculated and presented with respect to untreated groups. The positive control, rutin showed a significant increased value of ALP by 30.02%, 34.31%, and 51.47% at 0.001, 0.01, and 0.1μg/mL with respect to the untreated cells. The experimental test group’s viz. untreated medium and Biofield Treated Test item (UT-DMEM+BT-TI) showed a significant increase in the ALP level by 136.1% at 10 μg/mL, while 191.7% increased ALP at 0.1μg/mL in BTDMEM+ UT-TI group with respect to untreated group. Biofield Energy Treated medium and Biofield Energy Treated Test item (BT-DMEM+BT-TI) showed a significant increased ALP level by 127.8% at 1μg/mL as compared with the untreated test item and DMEM group. The scientific literature suggested that the ALP enzyme activity alteration results in serious bone health diseases such as post-menopausal women, osteoporosis, bone cancers, Paget’s disease of bone, healing fracture, bone growth, acromegaly, myelofibrosis, osteogenic sarcoma, or bone metastases, leukemia, and rarely myeloma. The decreased level of ALP can be overcome using some nutraceutical supplements or vitamin D3, calcium, etc. [35-38]. The experimental data concluded that the Biofield Energy Healing Treatment in the test samples showed a significant improved level of the ALP, which could be the best supplementation to treat various bone and age related diseases such as osteoporosis [36]. In conclusion, the experimental data proposed that The Trivedi Effect®-Energy of Consciousness Healing based vit D3 and DMEM could be used to improve the ALP concentration in many bone disorders.
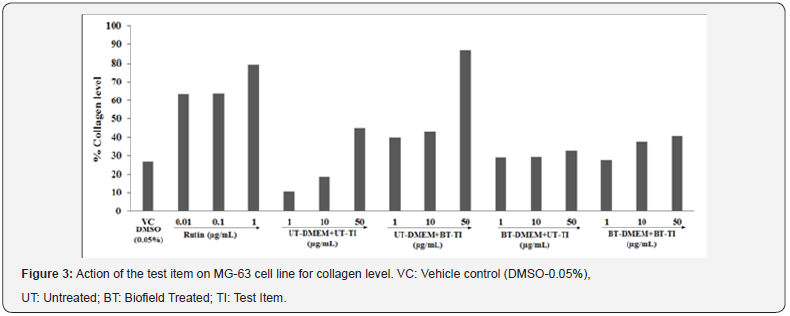
Assessment of collagen synthesis
Collagen level in term of percentage was calculated in all the test samples i.e. Biofield Energy Treated vit D3 and DMEM were estimated among various safe tested concentrations. The collagen data are presented as % values with respect to the untreated cells in Figure 3. The rutin hydrate showed a significant increased value of collagen by 63.44%, 63.83%, and 79.45% at 0.01, 0.1, and 1μg/mL, respectively. Besides, the experimental test groups such as UT-DMEM+BT-TI showed a significant increased collagen level by 267.9%, 128.9%, and 91.8% at 1, 10, and 50μg/mL, respectively while BTDMEM+ UT-TI group showed a significant increased collagen level by 169.6% and 56.7% at 1 and 10μg/mL, respectively as compared with the untreated test item and DMEM group. However, BT-DMEM+BT-TI group showed a significant increased collagen level by 155.4% and 100% at 1 and 10μg/ mL, respectively as compared with the untreated test item and DMEM group. Collagen, important constituent of bone health and its architecture. The turnover of the bone matrix is completely affected by the physical activity along with the collagen synthesis and degradation of the metalloprotease enzymes. Bone mineralization has significant role of collagen type I, which is the most abundant matrix protein [38]. Thus, for strong bone strength and its mechanical properties, structure and microarchitecture are considered as the determinant aspects. However, the level of collagen decreased with age, results in damage and bone deformities. Thus, collagen supplementation along with other nutritional factors is considered as the therapeutic agent to fight against bone diseases in case of osteoarthritis and osteoporosis [39]. The Biofield Energy Treated vit D3 and DMEM groups showed a significant improved level of collagen compared with the untreated group. Biofield Energy Treated vit D3 (The Trivedi Effect®) that would improve the collagen level for bone health, which can be used to decrease aging process and bone inflammation.
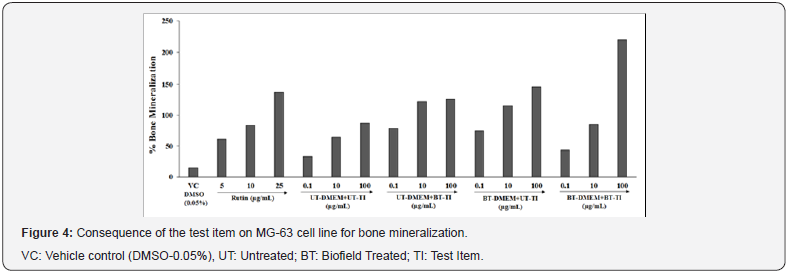
Bone mineralization
The quality of the bone depends on various set of parameters such as micro- and macro-architecture, bone remodeling rate, microdamage, apoptosis of bone cellular populations, and properties of the bone matrix i.e. crystals size, mineralization, collagen structure, and cross-linking. Bone fracture tendency depends on the quantity of mineralized tissue present in the bone (size and density) represented as the bone mineral density (BMD) and various other factors, grouped together defined as the ‘bone quality [40]. Bone mineralization has significant correlation with an important role in the treatment of osteoporosis or other bone diseases. The present study was conducted to check the alteration in percentage of bone mineralization in Biofield Energy Treated test samples with respect to the untreated test samples. Biofield Energy Treated vit D3 and DMEM groups showed a significant improved bone mineralization on MG-63 cell line. The results are presented in term of percentage change of bone mineralization among different experimental groups in Figure 4. The positive control, rutin group showed a significant increased value of bone mineralization by 60.89%, 83.68%, and 137.12% at 5, 10, and 25μg/mL, respectively. The experimental data among test group’s viz. UT-DMEM+BT-TI showed a significant increased bone mineralization by 139.8%, 91%, and 44.7% at 0.1, 10, and 100μg/mL, respectively while BT-DMEM+UT-TI group showed a significantly increased bone mineralization by 126.7%, 79.3%, and 67.5% at 0.1, 10, and 100μg/mL, respectively as compared with the untreated test item and DMEM group. However, BTDMEM+ BT-TI group showed a significant increased bone mineralization by 31.3%, 33.2%, and 152.5% at 0.1, 10, and 100μg/mL, respectively as compared with the untreated test item and DMEM group. A low value of BMD defines loss in bone mineralization, which results in poor calcium and vitamin D3 absorption and leads to various structural abnormalities [41]. The experimental test groups showed that Biofield Energy Healing Treatment significantly improved the rate of bone mineralization compared with the untreated groups.
Conclusion
The cell viability using MTT assay results showed a significant improved viability with more than 70% among the tested groups, which suggested that the test samples are safe and nontoxic. Bone health parameters such as the level of ALP was increased by 136.1% at 10μg/mL in the UT-DMEM+BTTI, 191.7% at 0.1μg/mL in BT-DMEM+UT-TI, while 127.8% at 1μg/mL in the BT-DMEM+BT-TI group as compared with the untreated test item and DMEM group. The level of collagen was significantly increased by 267.9%, 128.9%, and 91.8% at 1, 10 and 50μg/mL, respectively in the UT-DMEM+BT-TI, while 169.6% and 56.7% at 1 and 10μg/mL, respectively in the BTDMEM+ UT-TI group. In addition, collagen level was increased by 155.4% and 100% at 1 and 10μg/mL, respectively in BTDMEM+ BT-TI group as compared with the untreated test item and DMEM group. Similarly, the bone mineralization percent was significantly increased by 139.8%, 91%, and 44.7% at 0.1, 10 and 100μg/mL, respectively in the UT-DMEM+BT-TI group, while 126.7%, 79.3%, and 67.5% at 0.1, 10, and 100μg/ mL, respectively in the BT-DMEM+UT-TI group as compared with the untreated group. In addition, BT-DMEM+BT-TI group showed a significant increased bone mineralization by 31.3%, 33.2%, and 152.5% at 0.1, 10, and 100μg/mL, respectively as compared with the untreated group. Overall, the Biofield Energy Treated (The Trivedi Effect®) test samples were found to have a significant impact on tested bone health parameters viz. collagen, bone mineralization, and ALP, which are very vital to combat the bone disorders. Therefore, the Consciousness Energy Healing based vitamin D3 might be a suitable alternative nutritional supplement, which could be useful for the management of various bone related disorders viz. osteoporosis, Paget’s disease of bone, rickets, deformed bones, osteomalacia, bone and/or joint pain, increased frequency of fractures, osteoma, hormonal imbalance, stress, aging, bone loss and fractures, and other bone diseases that are caused by poor nutrition, genetics, or problems with the rate of bone growth or rebuilding. Biofield Energy Treated Vitamin D3 c an b e u seful a s a nti-inflammatory, a nti-aging, anti-stress, anti-arthritic, anti-osteoporosis, anti-cancer, antiapoptotic, wound healing, anti-psychotic and anti-fibrotic roles. It also influences cell-to-cell communication, normal cell growth, cell differentiation, neurotransmission, cell cycling and proliferation, hormonal balance, skin health, immune and cardiovascular functions. Besides, it can also be utilized in organ transplants (for example kidney transplants, liver transplants and heart transplants), hormonal imbalance, aging, and various immune related disease conditions such as Ulcerative Colitis, Alzheimer’s Disease, Dermatitis, Irritable Bowel Syndrome, Asthma, Hashimoto Thyroiditis, Pernicious Anemia, Sjogren Syndrome, Multiple Sclerosis, Aplastic Anemia, Hepatitis, Diverticulitis, Graves’ Disease, Dermatomyositis, Diabetes, Myasthenia Gravis, Parkinson’s Disease, Atherosclerosis, Systemic Lupus Erythematosus, stress, etc. with a safe therapeutic index to improve overall health, and quality of life.
Acknowledgement
Authors are grateful to Dabur Research Foundation, Trivedi Global, Inc., Trivedi Science, Trivedi Testimonials, and Trivedi Master Wellness for their support throughout the work.
References
- Holick MF (1996) Vitamin D and bone health. J Nutr 126(4 Suppl): 1159S-1164S.
- van Leeuwen JP, van Driel M, van den Bemd GJ, Pols HA (2001) Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit Rev Eukaryot Gene Expr 11(1-3): 199-226.
- Bikle DD (2012) Vitamin D and bone. Curr Osteoporos Rep 10(2): 151- 159.
- Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Rev 22(4): 477-501.
- Hossein-nezhad A, Holick MF (2013) Vitamin D for health: A global perspective. Mayo Clin Proc 88(7): 720-755.
- Czekanska EM, Stoddart MJ, Richards RG, Hayes JS (2012) In search of an osteoblast cell model for in vitro research. Eur Cell Mater 24: 1-17.
- Luo XH, Liao EY (2003) Effects of estriol on the proliferation and differentiation of human osteoblastic MG-63 cells. Endocrine Res 29(3): 343-351.
- Iba K, Takada J, Yamashita T (2004) The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab 22(6): 594-596.
- Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17(3): 319-336.
- Bhattarai T, Bhattacharya K, Chaudhuri P, Sengupta P (2014) Correlation of common biochemical markers for bone turnover, serum calcium, and alkaline phosphatase in post-menopausal women. Malays J Med Sci 21(1): 58-61.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8(6): 703-717.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 10(12): 1-23.
- Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, et al. (2012) Use and acceptance of complementary and alternative medicine among the general population and medical personnel: A Systematic Review. Ochsner J 12(1): 45-56.
- Trivedi MK, Tallapragada RM (2008) A transcendental to changing metal powder characteristics. Met Powder Rep 63(9): 22-28, 31.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4: 161.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Effect of biofield energy treatment on physical and structural properties of calcium carbide and praseodymium oxide. International Journal of Materials Science and Applications 4(6): 390-395.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L.). Journal of Food and Nutrition Sciences 3(6): 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Evaluation of biochemical marker - Glutathione and DNA fingerprinting of biofield energy treated Oryza sativa. American Journal of BioScience 3(6): 243-248.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, Jana S (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3(1): 129.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. J Antivir Antiretrovir 7: 083-088.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) An impact of biofield treatment: Antimycobacterial susceptibility potential using BACTEC 460/MGIT-TB System. Mycobact Dis 5: 189.
- Trivedi MK, Patil S, Shettigar H, Bairwa K, Jana S (2015) Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. J Microb Biochem Technol 7(4): 203-206.
- Nayak G, Altekar N (2015) Effect of biofield treatment on plant growth and adaptation. J Environ Health Sci 1(2): 1-9.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. International Journal of Clinical and Developmental Anatomy 3(3): 9-15.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. American Journal of Clinical and Experimental Medicine 5(4): 138-144.
- Branton A, Jana S (2017) Effect of The biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3 [25(OH) D3] in rats after a single oral dose of vitamin D3. American Journal of Pharmacology and Phytotherapy 2(1): 11-18.
- Kinney JP, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. American Journal of Life Sciences 5(2): 65-74.
- Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. American Journal of Pharmacology and Phytotherapy 2(1): 1-10.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. International Journal of Bioorganic Chemistry 2(3): 135-145.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) Chromatographic and spectroscopic characterization of the consciousness energy healing treated Withania Somnifera (ashwagandha) root extract. European Journal of Biophysics 5(2): 38- 47.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253- 257.
- Czekanska EM, Stoddart MJ, Richards RG, Hayes JS (2012) In search of an osteoblast cell model for in vitro research. Eur Cells Mater 24: 1-17.
- Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity (2009).
- Jesudason D, Need AG, Horowitz M, O’Loughlin PD, Morris HA, et al. (2002) Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone 31(5): 626-630.
- Seeman E (2009) Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 19(3): 219-233.
- Golub EE, Boesze-Battaglia K (2007) The role of alkaline phosphatase in mineralization. Curr Opin Orthop 18(5): 444-448.
- Paschalis EP, Recker R, Dicarlo E, Doty SB, Atti E, et al. (2003) Distribution of collagen cross-links in normal human trabecular bone. J Bone Miner Res 18(11): 1942-1946.
- Takeda S, Park J-H, Kawashima E, Ezawa I, Omi N (2013) Hydrolyzed collagen intake increases bone mass of growing rats trained with running exercise. J Int Soc Sports Nutr 10(1): 35.
- Ruppel ME, Miller LM, Burr DB (2008) The effect of the microscopic and nanoscale structure on bone fragility. Osteoporos Int 19(9): 1251- 1265.
- Bikle DD (2014) Vitamin D metabolism, mechanism of action, and






























