Nutritional Enhancement of Barley in Solid State Fermentation by Rhizopus oligosporus ML-10
Rubina Nelofer1*, Muhammad Nadeem1, Muhammad Irfan2 and Quratulain Syed1
1Department of Biotechnology and Food Research Center, Pakistan Council of Scientific and Industrial Research (PCSIR), Pakistan
2Department of Biotechnology, University of Sargodha, Pakistan
Submission: April 09, 2018;Published: June 18, 2018
*Corresponding author: Dr. Rubina Nelofer, Department of Biotechnology and Food Research Center, Pakistan Council of Scientific and Industrial Research (PCSIR), Laboratories Complex, Pakistan, Email: rubinanelofer@gmail.com
How to cite this article: Rubina N, Muhammad N, Muhammad I Quratulain S. Nutritional Enhancement of Barley in Solid State Fermentation by Rhizopus oligosporus ML-10. Nutri Food Sci Int J. 2018; 6(5): 555700. DOI:10.19080/NFSIJ.2018.06.555700.
Abstract
The present study was undertaken for nutritional enhancement of whole barley grains by optimizing solid state fermentation conditions with Rhizopus oligosporus ML-10 in polythene bags. The influence of various soaking time, boiling time, inoculum size, incubation time and temperature on the levels of proteins, fats and carbohydrates were evaluated during fermentation of barley. 500grams whole barley grains after soaking for 12h in 1L distilled water (pH, 4.5) were allowed to boil for 20min followed by drying at 80 °C for 15min. After this pretreatment, dehulled barley grains were inoculated with 1.5 % (v/w) spore suspension containing 1.0 x106 spores per mL of 96h aged R. oligosporus ML-10 in 25 x 25 cm2 polythene bags and incubated at 30 °C for 36h. The nutritional value of barley in terms of protein contents increased significantly from 10.25% to 16.85% after solid state fermentation. However, the level of total carbohydrate and fat after fermentation process was found to decrease from 68.6% to 63.55 and 2.13 % to 1.62 % respectively. From the results of present study it was concluded that the solid state fermentation of whole grain barley resulted in increasing the protein contents up to 64.3 % under optimized conditions by Rhizopus oligosporus ML-10 in polythene bags.
Keywords: Barley; Solid state fermentation; Protein; Fat; Carbohydrates; R. oligosprous; Nutritional value; Protein; Calorie; Hordeum vulgare
Introduction
Fermented foods either from plant or animal origin are commonly used in all parts of the world as a low cost protein source which fulfill the protein/calorie deficiency problems especially in developing countries where meat products are in poor supply [1- 4]. Barley is one of the most popular staple foods in Pakistan and many other countries. The barley (Hordeum vulgare) is reported to contain (w/w) 6.75 % crude fiber, 11.67% crude protein, 2.31 % crude fat and 2.22 % ash [5] .The consumption of barley and its products is found to assist in decreasing the risk of type 2 diabetes [6-7]. Moreover, it also helps in reducing the total serum lipids and LDL-cholesterol which in turn reduces the risk of cardiovascular diseases [8-9]. Whole grain barley has also been reported as a rich source of phytochemicals [10]. Therefore, increasing consumption of fermented cereals, would lower food cost and promote better health [11].
Microbial fermentation is also considered as one of the oldest and most economical methods for value additions and preservation of food. The fermentation process helps in increasing the digestibility and bioavailability of proteins, carbohydrates, lipids, minerals and vitamin contents in cereals. Moreover, it shortens the cooking time and increases the microbial safety [12].
The present study was undertaken to enhance the nutritional value of barley by optimizing the process parameters such as soaking and boiling time, inoculum size, incubation time and temperature, in solid state fermentation to study their effect on protein, fat and carbohydrates levels.
Materials and Methods
Microorganism maintenance
The fungal strain of Rhizopus oligosporus ML-10 used in the present study was obtained from Microbiology Lab, Biotechnology and Food Research Centre, PCSIR Labs Complex, Lahore. The culture was grown on potato dextrose agar (Oxoid) slant at 30 °C for 96h.The culture was then preserved at 4 °C in refrigerator and sub-culturing was done after every 4-6 week for further study.
Preparation of inoculums
Ten ml of sterilized distilled water was transferred to 96h aged potato dextrose agar slant of Rhizopus oligosporus ML-10. The spores were dislodged by using a sterile inoculation needle under aseptic conditions. The spore suspension containing approximately 1.0 x106 spores per mL was used as inoculum for the fermentation of pretreated barley grains with Rhizopus oligosporus ML-10.
Substrate: The whole barley grains used in the present study was purchased from local
Market of Lahore, Pakistan.
Solid-state fermentation
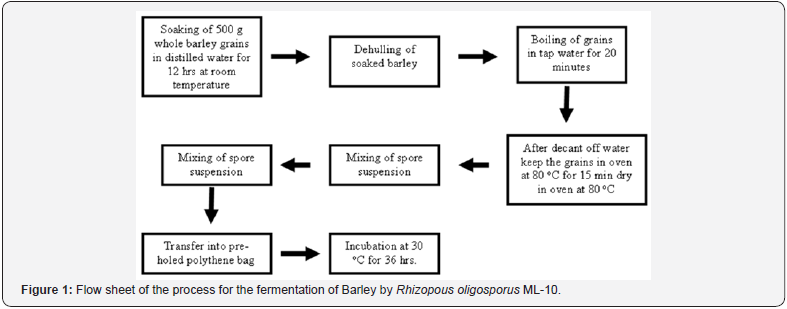
The solid state fermentation of barley was carried out in accordance with the modified method of Berg et al. [11]. 500g of whole barley grains were soaked for 12h in 1L distilled water in plastic beaker at room temperature. After manual dehulling, the soaked barley grains were allowed to boil in tap water for 20 min. Decant off water and kept the grains in oven at 80 °C for 15 min to remove excess of water. Then the spore suspension of Rhizopus oligosporus ML-10 at the rate of 1.5 % (V/W) was mixed well with pretreated barley grains at room temperature. Packed the inoculated barley grains in pre- holed 25 x 25cm2 polythene bags and kept at 30 °C for 36h. All the experiments were conducted in triplicate. The flow sheet of the process is given in Figure 1.
Proximate composition: Moisture, Fat, total carbohydrates and total nitrogen (microkjeldahl) were determined according to AOAC [13].
Result and Discussion
Effect of soaking time
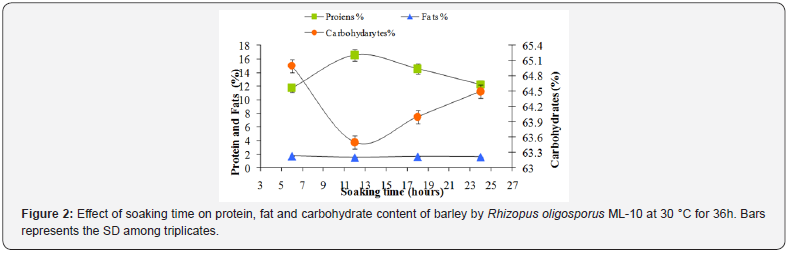
The soaking time on the fermentation of barley is important in solid state fermentation due to its effect on the softening of kernels for smooth mycelial growth of the Rhizopus oligosporus ML-10. Therefore, soaking time was varied from 6, 12, 18 and 24h in distilled water at room temperature. The data in Figure 2 revealed that the soaking time of 12h was found to be optimum for the production of nutritionally rich fermented barley with the increase in protein contents from 10.25 % to 16.55 %. Further increase in soaking time resulted in decreasing the protein contents as evident from Figure 2. However, the percentage of carbohydrates and fat in fermented barley was found to decrease slightly from 65 to 63.5 % and from 1.72 % to 1.52 %, respectively, with the increase of soaking time. It has been reported earlier that during soaking of soybean, organic acids are formed which resulted in lowering the pH from 6.0 to 3.9 [14]. Moreover, during soaking, the bacterial fermentation occurred resulting in acidification. Many workers, described the addition of < 0.5% lactic acid or < 0.25 % acetic acid during soaking stage which lowered the initial pH, allowing the mould to grow and suppress the bacterial growth [15]. In the present study, the pH was also noted to decrease from 6.5 to 4.3. Our results are in good agreement with earlier investigations that a natural microbial acidification occurred during the soaking process in Indonesia soybeans fermentation [16]. According to other investigators, the changes observed during soaking were due to leaching of soluble components and also due to enzyme activities during sprouting and fermentation [17,18].
Effect of boiling time
Partial cooking or boiling of the cereals was also known to play a vital role in the production of fermented food. Therefore, boiling time ranging from 15 to 30mints was evaluated for the production of nutritionally rich barley in solid state fermentation as given in Figure 3. Boiling of cereals in water has been reported to destroy contamination bacteria and anti nutritional factors and also release of some nutrients which are essential for mould growth [15]. According to Steinkraus et al. [12] a heat stable and water soluble mold inhibitor is leached out during boiling process, which is discarded later. The results in Figure 3 revealed that combination of soaking and boiling was most favorable for the growth of Rhizopus oligosporus ML-10. Longer boiling time alone did not give the same elasticity of the seed as did the combination of soaking and boiling. For longer boiling the gelatinization of starch content occurred and the cake formation deteriorated. Therefore, the boiling time of 15min was found to be suitable for the production of fermented whole grain cereal by Rhizopus oligosporus ML-10 in solid state fermentation. Mulyowidaro et al. [19] reported that mild boiling (15 min at 95 °C) of soaked and biologically acidified beans results in sufficient survival of a mixed flora of lactic acid bacteria and acillus spp. A strong proteolytic Bacillus spoilage was also observed in beans which had been heated for 60 min at 95 oC or 15 min at 121 °C [14]. Moreover, these workers also hypothesized that the lack of beany flavor in tempeh was due to the result of inactivation of the lipooxygenase associated with the formation of such flavors during the boiling stage. According to earlier investigations, the combination of cooking and fermentation improved the nutrient quality of all tested sorghum seeds and reduced the content of anti nutritional factors to a safe level in comparison with other methods of processing [17].

Effect of inoculum size
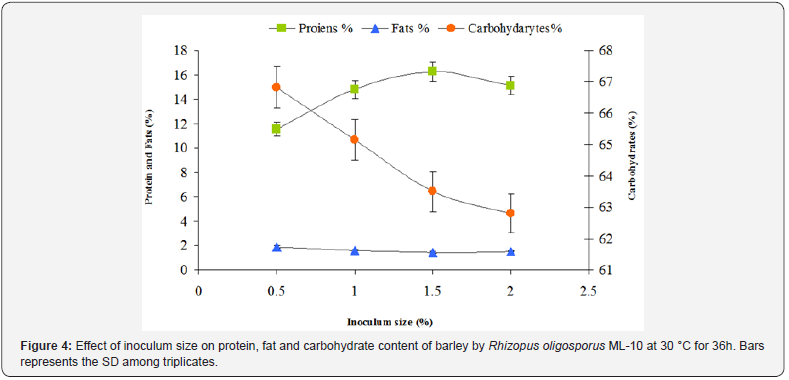
Homogeneous seedling of substrate with adequate spore inoculation along with rapid starting of growth is important to achieve successful fermentation of the cereals for improved nutritional quality. Rhizopus oligosporus is the most preferred fungus in tempeh fermentation due to rapid growth rate at high temperature, high proteolytic and lypolytic activities and strong antioxidant properties [20]. Therefore, various inoculum sizes of 96hrs aged Rhizopus oligosporus ML-10 containing 106 spores/ml was used for nutritional enhancement of barley by fermentation. In Figure 4, the data shows that with low inoculum size 0.5 % (v/w) the fungus grew slowly and barley dense cake formation did not form as also indicated from low protein contents 9.01 %. This slow growth may increase the risk of contamination in barley cereals with microbes. The optimum inoculum size of 1.5 % (v/w) containing 1.0 x106 spores of Rhizopus oligosporus ML-10 per ml was found to enhance protein contents up to 10.55 % in fermented barley. At high inoculum size (2 %), the fungus grew rapidly and the dense cake formation was uneven resulting in slight decrease in protein level, whereas the carbohydrates level significantly decreased down to 62.5 %.
These findings coincide with the earlier investigations regarding tempeh production by using different substrates [14, 21,22]. Some workers reported that 3.35x104cfu/gm is the optimum level of inoculum for quinoa tempeh [23]. Similar findings have also been reported by Xin-Mei Feng [10]. According to them Rhizopus oligosporus was inoculated at approximately 104 spores/g moist substrate. The data in Figure 4 shows that when Rhizopus oligosporus was inoculated at approximately 1.5x104spores/g moist barley, the time for obtaining dense mycelial growth was shortened to 36 h. However, the growth was uneven, probably due to oxygen limitation in the center. Similar results have also been reported for tempeh fermentation of soy bean by Nout [24]. Earlier investigation showed the widening effect of oxygen on hyphae grown in a gradient of limiting oxygen [25].
Effect of incubation temperature
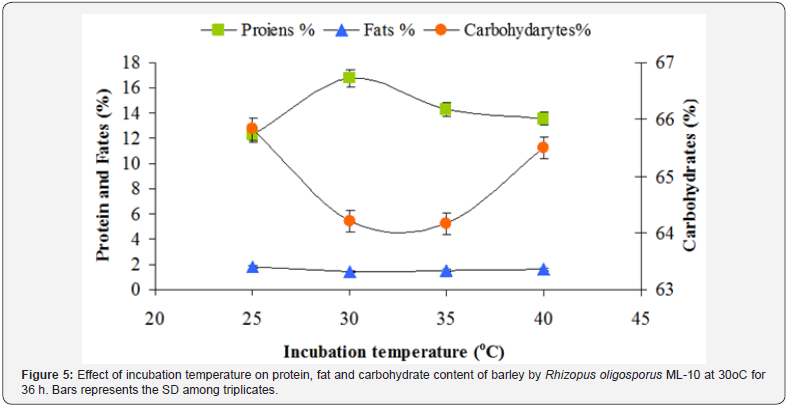
The incubation temperature was found to be considerable importance for the production of nutritionally rich fermented barley. The best mycelia growth was reported at 30 °C as shown in Figure 5 in which maximum protein contents i.e. 16.75 % were found. These results are in agreement with the work reported by Steinkraus et al. [26]. The workers observed good mycelial growth at 30 °C. Reu et al. [27] also supported that inoculation of beans with Rhizopus oligosporus at various temperatures followed by incubation at 30 °C resulted in both increased and decreased periods for the lag phase of fungal growth. According to them maximum difference of 3 h lag phase was found between initial bean temperatures of 25 and 37 °C. However, in another investigation, the optimum growth of Rhizopus oligosporus was reported as 35 °C [11]. The data in Figure 5 also revealed that with the increase in temperature the fat and carbohydrates contents did not show significant variation.
Effect of incubation time
The results shown in Figure 6 demonstrate that the 36h of incubation time is optimum for the fungal mycelia growth because after this period , the sporulation would be started which had adverse effect on the production of a fermented cereal product in respect of protein contents, and in particular fermented whole grain barley based product of tempe type. It was found that during this time period the fungus growth was very good, and the fungus mycelia evenly distributed in the entire grains. As the time of the incubation was increased the sporulation would be started.
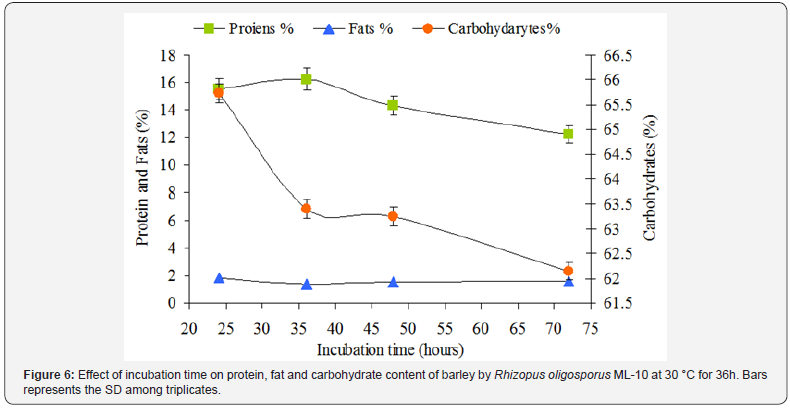
The fermented barley in solid state fermentation with 1.5 % (v/w) spore suspension of 96h aged R. oligosporus ML-10 in 25 x 25cm2 polythene bags at 30 °C for 36h was analyzed for proximate composition to evaluate the level of protein, fat and carbohydrates as compared with unfermented barley.
The results in Table 1 revealed that protein contents increased significantly whereas the carbohydrates and fats were found to decrease after fermentation with Rhizopus oligosporus ML-10 in pretreated barley. It was reported by other workers that the analysis of fermented cereals showed significant decreased in ash contents after fermentation [28]. In fermented soybean, a slight increase was found in crude protein and crude fiber content of Tempe as compared with unfermented soybeans [29]. It was also observed that the growth of fungus reduced the concentration of low molecular carbohydrates and increased the dietary fiber content in fermentation [14]. A decrease in carbohydrate level after fermentation might be due to the partial removal of non starch constituents during solids state fermentation process [30]. It has been reported that during the fermentation process of cereals, proteases, lipases, phytases and a variety of carbohydrases are produced resulting in the degradation of macromolecules into lower molecular weight products thereby improving the nutritional quality of fermented product [24].
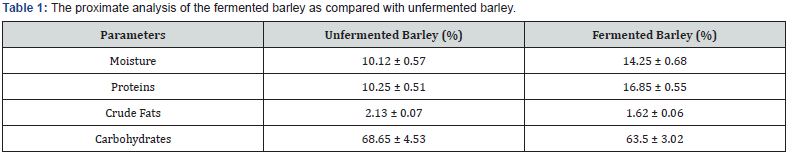
Nutritionally rich fermented barley has earlier been reported by Hesseltine et al. [31]. A patented barley tempe product has been described by fermenting pearled barley kernel with selected strains of Rhizopus sp [11]. The barley fermentation process on a new barley genotype has been reported by Eklund- Jhonson et al. [32]. Variations in total fat contents of fermented and unfermented samples were observed under the influence of different parameters as presented in (Figure 2-6). According to Khaterpaul [33] that natural fermentation increases whereas the pure culture fermentation decreased the fat contents in cereals. Fatty acids present in glycerides have been reported to decrease during fermentation of soy bean from 30% natural lipid by the action of lipases activity [24]. The protein contents increased initially as a result of fermentation carried out from 6 to 12h. This enhancement in protein level can be attributed to microbial synthesis from metabolic intermediate during the growth [34]. Similar findings were reported regarding the level of protein in the pearl millet after fermentation [35]. It has also been reported that the water soluble protein increased from two to three times after soy fermentation and four to six times after miso fermentation [36,37]. However, in another investigation, it was found that the fermentation process either decreased or did not change the protein contents of pearl millet flour [33].
From the results of present study it was concluded that the solid state fermentation of whole grain barley resulted in increasing the protein contents up to 64.3 % under optimized conditions by Rhizopus oligosporus ML-10 in polythene bags.
References
- Danish BP, Bhakyatraj R, Vidhyalakshmi R (2009) A low cost nutritious food tempeh - A Review. World Journal of Dairy & Food Science 4(1): 22-27.
- Farsworth RE (2008) Handbook of Fermented Functioan Foods, CRC Press, USA.
- Mohammad MA, EI Abu, Gasim A, Yagoub A (2007) Furundu, from Fermented sprouted roselle (Hibiscus sabdariffa L) seed: investigation on chemical composition, antinutritional factors, HCl-extractability of minerals, amino acids composition in vitro protein digestibility and microbial growth research. Journal of Agricultural and Biological Science 3(6): 876-885.
- Kuswanto RK (2004) Industrialization of Tempe fermentation. In: Steinkraus KH, Industrialization of indigenous fermented foods, (2nd edn.), Marcel Dekker, Inc., New York, USA, pp. 587-635.
- Din A, Anjum FM, Zahoor T, Nawaz H (2009) Extraction and Utilization of Barley β-glucan for the Preparation of Functional Beverage. International Journal of Agriculture & Biology 11(6): 737-740.
- Granfeldt Y, Liljeberg H, Drews A, Newman R, Bjöck I (1994) Glucose and insulin responses to barley products influence of food structure and amylase amyl pectin ratio. American Journal of Clinical Nutrition 59(5): 1075-1082.
- McKeown NM (2004) Whole grain intake and insulin sensitivity: evidence from observational studies. Nutr Rev 62(7 pt 1): 286-291.
- Behall KM, Scholfield DJ, Hallfrisch J (2004) Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. Am J Clin Nutr 80(50): 1185-1193.
- Behall KM, Scholfield DJ, Hallfrisch J (2004) Lipids significantly reduced by diets containing barley in moderately hypercholesterolemia men. Am J Clin Nutr 23(1): 55-62.
- Feng X (2006) Microbial dynamics during barley tempeh fermentation. PhD Thesis Swedish University of Agricultural Sciences, Sweden.
- Berg S, Olsson J, Swanberg M, Schnürer J, Eriksson A (2001) Method for the production of fermented cereal food products and products thereof, International patent application number: PCT/SE02/00357.
- Steinkraus KH, Cullen RE, Pederson CS, Nellis LF, Gavitt BK (1983) Indonesian tempeh and related fermentations. In: Handbook of indigenous fermented foods. Steinkraus KH, et al. (Eds.), New York, USA, pp. 1-94.
- AOAC (1990) Official Methods of Analysis, (15th edn); Association of Official Analytical Chemists: Washington DC, USA.
- Nout MJR, Rombouts FM (1990) A review recent development in tempeh research. Journal of Applied Bacteriology 69: 609-633.
- Daniel YCF, Crozier-Dodson BA (2008) A Mold-modified indigenous fermented food. In: Handbook of fermented food. Farnworth ER, (Eds.), Functional food and nutraceuticals series, Taylor and Francis group, USA.
- Winnaro FG, Reddy NR (1986) Tempe legume-Based Fermented Food. Reddy NR, et al. (Eds.), CRC Press, Boca Raton, USA, pp. 95-117.
- Obizoba IC (1992) Evaluation of the effect of processing techniques on the nutrient and antinutrient contents of pearl millet (Pennisetum glaucum) seeds. Plant Foods Hum Nutr 45(1): 23-34.
- Yagoub AA, Abdalla AA (2007) Effect of domestic processing methods on chemical, in vitro digestibility of protein and starch and functional properties of bambara groundnut (Voandzeia subterranea) seed. Research Journal of Agriculture and Biological Science 3(1): 24-34.
- Mulyowidarso RK, Fleet GH, Buckle KA (1989) The microbial ecology of soyabean soaking for Tempe production. Int J Food Microbiol 8(1): 375-377.
- Sharma R, Sarbhoy AK (1984) Tempeh: a fermented food from soyabean. Current Science 53(6): 325-326.
- Wang HL, Swain EW, Hesseltine CW (1975) Mass production of Rhizopous oligosporous spores and their application in tempeh fermentation. Journal of Food Science 40(1): 168-170.
- Ko SD, Hesseltine CW (1979) Tempeh and related foods. In: Economic Microbiology, In: Rose AH (Ed.), Academic Press, London, UK, pp. 115- 140.
- Penaloza W, Christopher LD, John NH, Kell DB (1992) Physiological studies on the solid state quinoa tempe fermentaion, using on - line measurements of fungal biomass production. Journal of Science of Food and Agriculture 59: 27-235.
- Nout MJR, JL Kiers (2005) Tempe fermentation, innovation and functionality: update into the third millinium. Journal of Applied Microbiology 98(4): 789-805.
- Gow NAR (1989) Relationship between growth and the electrical current of fungal hyphae. Biol Bull 176(2S): 31-35.
- Steinkraus KH, Hwa van YB, Providenti MIB, Hand DB (1960) Studies on tempe- an Indonesian fermented soyabean food. Food Research 25(6): 777-788.
- De Reu JC, Rombouts FM, Nout MJR (1995) Influence of acidity and Initial substrate temperature on germination of Rhizopous oligosporoous sporangiospores during tempe manufactuire. Journal of Applied Biotechnology 78(2): 200-208.
- Parveen S, Hafiz F (2003) Fermented cereals from indigenous raw materials. Pakistan Journal of Nutrition 2(5): 289-291.
- Murata K, Ikehata H, Miyamoto T (1967) Studies on the nutritional value of tempe. Journal of Food Science 32(5): 580-586.
- Bejoran PA, Nadia M, Montoya V, Rodriquez EC, Carrillo JM, et al. (2008) Tempeh flour from chick pea, nutritional and physiochemical Guachtenoc Reyes-Moreno. Food Chemistry 106: 106-112.
- Hesseltine CW, Smith M, Wang HL (1967) New fermented cereal products. Developments Industrial Microbiology 8: 179-186.
- Eklund-Johnson C, Sandberg AS, Alminger M (2006) Reduction of phytate content while preserving mineral content during whole grain tempe fermentation. Journal of Cereal Science 44(2): 154-160.
- Khaterpaul N, Chauhan BM (1991) Effect of natural fermentation on phytate and polyphenolic content and in vitro digestibility of starch and protein of pearl millet (Pennisteum typhoideum). Journal of Science of Food and Agriculture 55(2): 189-195.
- Zamora RG, Venum TL (1979) The nutritive value of dehulled soyabean fermented with Aspergillus oryzea or Rhizopous oligosporous as evasluated by rates. J Nutr 109(7): 1333-1339.
- Elyas SHA, Abdullahi HTE, Yousif NE, Elsheikh EAE (2002) Effect of natural fermentation on nutritive value and in vitro protein digestibility of pearl millet. Food Chemistry 78(1): 75-79.
- Robinson RJ, Kao C (1977) Tempe and miso from chickpea, horse bean, and soyabean. Cereal Chem 54: 1192-1197.
- Moore JM, Cheng Z, Hao J, Guo G, Jiu JG, et al. (2007) Effect of solid state yeast treatment on the antioxidant properties protein and fiber compostion of common hard wheat bran. J Agric Food Chem 55(25): 10173-10182.






























