Comparative Study on Macro and Micro Nutrient Profiling of Selected Freshwater, Brackish Water and Marine Water Food Fishes Available in Kerala, India
Dilip Kumar Singh1 and Amit Ranjan2*
1Department of Fish Nutrition and Feed Technology,Central Institute of Fisheries Education, Kolkata Centre, India
2Department of Fish Nutrition and Feed Technology ,Central Institute of Fisheries Education,Mumbai, India
Submission: October 10, 2016; Published: November 16, 2016
*Corresponding author: Amit Ranjan, Department of Fish Nutrition and Feed Technology, Mumbai, India, Email: amitranjanfcri@gmail.com
How to cite this article: Dilip Kumar S, A Ranjan. Comparative Study on Macro and Micro Nutrient Profiling of Selected Freshwater, Brackish Water and Marine Water Food Fishes Available in Kerala, India. Nutri Food Sci Int J. 2016; 1(4): 555569. DOI: 10.19080/NFSIJ.2016.01.555569
Abstract
An experiment was conducted to study the macro and micro- nutrient profiling of selected freshwater, brackish water and marine water food fishes available in India. The raw fishes have been collected from the Polakandam Fish Market, Kochi, and Kerala during the early morning hours of the day and carried in fresh condition to the laboratory. The biochemical composition viz., Moisture (%), Crude Protein (%), Fat (%), Ash (%), Fatty acid Profile, Amino acid Profile, Mineral Profile of selected fish species Etroplussuratensis (Cichlidae), Scatophagusargus (Scatophagidae), Channamarulius (Channidae), Scomberomorusguttatus (Scombridae), Channapunctatus (Channidae), Horabagrusbrachysoma (Bagridae) were analysed. This study suggested that fishes are good source of protein, essential amino acids, essential fatty acids and minerals which can be directly used in human diet to cope up with the protein malnutrition in developing countries.
Introduction
The global consumption of fish and fisheries products has greatly increased during recent decades. Fish demand is also increasing as a result of the increasing world population, higher living standards and the good overall image of fish among consumers [1]. Fish is a key ingredient on the global menu, vital factor in the global environmental balance, and an important basis for livelihood worldwide (UNICEF, 2006). Fish has no cultural or religious restrictions which make it more advantageous than pork, beef and mutton [2]. Fish is widely acceptable in global menu due to its palatability, low cholesterol level and tenderness of its flesh [3].
Change in consumer trend could be based on a number of distinct factors; foremost among these is the growing knowledge that fish constitute an important and healthy part of the human diet, it provides superior quality protein to that of meat, milk and eggs and well balanced essential amino acid profile, necessary minerals and fatty acids. In addition to the fact that, fish flesh is tasty and highly digestible; it also minimizes the risk of heart diseases and increases life expectancy. About 85 to 90% fish protein is digestible and all the dietary essential amino acid is in fish flesh [4]. The crude protein content of fish can be of immense nutritional value to pregnant women for proper development of the foetus and prevention of abortion.
It will also enhance the proper mental and immunity development against disease among growing children [5]. Fish meat contains significantly low lipids and high water compared to that of beef or chicken and is favoured over other white or red meats [6]. Lipids from fish are well known as a rich source of long-chain n- polyunsaturated fatty acids (LC n-3 PUFA) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which cannot be synthesized by humans and commonly obtained from the diet [7]. Polyunsaturated fatty acids from fish have been reported to have preventive and/or curative effects for several diseases including arterial hypertension, cancers and inflammatory diseases (Turkmen et al.,2005). It may also aid in lowering the risk of Dementia, Alzheimer’s diseases [8], and prevent the cardiovascular diseases [1].
Fish is a good dietary source of vitamin B complex and vitamins A and D; fatty fish have higher level of vitamins than the lean fish. Vitamin content may be considerably influenced by methods of handling, storage and preparation of fish food. Fish food includes the following important macro minerals: Ca, P, Mg and the electrolytes (Na and K). Trace minerals present in fish are Mn, Fe, Co, Cu, Zn, Ni, Mo and Cr (essential), Al, Ti, V and Ag (nonessential) and Pb and Cd (toxic). Fish are good sources of I, Ca and P which ranged from 70 to 80% in the skeleton of fishes. The knowledge on biochemical composition of any edible organisms is extremely important since the nutritive value is reflected in its biochemical contents. A new species should be recommended for human consumption only after assessing the nutritive value of the species with regards to its nutritional qualities.
The demand for protein rich food is increasing, especially in developing countries, stimulating the exploration of unexploited or non-traditional resources. Hence, the present work was planned to study the biochemical composition of Etroplussuratensis (Cichlidae), Scatophagusargus (Scatophagidae), Channamarulius (Channidae), Scomberomorusguttatus (Scombridae), Channapunctatus (Channidae), Horabagrusbrachysoma (Bagridae) through estimating their major biochemical components such as total protein and lipid content in the whole body tissue apart from the amino acids, fatty acids profile, vitamins and minerals content (Figure 1A-1F).
Materials and Methods
The whole experiment was conducted at the ICAR-Central Institute of Fisheries Technology (CIFT), Willingdon Island, Matsyapuri PO, Cochin-682 029, Kerala (India). The raw fishes have been collected from the local markets (Polakandam Fish Market, Kochi, and Kerala) during the early morning hours of the day and carried in fresh condition to the laboratory. All the fishes were evaluated through organoleptic method. The collected samples were washed thoroughly with clean water and weight was then taken using electric balance. Boneless muscles, used for analysis, was collected from the dorsal, caudal and ventral region of the fish and then mashed. Mashed muscle sample of each group was mixed and stored in a plastic bag, and then kept in refrigerator at -20 °C till further use.
Chemicals
All glassware, chemicals and reagents used in this study were procured from Qualigens, HiMedia & Sigma Aldrich.
Proximate composition of the collected samples
Determination of Moisture, Ash, Crude Protein and Crude Lipid of the collected sample was done as per the [9] Method.
Determination of Total Lipid
Total lipid was extracted according to the method of [10] using chloroform/methanol (2:1). Aliquot of the chloroform layer extract was evaporated to dryness under nitrogen and the lipids were quantified gravimetrically.
Procedure
A weighed amount of the tissue was subjected to lipid extraction using chloroform-methanol mixture (2:1). The extraction was repeated twice with fresh aliquot of chloroformmethanol mixture. The lipid extracts were transferred to a separating funnel and added 20% of water into it and left overnight. Next day the lipid extracts were drained through filter paper containing anhydrous sodium sulphate and was collected in round bottom flask and was evaporated to dryness in a flash evaporator. The lipid in the round bottom flask was made up to 10ml by using chloroform. From this 1.0ml was taken into a pre-weighed vial and allowed to dry in warm temperature to constant weight and total lipid content were calculated from the difference in weight. Sample made up to 10ml was used for the estimation of various lipid components.
Analysis of fatty acid composition (FAME)
Fatty acids methyl esters (FAMEs) were analysed by the method of Sankar et al. [11]. A fraction of the lipid extract was saponified with 0.5N NaOH in methanol followed by methylation in 14% boron trifluoride in methanol (BF3/MeOH).
Analysis of total fatty acid profile using GC
The fatty acids extracted by folch method were derivatized using BF3 methanol and the methyl esters of the fatty acids (FAME) thus obtained were separated and analysed in GC. Individual fatty acids are expressed by the percentage of total fatty acids.
Amino acids analysis
Total amino acid composition was determined following the method of [12] using a Hitachi amino acid analyzer equipped with an ion exchange column (Shodex CX Pak P-421S), fluorescence detector and post-column derivatization chamber. A binary gradient elution programming was used. Buffer A was prepared by dissolving trisodium citrate (13.31g) in 70mL ethanol, followed by addition of citric acid monohydrate (12.8mL), NaCl (3.74g) and Brij® (4mL). Finally the pH was adjusted to ~3.2 with drop wise addition of perchloric acid and volume was made up to 1L. Buffer B was prepared by dissolving tri-sodium citrate (117.6g) and Boric acid (24.8g) in 500mL distilled water followed by addition of 45mL 4N NaOH. Final volume was made up to 2L with distilled water (~pH10).
Elution programme (flow rate 0.4mL/min) started with 100% Buffer A and was kept constant for 12min, followed by stepwise gradient to 100% Buffer B at 35th min. Buffer B was kept constant up to 55th minute. Next 1min, again 100% buffer A concentration was achieved. Total run time was 70min. The oven temperature was set at 60 °C. Samples (100mg each) were hydrolyzed in 6N Hcl in vacuum sealed tubes at 110 °C for 24h. Following a post-column derivatization with O-phthalaldehyde in the presence of sodium hypochlorite solution, amino acids were detected in fluorescence detector (Excitation 340nm, Emission 450nm). Quantification was done with the help of external standard mixture of amino acids (Sigma). The results were expressed in % of amino acid in terms of total amino acid.
Mineral content
Moisture free fish sample was used for the preparation of the ash solution. Approximately, 1 ± 0.01 g of sample was taken individually in a silica crucible and placed in the Muffle furnace set at 500-550 °C for 4-6 h until the residue is white or nearly white. To the residue, conc. HCl acid was added to dissolve the ash. The ash solution was then filtered through Whatman No. 1 filter paper and rinsed again with hot distilled water and made up to 50ml with distilled water. Macro elements were determined by flame photometry using working standards in the range of 10-40ppm for each element (Na, K and Ca). Samples were aspirated into the flame and the corresponding absorption of the characteristic radiation by each element was recorded. Micro elements were determined by Atomic absorption spectroscopy. Values are expressed in ppm.
Statistical analysis
The data were statistically analysed by statistical package SPSS version 16. Comparison between two treatments was made using Duncan's Multiple Range Test (DMRT). Comparison among all the treatment was done by one way ANOVA. Comparisons were made at the 5% probability levels.
Results and Discussion
Proximate composition (moisture %, cp%, fat %, ash %) of freshwater, brackish water and marine water food fishes
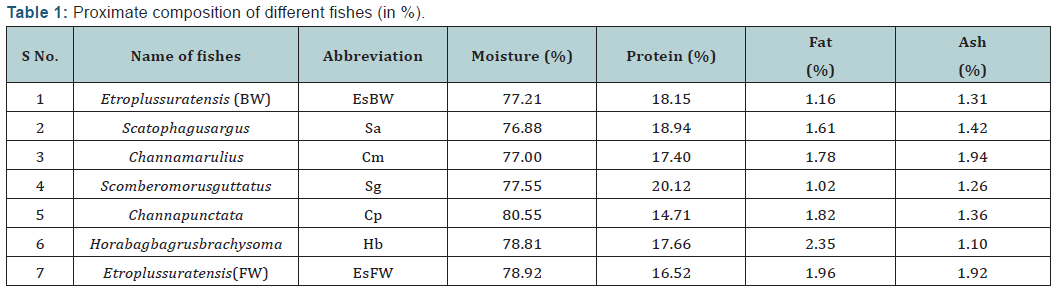
The proximate composition of different fish species are shown below in Table 1. The moisture, crude protein, crude fat and ash content are expressed in % of wet weight basis. The moisture content (%) of different fishes varied from 76.87 to 80.55, crude protein content (%) varied from 14.70 to 20.12, and crude fat content (%) varied from 1.02 to 2.34 and ash content (%) from 1.09 to 1.93. The highest value of crude protein was found in marine fish (Scomberomorusguttatus) and lowest protein content was reported in fresh water fish (Channapunctata). In terms of crude fat, the highest value was recorded in Horabagrusbrachysoma whereas lowest value was in Scomberomorusguttatus. Nutritional point of view, marine fish and brackish water fishes are good source of crude protein compare to fresh water counterparts. Similar results related to proximate composition of fresh fish were observed by [13].
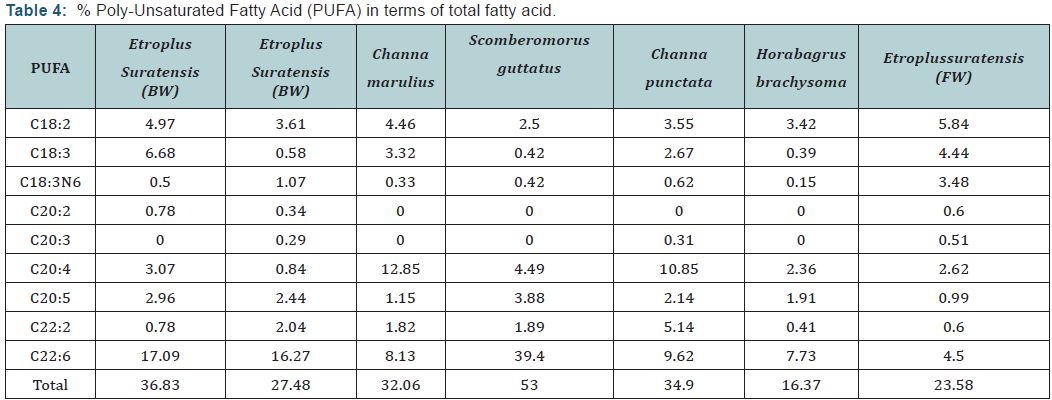
Fatty acid profile of different fishes
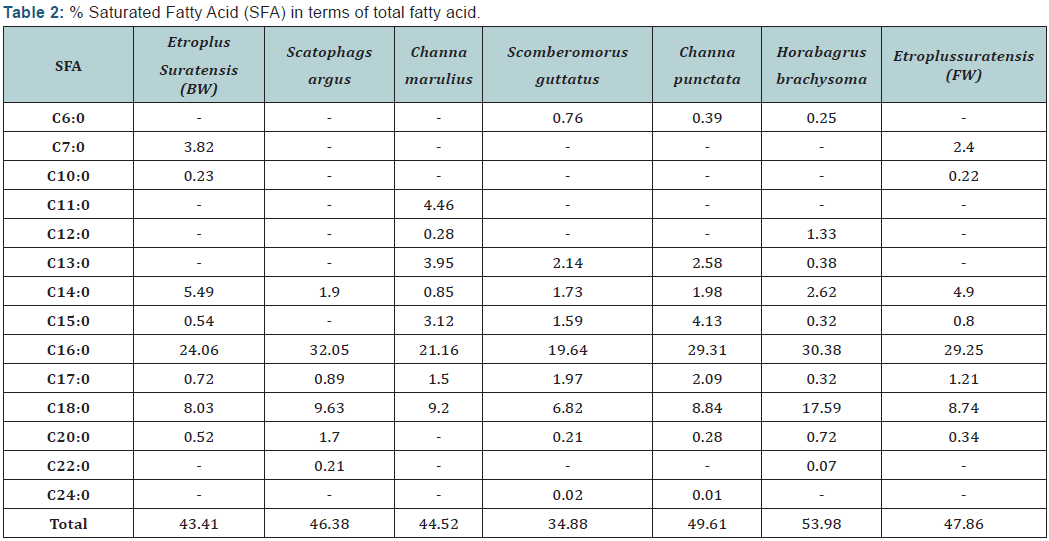
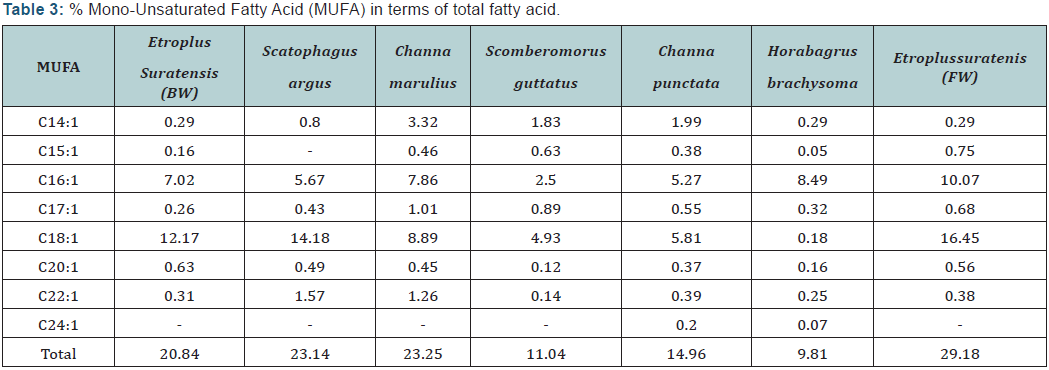
The fatty acid profiles of different species are shown in Table 2. The predominant fatty acid among all fish species was Palmitic acid (C16: 0) which varied from 19.64 (scomberomorusguttatus) to 32.05 (scatophagusargus). EPA and DHA content was found to be higher in marine water and brackish water fish with highest amount was recorded in Scomberomorusguttatus of about 3.88 and 39.4 respectively. The lowest EPA and DHA were encountered in Etroplussuratensis (cultured), of about 0.99 and 4.4 respectively. The PUFA content especially EPA and DHA were predominant in marine fish than fresh water fish counterparts. This may be due to fish residing in marine water have great food chain (marine algae) which are rich in HUFA content.
Results of fatty acid revealed that marine and brackish water fish are rich in n-3 fatty acids whereas fresh water fish are rich in n-6 fatty acids. Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) prevent human coronary artery diseases. Therefore, fish have been suggested as a key component in the healthy diet of humans. Saturated fatty acids (SFA) were found to be higher in fresh water fish than marine and brackish water fishes with highest value was in Horabagrusbrachysoma (53.98). The higher values of PUFA were recorded in marine fish with highest amount in Scomberomorusguttatus (53%). All results are supported with the earlier results.
Total amino acid profile
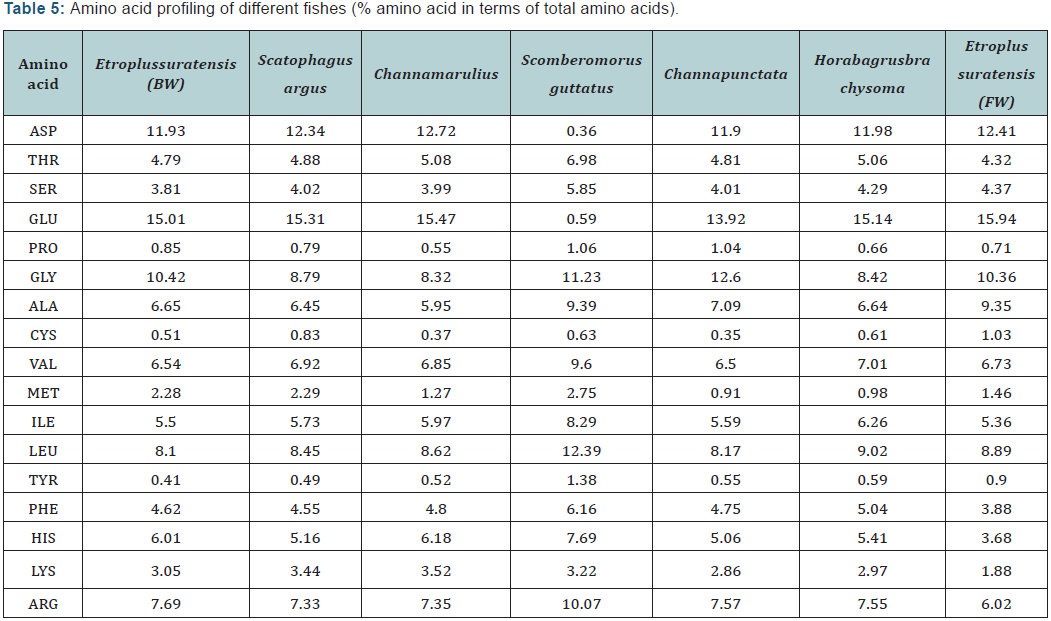
The sequence of amino acids in a protein or peptide determines the properties of the molecule. Amino acid present in muscle tissue was detected using HPLC. The chromatogram of sample is compared with the retention time of standard. The HPLC method for the detection of amino acids has been shown to be capable of measuring various types of biological molecules in a sensitive, accurate, and comprehensive fashion. Muscle tissue of fish is an important source of protein for humans. The amino acid composition is one of the most important nutritional qualities of protein and the amino acid score [14] and is used to evaluate protein quality world-wide [15]. Comparative amino acid profile (% amino acid in terms of total amino acid) of the muscle tissue of different fish is represented in (Table 3).
The muscle tissue of all fishes contains high concentration of glutamic acid ranging from 13.92 to 15.94, followed by Aspartic acid (11.9 to 12.4) and glycine (8.32-12.6). According to Zhao et al, amino acid composition of pomfret muscle showed a higher amount of glutamic acid followed by lysine, leucine, aspartic acid, arginine, valine and alanine in that order. Glutamine is the most abundant free amino acid in the body, comprising nearly 60% of the free intra-cellular amino acids in skeletal muscle. The functional role of the protein from a particular source is determined by the content and arrangement of amino acids in the protein molecule. An optimal balance among amino acids in the diet and circulation is crucial for whole body homeostasis. Amino acids such as arginine, glutamine, glutamic acid, aspartic acid, proline, methionine, serine and tryptophan involved in metabolic pathways that are necessary for maintenance, growth, reproduction and immunity [16].
Glutamine is one of the most abundant free α-AA in fish plasma and muscle, whereas glutamate and its decarboxylation product (GABA) are neurotransmitters present at high concentrations in the brain. Moreover, glutamine is essential for the synthesis of purine and pyrimidine nucleotides in all cells. Through renal-ammoniagenesis, glutamine also plays an important role in regulating the acid-base balance in the body. As a major energy substrate for leukocytes and a key modulator of cytokine and NO production, glutamine is crucial to the immune response in fish [17,18-23]. Aspartate is the major glucogenicprecursorsand important energy substrates for fish. In addition, aspartateis essential to purine nucleotide synthesis in all cell types. All fish muscle tissue also contain noteworthy amount of histidine (5.06-6.01).
Histidine is an abundant AA in plasma albumin of fish. It is also rich in fish muscle as a free AA or carnosine. Histidine participates in one-carbon unit metabolism, therefore affecting DNA and protein synthesis. In addition to serving as an energy fuel during starvation, histidine is a major component of noncarbonated buffers protecting fish against changes in pH resulting from hypoxia, burst-swimming, and increased lactacidosis. The capacity of the non-carbonate buffer system among various fish species varies considerably, which is likely attributable to long-term adaptation to environments.
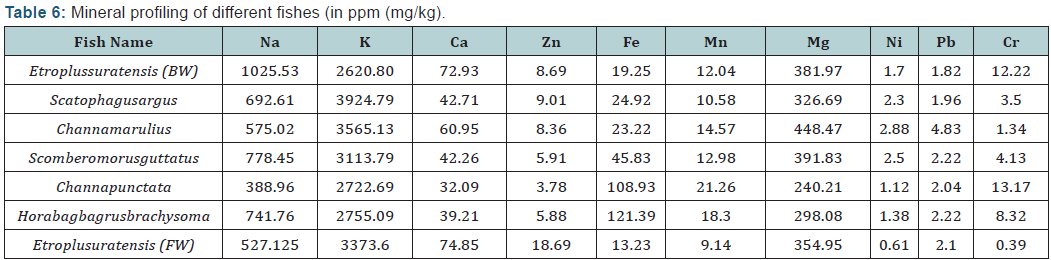
The mineral composition and heavy metal level of different fish species are given in Table 4. The major minerals such as sodium (Na), Potassium (K), magnesium and calcium were analyzed. Potassium was the predominant major mineral found among all fish species, ranged from 2620.80 (Etroplussuratensis) to 3924.79 (Scatophagusargus). Sodium values varied from 388.96 (Channapunctata) to 1025.53 (Etroplussuratensis from brackish water). Potassium is required for the normal functioning of the nerves and muscle, the sugar metabolism, acid-base balance and oxygen metabolism in the brain. The heart also needs potassium. Sodium regulates the electrolyte and acidalkali balances, the conductive capacity of the nerves and muscle contractions. Magnesium is an essential mineral for cell function and it occupies a key role in all reactions with phosphate.
The cells also require Mg for cell division and enzyme production. Calcium is major constituent of the inorganic portion of the diet and function primarily as the structural component of hard tissues viz. bone, exoskeleton, scales and teeth. In addition to its structural function Calcium is essential for blood clotting, muscle function, proper nerve impulse transmission, osmo regulation and as a cofactor for enzymatic process. Iron (Fe) were present in considerable amount in all fishes with higher amount in Horabagbagrusbrachysoma 121.39) and lower in Etroplussuratensis (FW) of about 13.23. Fish is a major source of Fe for adults and children, and is a vital mineral for better health, especially for women. Notably the highest chromium (12.22) amount was found in Etroplussuratensis (BW) whereas least amount was recorded in Etroplussuratensis (FW).
Summary
The demand for marine, brackish and fresh water fishes can still be improved on, if information dissemination on the valuable nutritional composition of fishes reaches the grass route. Besides this, there is the need to raise consumer awareness to ensure that contaminated fishes are avoided. This study suggested that fishes are good source of protein and can be used as a dietary component in developing countries as well as alleviate protein malnutrition. This study revealed the importance of marine and brackish water rather than fresh water fishes as good sources of PUFA (EPA & DHA). Hence, marine fish could be a dietary ingredient to alleviate cardiovascular disease, brain development in infants etc. On the other hand, the information will be useful to the consumers in choosing fish based on their nutritional values rather than taste, appearance and other physical features. Overall, present study revealed that fish is a prime ingredient in human diet to balance all essential and nonessential amino acids as well as minerals which are important for several physiological processes.
References
- Cahu C, Salen E, Lorgeril MD (2004) Farmed and wild fish in the prevention of cardiovascular diseases: Assessing possible differences in lipid nutritional values. Nutr Metab Cardiovasc Dis 14(1): 34-41.
- NIFFR (1999) National Institute for Freshwater Fisheries Research, Newsletter, 16(3).
- Eyo AA (2001) Fish Processing: Technology in the tropics. National Institute for Freshwater Fisheries Research, University of Ilorin Press, Nigeria, pp. 403.
- Cappell R, Wright S, Nimmo F (2007) Sustainable production and consumption of fish and shellfish. Environmental impact analysis. Final report of Defra LCA project. Haskoning UK Ltd, United Kingdom.
- NAFDAC (2003) National Agency for Food and Drug Administration and Control, Consumer Safety Bulletin 2(2): 1394-1596.
- Nestel PJN (2000) Fish oil and cardiovascular disease: lipids and arterial function. Am J Clin Nutr 71(1): 228-231.
- Alasalvar C, Taylor KD, Zubcov E, Shahidi F, Alexis M (2002) Differentiation of cultured and wild sea bass (Dicentrarchuslabrax): total lipid content, fatty acid and trace mineral composition. Food Chemistry 79(2): 145-150.
- Grant WB (1997) Dietary links to Alzheimer’s disease. Alzheimer’s Disease Review 2: 42-55.
- AOAC (2000) Official methods of analysis (17th edn), Gaithersburg, MD: Association of Official Analytical Chemist.
- Folch J, Lees M, Sloane Stanley GH (1957) A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J Biol Chem 226(1): 497-509.
- Sankar TV, Suseela Mathew, Anandan R, Asha KK, Mohanty BP (2010) Central Institute of Fisheries Technology handbook on Nutrient Profiling of Fish, p. 50-51.
- Ishida Y, Fugita T, Asai K (1981) New detection and separation method for amino acid by high performance liquid chromatography. J Chromatogr 204: 143-148.
- Paul AK (1994) Bangladesher Raptani jogga Matso Sampod (Exportable Fish and Fisheries Products of Bangladesh), Tuli-Mili Prokashani, Khulna, pp. 103-105.
- FAO/WHO (1990) Report of the joint FAO/WHO Expert Consultation on Protein Quality Evaluation. Bethesda, MD, USA.
- Iqbal A, Khalil IA, Atgeeq N, Khan MS (2006) Nutritional quality of important food legumes. Food Chemistry 97(2): 331-335.
- Guoyao Wu (2009) Amino acids. Springer 37: 1-7.
- Buentello JA, Gatlin DM (1999) Nitric oxide production in activated macrophages from channel catfish (Ictalurus punctatus): influence of dietary arginine and culture media. Aquaculture 179(1/4): 513-521.
- Li P, Yin Y, Li D, Kim WK, Wu G (2007) Amino acids and immune function. Br J Nutr 98(2): 237-252.
- Gurr MI (1992) Role of fats in food and nutrition (2nd edn), London, UK: Elsevier Applied Science.
- Haruna AB (2003) Aquaculture in the tropics Theory and Practice. Al- Hassan Kano pp. 432.
- Pigott GM, Tucker BW (1990) Effects of technology on nutrition. Marcel Dekker, New York.
- Pigott GM, Tucker BW (1990) Seafood: Effects of Technology on Nutrition. (1st edn), CRC Press LLC, Boca Raton, Florida, p. 45-46.
- Saito M, Kunisaki N (1998) Proximate composition, fatty acid composition, free amino acid contents, mineral contents, and hardness of muscle from wild and cultured puffer fish Takifugurubripes. Nippon Suisan Gakkaishi, 64(1): 116-120.







