Functional Properties of Crotalaria Pallida Seed Protein Isolate
Sushobhan Ukil, Suman Ray, Alak Kumar Ghosh and Subrata Laskar*
Department of Chemistry, University of Burdwan, India
Submission: April 21, 2016; Published: August 10, 2016
*Corresponding author: Subrata Laskar, Department of Chemistry, University of Burdwan, West Bengal, India.
How to cite this article: Sushobhan U, Sumon R, Alak Kumar G, Subrata L. Functional Properties of Crotalaria Pallida Seed Protein Isolate. Nutri Food 005 Sci Int J. 2016; 1(3): 555561. DOI: 10.19080/NFSIJ.2016.01.555561
Abstract
Solubility and functional properties of Crotalaria pallida seed protein isolate have been evaluated. The effects of pH on some of these properties were also investigated. Solubility of this protein was minimum at pH 5 (which is supposed to be close to its iso electric point) and maximum at pH 8. The protein content of the total seed protein isolate was 62.43%. Oil holding capacity and water holding capacity were estimated to be 4.62 g/g and 3.21 g/g respectively. The emulsifying activity and emulsion stability as well as foaming capacity and foam stability were largely affected by pH levels. Viscosity of the seed protein isolate was also found to be pH and concentration dependent. Gelation properties of the protein isolate have been investigated and least gelation concentration was found to be 10% in distilled water and 8% in 0.5 (M) NaCl solutions respectively.
Keywords:Crotalaria pallida; Seed Protein Isolate; Nitrogen Solubility; Functional Properties
Introduction
Scientists and researchers have constantly been trying to find alternative protein foods to combat malnutrition and diseases due to protein inadequacy in exponentially increasing population. Shortage of protein foods is a serious threat to mankind especially in under-developed and developing nations. Hence it has become utmost important to find non-conventional seeds as new protein source and nutritional supplements. As a consequence, during the last few decades the food industries have paid special attention on utilizing seed protein from both conventional and non-conventional sources [1]. For plant proteins to be acceptable and successful in food application, it must behave ideally in some physico-chemical properties, referred to as ‘functional properties’, such as solubility, viscosity, emulsifying property, foaming property, gelation capacity etc. [2].
Our intention was to find new proteins for human and animal consumption from non-conventional seeds. One of such non-traditional seeds is Crotalaria pallida, (also known as ‘smooth rattle pod’) which belongs to the family ‘Fabaceae’. This plant is widely distributed in the tropical and subtropical region across the world, though, very little effort has been made to isolate and characterize protein from the seeds of this plant. This plant is a good cover crop in tea, coconut and rubber plantations [3].
Flowers of this plant are used as vegetables. Seeds of this plant are roasted and grounded to prepare a sort of coffee beverage. The seeds of this plant are rich in nitrogen and protein content. The main objective of our group is to examine various functional properties of its seed protein isolate as a key step to check its usability as a non-traditional protein source. This present study also deals with the first time report of functional properties of Crotalaria pallida seed protein.
Materials and Methods
Materials:
Fresh and mature fruits of C. pallida were collected from the Golapbag campus of the University of Burdwan and identified by A. Mukherjee, Department of Botany, The University of Burdwan, Burdwan, West Bengal, India. Voucher specimen (labeled as ‘BURD Sushobhan 203’) has been preserved at the herbarium of Botany Department. Collected seeds were air-dried and then finely powdered and kept in a refrigerator at 40C for future uses. All the chemicals used in our present study were of analytical standard. Corn oil was obtained from Sigma-Aldrich, USA.
Methods:
Preparation of Total Protein Isolates (TPI):Powdered seed material was defatted using pet ether (600C-800C) in a Sox let apparatus for 72 hours, followed by washing with the help of Chloroform: Methanol (1:1) solvent mixture. As the nitrogen solubility is maximum at pH 8.0, powdered seed was extracted using distilled water at the same pH. The defatted flour was stirred with distilled water (10 volumes than the weight of seed flour taken) for 30 minutes at constant pH (pH 8.0) and filtered through Whatman-41 filter paper and finally centrifuged at 5000g for 20 minutes. The supernatant was collected and precipitate was obtained from this solution by lowering the pH to a 4.0 using Trichloroacetic acid(TCA). The precipitate thus obtained was recovered by centrifuge at 5000g for 20 min. The precipitate was re-dissolved in deionizer water at pH 7.0 and then freeze-dried. The freeze-dried protein (total protein isolate) was preserved in a refrigerator at 40C until further use.
Proximate analysis::The ash and moisture contents of the Total protein isolate (TPI) were estimated according to the method of AOAC [4]. Micro-Kjeldahl methodology was followed to determine its nitrogen content (AOAC, 1990)[4].The percentage of nitrogen was converted to the percentage of crude protein by multiplying by a factor of 6.25. All the results were placed in (Table 1).
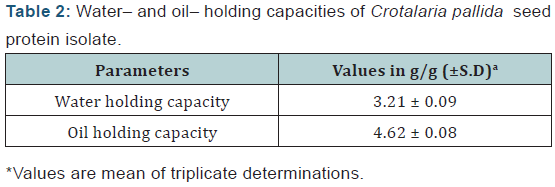
Water-holding capacity (WHC) and Oil-holding capacity (OHC) :Water- and Oil-holding capacity was measured according to the method of Carcea Bencini with slight modification [5]. One gram of protein sample in each case was stirred in 10 ml distilled water at pH 7.0 or 10 ml corn oil in a centrifuge tube. Then the samples were allowed to stand at room temperature for 30 min and centrifuged at 5000g for another 15 min. The volume of the supernatant was measured in a graduated cylinder. The number of grams of water or the number of grams of oil held by 1g of protein represents WHC and OHC respectively. Density of the corn oil was found to be 0.9 g/ml. The triplicate determinations of the capacities (WHC & OHC) were carried out and the results were placed in (Table 2).

Nitrogen solubility:The method of Were et al. was used to evaluate nitrogen solubility of the TPI at different pHs (2- 10) [6] (Figure 1). Sample (125 mg) in each case was dispersed in 25 ml distilled water and the solution was adjusted to 2, 3, 5, 5, 6, 8, 9, 10, 11 and 12 using either 0.5 (M) HCl or 0.5 (M) NaOH. The dispersion was stirred for 30 min with the aid of a magnetic stirrer and then centrifuged at 10,000g for 15 min. The solubility was determined by nitrogen estimation with the aid of Kjeldahl method of AOAC (1990) [4]. Results are represented as mean ± SD, n=3. Solubility profile was obtained plotting protein solubility (%) against pH. The percentage of soluble protein was calculated as follows:

Emulsifying activity (EA) and Emulsion stability (ES):Emulsions were prepared according to the method described by Sathe and co-workers with a little modification [7]. 5 ml of protein suspensions at different pHs (2, 4, 6, 8 and 10) were homogenized at 10,000 g for 1 minute and then with corn oil for another 1 minute. The emulsions obtained were centrifuged at 3000 g for 5 minutes and the volumes of the emulsified layers were noted.
Emulsion activity (EA) can be calculated as follows:

Determination of Emulsion stability involves heating of the above emulsion at 800C for 30 min, followed by cooling at room temperature and measuring the volume of remaining layer after rotating at 3000 g for 5 min in a centrifuge.

Foaming properties:The method described by Sze-Tao and Sathe[8] was used with slight modification for the determination of Foaming capacity and Foam stability. 1.5% aqueous solution (w/v) of TPI was prepared using ultra-sonic vibrator (Model No. Transsonic T660/H, Elma, Germany) and pH of the suspensions was adjusted to 2, 4, 6, 8 and 10 with 0.5 (M) Hcl or 0.5 (M) NaOH solutions. Then it was whipped with an electrical stirrer at medium speed for 5 minutes and the foam volume was recorded after 30 s. Foam stability was determined by measuring the decrease in foam volume as function of time up to a period of 120 minutes.

Viscosity:An appropriate sample was dispersed in distilled with the help of the ultra-sonic vibrator (aforementioned model). Each sample was prepared at concentrations of 1, 1.5, 2, 2.5, 3, 4 and 5% (w/v) and relative viscosity of each sample was measured in an Ostwald type viscometer. In another set of experiment, relative viscosity of 1.5% protein solution was recorded at various pHs [4,6,8,10]. All these experiments are repeated thrice and means are presented in Table 3.
Least Gelation concentration:This was measured with slight modification of the method of Abbey and Ibeh [9], where TPI was suspended in 5 ml distilled water to obtain 4%, 6%, 8%, 10%, 12%, 14%, 16%, 18% and 20% (w/v) concentrations with the use of ultrasonic vibrator(same model used earlier). The test tubes containing the solutions/suspensions were heated in water bath for an hour, followed by rapid cooling in running tapwater. These test tubes were kept in a refrigerator for another 2 hours at 40C. The least gelation capacity was regarded as the concentration at which the sample from the inverted tubes did not fall down or slip.
Results and Discussion
Proximate analysis: The moisture, ash and protein content of Crotalaria pallida seed isolates are given in (Table 1). The protein content of Crotalaria pallida seed protein isolate is sufficiently high, which makes it a good source of vegetable protein.

Water- and Oil-holding capacity:Water- and oil holding capacity of TPI were found to be 3.21 g/g and 4.62 g/g respectively (Table 2). WHC for this protein is higher than Lupin seed protein concentrates (1.37 g/g) and Cashew nut protein concentrates (1.74 g/g) [10-11]. This value resembles with Soybean protein concentrates (3.06 g/g) and three Chinese indigenous legume seeds as reported by Chau et al. [12]. Such high value of WHC makes it a suitable ingredient for meat, bread and cakes industries. Oil holding capacity of Crotalaria pallida seed protein isolates is found to be higher than that of Soybean protein concentrate (1.37 g/g) [12]. High value of OHC for Crotalaria pallida seed protein concentrates is probably attributed to a higher level of non-polar side chains in the molecule. This is in agreement of the report of Campell, Shih and Marshal that the value of OHC increases with increasing protein concentration in sunflower and soya products [13]. Such high OHC makes the protein potentially useful in structural interactions in food, especially in flavor retention, improvement of palatability and extension of shelf life in meat products through lowering of fat and moisture loss. The ability of protein to bridge fat and water molecules simultaneously is an important criterion for coldmeat industry especially for sausages.
Nitrogen solubilityThe nitrogen solubility profile of Crotalaria pallida seed protein isolate indicates a dip at pH 5.0 which is close to its isoelectric point. On either side of pH 5.0, there is a sharp increase in the nitrogen solubility of protein isolate having maximum solubility at pH 8. At pH above 8.0, solubility does not change significantly with increase in pH value. The percentage of soluble protein is 58.12 (± 0.22) at pH 5.0 and 96.28 (± 0.06) at pH 8.0. These are comparable to the solubility of bitter lupin concentrate (98.43%) and sweet lupin concentrate (98.79%) as reported by El-Adawy et al. [14]. Also the data indicates that solubility is slightly greater in alkaline medium than that of acidic medium Crotalaria pallida seed protein exhibits good solubility in both acidic and alkaline pH ranges, an important characteristic for food formulation. Moreover, the study of nitrogen solubility over a wide range of pH values is an important guide to protein functionality, since it is directly related to many important properties such as emulsification, foaming capacity, gelation etc [15].
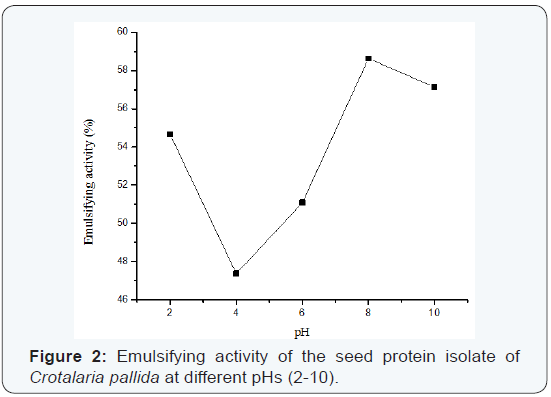
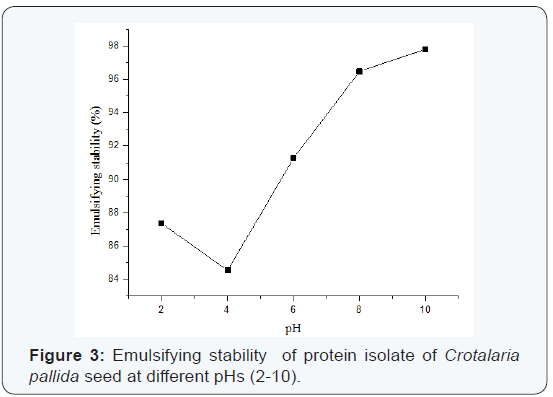
Emulsifying properties:The effects of pH on emulsifying activity (EA) and emulsifying stability (ES) are shown in Figure 2 and Figure 3 respectively. Minimum emulsifying activity is observed at pH 4.0 with coincidental decrease in solubility, and is slightly increased to attain maxima at pH 10.0. Emulsifying stability is also found to be pH dependant, where ES is 87.36% at pH 2.0, 84.54% at pH 4.0 and 97.80% at pH 10.0 resulting in a V-shaped pattern (Figure 3). A good number of studies have shown that the pH-emulsifying properties profile for different proteins including soya protein is quite similar to their corresponding pH-solubility profile [12]. This means that solubility and/or electrostatic charge largely affects emulsifying properties. Most food proteins are generally poor emulsifier near their is electric pH and they cannot move rapidly to the interface due to lack of electrostatic repulsive force at this pH. These proteins may, however, be effective emulsifier when moved away from their isoelectric pH [16-17].
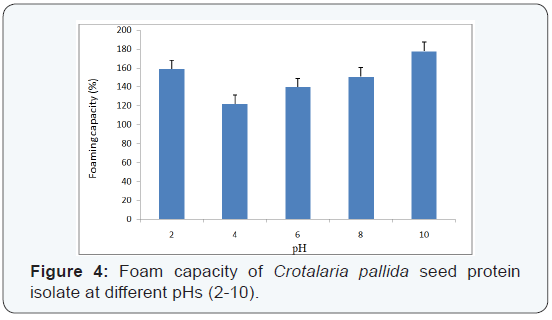
Foaming properties:The results of foaming capacity (FC) indicate that pH of the protein solution is an important factor governing the extent of foam formation as well as its stability in the medium. Foam formation is dependent on three crucial factors: transportation, penetration and reorganization at the air-water interface. Therefore, high foaming capacity is normally being observed apart from its is electric pH, where net charge increases on the protein resulting in unfolding of peptide chains. This allows protein to diffuse rapidly into the air-water interface to encapsulate air particles and then enhance foam formation [18]. The lowest FC (122%) is observed at pH 4.0 and the highest FC (178%) at pH 10.0. Maximum foam stability is observed at pH 4.0 and it gradually decreases with increase in pH value (alkaline medium). Maximum foam stability near the isoelectric pH is is mainly attributed to the formation of stable molecular layers in air-water interface where the net charge on peptide is minimal. Such pH dependency is also observed for Soybean and Sunflower protein [19]. The results are presented at (Figures 4 & 5).

Viscosity:Viscosity measurement over a wide range of pH and concentration is very important in food formulation. Concentration dependency of viscosity was also reported for sunflower protein isolate and soybean protein isolate [20]. The result of viscosity of Crotalaria pallida has been presented in (Table 3). Generally it has been observed that viscosity increases with the increase in concentration at room temperature (260C). It is also observed that the viscosity of Crotalaria pallida seed protein isolate in slightly greater in alkaline range than that of acidic range. As the conformation of protein chain changes with pH, viscosity becomes pH dependent also.

Gelation capacity:The least gelation capacity (LCG) for Crotalaria pallida seed protein isolate is found to be 10% in distilled water medium and 8% in 0.5 (M) NaCl solution as a medium. These values are in agreement with the values reported by Okezie and Bello for soybeans powder (14%) [21], Lqari et al. reported that LGC of lupin protein concentrate is 12% [22], while Schmidt reported 7.5% for wheat protein isolate [23]. According to Schmidt, for a given type of protein, a critical concentration is required for the formation of a gel and the type of gel varies with the protein concentration. Considerably, higher protein concentration is usual required for the gelation of globular proteins. Thus, C. pallida seed protein may be globular in nature.
Conclusion
Functional properties of Crotalaria pallida seed protein isolate have been evaluated to screen its usability in food systems. Due to its high solubility in wide range of pH values, it can be a good source of protein. The values are quite comparable with commercially available protein foods such as soybean, sunflower, cashew nuts, pea nuts etc. Experimental results reveal that it can be useful in food systems such as whipped toppings, chiffon deserts, angel and sponge cakes etc. (owing to its high FC and FS), meat processing industry and sausages (requiring high EA and ES), doughnuts (requiring high OHC) etc. For these mentioned properties, the seed protein isolate of Crotalaria pallida is very attractive as functional ingredients in food systems but sensory and texture analysis of the product would be necessary.
Acknowledgement
The authors are grateful to The University of Burdwan, Burdwan, West Bengal, India for infrastructural and financial help. One of the authors is indebted to the same for providing necessary fellowship. Thanks are due to Bimalendu Ray for giving necessary permission to freeze dry protein solutions.
Statement
This article has not been submitted anywhere else for publication. Neither it has been published before in any other journal nor has it been presented in any conference or symposium.
References
- Onweluzo JC, Obanu ZA, Onuoha KC (1994) Functional properties of some lesser known topical legumes. J Food Sci Tech 31(4): 1-15.
- Satterle LD (1981) Proteins for use in foods. Food Tech 35: 53-70.
- Editorial Board (1986) CSIR Publications and Information Directorate. CSIR, New Delhi, India, p.147.
- Official Methods of Analysis, (15th edn) (1990) Association of Official Analytical Chemists Washington DC, USA.
- Carcea Bencini M (1986) Functional properties of drum dried chickpea (Cicer arietinum L) flours. J Food Sci 51(6): 1518-1521.
- Were L, Hettiarachchy L, Kalapathy U (1997) Modified soy proteins with improved foaming and water hydration properties. J s Food Sci 62(4): 821-824.
- Sathe SK, Deshpande SS, Salunkhe DK (1983) Functional properties of black gram (Phaseolus mungo L.) proteins. Lebensm-Wiss. Techno 16: 69-72.
- Sze-Tao KWC, Sathe SK (2000) Functional properties and in vitro digestibility of almond (Prunus dulcis L) protein isolate. Food Chems 69(2): 1530-160.
- Abbey BW, Ibeh GO (1988) Functional properties of raw and heat processed cowpea flour. J Food Sci 53(6):1775-1777.
- Sathe SK, Deshpande SS, Salunkhe DK (1982) Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrate. J Food Sci 47: 491-497.
- Ogunwolu SO, Henshaw FO, Mock HP, Santros A, Awonorin SO, et al. (2009) Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L) nut. Food Chem 115(3): 852-858.
- Chau CF, Cheung PCK, Wong YS (1997) Functional properties of protein isolates from three Chinese indigenous legume seeds. J Agri Food Chem 45(7): 2500-2503.
- Campell NF, Shih FF, Marshall WE (1992) Enzymatic phosphorylation of soy protein isolate for improved functional properties. J Agri Food Chems 40(3): 403-406.
- El-Adawy TA, Rahma EH, Bedawey AA, Gafar AF (2001) Nutritional potential and functional properties of sweet and bitter lupin seed protein isolates. Food Chems 74(4): 455-462.
- Betschart AA, Kinsela JE (1973) Extractability on solubility of leaf protein concentrate J Agri Food Chem 21(1): 60-65.
- Damodaran S, Paraf A (1997) An overview of Food proteins and their applications Marcel Dekker. New York, USA, p. 1-21.
- Klompong V, Benjakul S, Kantachote D, Shahid F (2007) Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influence by the degree of hydrolysis and enzyme type. Food Chem 102(4): 120-131.
- Hallingn PJ (1981) Protein stabilized foams and emulsions. CRC Critical Rev. Food Sci 15(2): 155-203.
- Lin MJY, Humbert ES, Sosulski FW (1974) Certain functional properties of sunflower meal products. J Food Sci 39(2): 368-370.
- Fleming SE, Sosulski FW, Kilara A, Humbert ES (1974) Viscosity and water absorption characteristics of slurries of sunflower and soybean flours, concentrates and isolates. J Food Sci 39(1): 188-191.
- Okezie BO, Bello AB (1988) Physicochemical and functional properties of winged bean flour and isolate compared with soy isolate. J Food Sci 53(2): 450-454.
- Lqari H, Vioqu J, Pedroche J, Millan F (2002) Lupinus gustifolius protein isolates: Chemical composition, functional properties and protein characterization. Food Chem 76(3): 349-356.
- Schmidt R H (1981) Gelation and coagulation; American Chemical Society, New York, USA, pp. 131-145.







