Comparative Study of the Properties and Release Kinetics of Two Different Types of Nanocapsules Based on Polysaccharide Encapsulants
*Surashree Sen Gupta and Mahua Ghosh
Department of Chemical Technology, University of Calcutta, India
Submission: May 12, 2016; Published: August 05, 2016
*Corresponding author: Surashree Sen Gupta, Dept. of Chemical Technology, University College of Science & Technology, University of Calcutta, 92, A.P.C. Road, Kolkata 700009 India.
How to cite this article: ASurashree S G, Mahua G. Comparative Study of the Properties and Release Kinetics of Two Different Types of Nanocapsules Based on Polysaccharide Encapsulants. Nutri Food Sci Int J. 2016; 1(2): 5555558. DOI: 10.19080/NFSIJ.2016.01.555558
Abstract
The aim of the present study was to synthesize polysaccharide nanocapsules by the emulsion technology using sodium alginate and trehalose as encapsulants and twine 20: lecithin (70:30) as emulsifiers. The size distribution, zeta potential, viscosity and lipid content of the emulsion formulation with sodium alginate was 220.5 nm, -80.5 mV, 32.4 cP and 83.4% respectively. Similarly, the corresponding values for emulsion formulation with trehalose was 202.4 nm, -67.2 mV, 22.66 cP and 84.6% respectively. The emulsions were freeze-dried and their encapsulation efficiencies were determined. For trehalose nanocapsules encapsulation efficiency was (92.3+0.97) % and for sodium alginate nanocapsules it was (89.7+0.557) %. From XRD data the nanocapsules were considered physically stable. Furthermore, the 45-day storage studies at ambient temperature also pin-pointed the shelf-life stability of both types of nanocapsules. Finally release study of the nanocapsules indicated a controlled release pattern, where both types of nanocapsules followed a zero order release rate. From the results it is evident that trehalose served as more deft encapsulants for protection and release of nutraceuticals than sodium alginate.
Keywords: Trehalose; Sodium alginate; Nanoencapsulation; Release; Release kinetics; Polysaccharides
Introduction
Polysaccharides are not very commonly used as encapsulants in the food and nutraceuticals industries. However, sodium alginate is one such compound which finds immense application in food and medical fields due to its nontoxic, biodegradable and low-cost properties [1]. It also shows extensive polymerisation properties. Trehalose, another type of polysaccharide, is also used in food and pharmaceutical industries due to its cryoprotectant nature. It is a low molecular weight carbohydrate which crystallizes to form trehalose dehydrates thus keeping water activity very low [2].
In our previous study microencapsulation of a bioactive lipid using sodium alginate and trehalose was carried out using calcium caseinate as the emulsion stabilizer following the emulsion technique [3]. As promising results were obtained from that study the work was further extended to prepare nanocapsules using the same encapsulants. Literature also showed that milk fat emulsions, stabilized with casein/sodium caseinate were prepared using trehalose/lactose as encapsulants when spray-dried and evaluated for functional properties, sodium caseinate emulsion showed better results in comparison to casein emulsion [4]. For nano-sized materials, the large surface area per unit mass of a substance enhances the bioactivity of the product [5]. This fact necessitates the development of nano-vesicles to fulfil the demands of the present day’s food and nutraceuticals industries. The efficiency of such nanocapsules invariably depends on the type of encapsulants chosen. Hence in the present study nanocapsules were synthesized by preparing oil-in-water emulsions using trehalose and sodium alginate as encapsulants. The emulsions were characterised in terms of particle-size and morphology. Properties of the nanocapsules were also evaluated and compared by studying their encapsulation efficiencies, diffraction studies, release, and shelf-life.
Material and Methods
Materials
D (+) Trehalose was procured from Sigma-Aldrich, Co., St. Louis, USA. Sodium alginate was obtained from Alpha Chemika, Mumbai, India. Oil that was encapsulated was extracted from flaxseed by pressing with hexane. The extracted oil was refined and then encapsulated. All other reagents were of analytical grade and procured from Merck India Ltd., Mumbai, India.
Methods
Emulsion Preparation: An aqueous solution with a 20% (w/v) preparation of trehalose in distilled water and 10% aqueous solution of sodium alginate in distilled water were formulated. Emulsions were prepared by mixing each of the two aqueous solutions with flaxseed oil (10%), added drop wise, while constantly stirring the core material. The ratio of the core material to the encapsulants was kept at 1:2 by weight, in case of trehalose encapsulants and 1:1 for alginate encapsulants for complete encapsulation of the core material (optimized after repeated experiments). While blending the mixtures, drop wise a 5% (by weight) of emulsifier mixture consisting of tween 20: lecithin (70:30) were added. The core material was combined with the aqueous solutions by the application of high speed stirrer (OMNI International GLH; Model: GLH-220, Ser. No.- 76014) at 30,000 rpm for 1 minute, to give pre-emulsions. The resultant emulsions were further homogenized at 8000 rpm including a re-mingling (six times) of the pre-emulsions with the homogenizer (Type: RQ-127A, Remi Motors Ltd., Mumbai-53, India) at 1.1 Amp and 220 V. Size reduction, Particle-size, Zetapotential analysis, Viscosity and Lipid Content of the emulsions.
The resulting coarse pre-emulsions were immediately passed through high pressure homogenization (NanoDebee [8799], B.E.E. International Inc., Easton, MA 02375, USA) with a hydraulic pressure of 3000 psi and a homogenization pressure of 40,000 psi and 5 cycles at 5°C. The particle size distribution and mean droplet diameter of the emulsions were measured using dynamic light scattering technique (Nano-ZS, Malvern Instruments, and Worcestershire, UK). Mean particle diameters were reported as Z-average diameters or the scattering intensityweighted mean diameter. Samples were diluted prior to making the particle size measurements to avoid multiple scattering effects, using a dilution factor of 1:10 sample-to-deionised water. Data reported is a mean of 3 consecutive readings. Emulsion viscosities were observed using Brookfield viscometer (DV-II+ Pro Viscometer, Brookfield, Brookfield Engineering Labs. Inc. Middleboro, USA) at 100 rpm with S21 spindle at a temperature of 25°C. Lipid was extracted from 1 g of each of the solvent washed emulsions after hydrolyzing the emulsions with acid (1 N HCl). The released core material lipid content was evaluated by extracting the fat with hexane. The emulsions were solvent washed prior to hydrolyzing to remove any un-emulsified oil from the system. Hence the amount of fat collected by hexane after hydrolysis of the emulsions was the amount emulsified. The hexane layer was evaporated to dryness and the amount of fat emulsified was estimated. The process was repeated for three times [6].
Powder production: Both the emulsions were initially frozen with liquid nitrogen (-196ºC) and then stored overnight at -70ºC. Next day the frozen emulsions were freeze-dried to allow the highest amount of freezable water to crystallize [2]. An Eyela Freeze Dryer (Type - FD-5N, Ser. No.-10160657, AC100V, and 50/60Hz 500W, Tokyo RIKAKIKAI Co. Ltd., JAPAN) was used for freeze drying which was operated at -20ºC and a chamber pressure of 13.3 Pa. The dried emulsions were crushed with mortar and pestle to get uniform powder-like products.
Efficiency of encapsulation and quality of encapsulated oil: The efficiency of the two polysaccharide encapsulants was determined by estimating the amount of fat encapsulated [7]. Both types of nanocapsules were washed with hexane (HPLC grade) to remove the non-encapsulated oil from the surface of the nanocapsules. The washing procedure was repeated twice. The solvent was removed by vacuum drying and amount of oil not encapsulated was determined. To measure the amount encapsulated, oil was extracted by acid hydrolysis followed by extracting the oil with hexane. Hexane was then evaporated and internal oil was scaled. From this study the amount of lipid that was encapsulated in the dried nanocapsules after undergoing the entire encapsulation procedure was recorded. Encapsulation efficiency was calculated using the following formula:
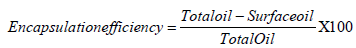
Free oil content was then calculated as percentage taking into account the total oil.
A fatty acid composition analysis of the extracted oil was conducted by means of gas chromatography (AGILENT; Model: 6890 N; FID detector; DB Wax column) to ascertain the quality of the encapsulated oil.
Crystallinity: Samples were analyzed for their Crystallinity by X-ray diffraction (XRD). A diffractometer (XPERT-PRO from Panalytical Diffractometer) using Cuα (λ = 1.5406) as X-ray source. Kα1α2β radiation from copper was used at 40 kV and 30 mA. Kα2 /Kα1 ratio was 0.50000. A scanning velocity of 1°/minute from 2° to 80° was maintained. Experiments were performed at ambient temperature (25°C).
Release study: Measured amount of dried capsules were placed in a glass bottle containing 100 ml dissolution media consisting of phosphate buffered saline (PBS) of pH 7.4. It was incubated in a shaking water-bath at 37°C, and 100 rpm. At definite time intervals of 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 hours definite amount of aliquots consisting of unhydrolyzed capsules and released oils were withdrawn, filtered and the oil was extracted with hexane. The solids were added back to the reaction mixture. An equal volume of dissolution media was added back to maintain a constant volume. Released oil quantity was determined on removal of the solvent. Experiments were performed in triplicate.
Release kinetics: Release pattern of the lipid was studied and the corresponding results were fitted to various kinetic models. These include the zero order models [8], first order model [9], Higuchi model [10], Hixson-Crowell cube root law [11] and the Korsmeyer-Peppas model [1].
Storage study: Measured quantity of dried capsules (2g) was taken in glass vials. They were then exposed to an environment of saturated salt solutions of water activities 0.75 (NaCl), 0.84 (KCl) and 0.97 (K2SO4) into evacuated desiccators at ambient temperature (25°C). At time intervals of 0, 5, 10, 15, 20, 25, 30, 35, 40 and 45 days, nanocapsules were withdrawn and then analysed for the amount of fat and water retained.
Statistical analysis: Statistical analysis was performed using one-way analysis of variance (ANOVA). When ANOVA detected significant differences between mean values, means were compared using Tukey’s test. For statistical studies Origin Lab software (Origin Lab Corporation, Northampton, UK) was used. Statistical significance was designated as P<0.05. Values were expressed as Mean± SEM.
Results and Discussion
Particle-size, Zeta-potential, viscosity and lipid content of the emulsions
Sodium alginate and trehalose were used as encapsulants and 20% lipid was encapsulated. Each of the two emulsions was subjected to particle-size, zeta potential, viscosity and lipid content analyses whose values are reported in (Table 1).
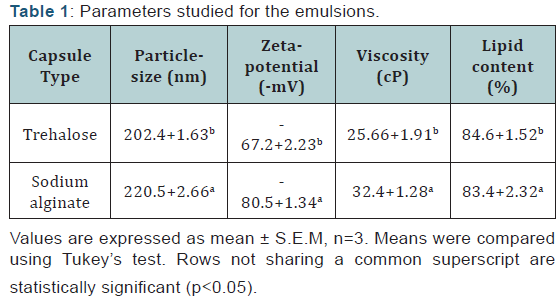
From (Table 1) it is evident that using polysaccharide encapsulants showed that trehalose encapsulated nanocapsules were better. The particle-size is lower for trehalose nanocapsules, and considerable stability in terms of zeta-potential were observed. The stability of the nanoemulsions is corroborated by the lower viscosity values of trehalose nanoemulsions in comparison to sodium alginate nanoemulsions. It is also observed that the trehalose nanoemulsions retain slightly higher amount of lipid in comparison to sodium alginate nanoemulsions. In fact, the values of the different parameters are significantly different from each other for each of the two encapsulants. The type of encapsulants and emulsifiers chosen while formulating an emulsion is critical in determining the emulsion stability. The emulsifier mixture used here shows superior emulsification activity by stabilizing both the emulsions. Furthermore the ultimate stability of emulsions against coalescence can be achieved only if the interfacial tension between oil and water is minimised. Evidently this is efficiently achieved using the emulsifier composition used. In addition the structural make-up of trehalose helps to generate an emulsion with smaller dropletsize leading to higher lipid content and good stability. Hence it shows a distinct resistance to structural deformities during the individual steps involved in the emulsion synthesis.
Efficiency of encapsulation and quality of encapsulated oil
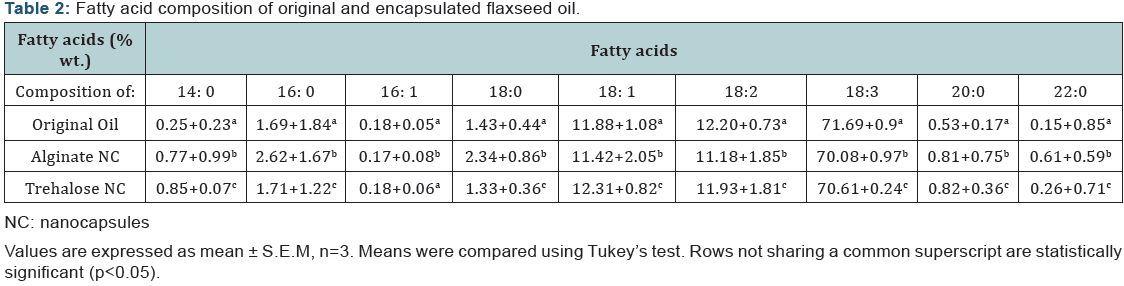
The encapsulation efficiency of both types of nanocapsules was evaluated after the nanoemulsions were freeze-dried. Encapsulation efficiency for trehalose nanocapsules was (92.3+0.97) %, whereas in case of sodium alginate nanocapsules encapsulation efficiency was observed to be (89.7+0.557) %. The efficiency of encapsulation was higher for trehalose nanocapsules. This can be attributed to the dense crystalline structure of trehalose. The drying process adopted here was the freeze-drying technique. Freeze-drying occurs at a very low temperature under vacuum. Trehalose is a well known cryoprotectant. As it crystallizes in a di-hydrate form the water activity is maintained at a very low level [2], minimizing the oxidation of fatty acids to undesired products (as evident from (Table 2), thereby enhancing the fat retention efficiency. The core material compositions of the nanocapsules were estimated by hydrolysis of the capsules and their fatty acid composition was determined by gas chromatographic analysis. The oil used as the core material was flaxseed oil. It is rich in alpha linolenic acid (ALA). The fatty acid is a polyunsaturated one with multiple unsaturations. This makes it a highly oxidation-prone fatty acid. As the proportion of ALA in flaxseed oil is almost 70-80% hence the oil itself is very sensitive to oxidation. The core material composition study helped us to understand the status of the oil after the completion of the entire procedure of encapsulation. As the procedure followed was similar in case of both types of nanocapsules, the only variable in both the cases was the encapsulants used. Hence on comparison of the oil extracted from the two types of nanocapsules and then comparing them with the original oil, a clear idea of the efficacy of the two types of encapsulants can be formed. It is evident that oil composition remains almost intact after encapsulation in both the cases (Table 2). Slightly better retention values were observed for trehalose nanocapsules. Also it is evident from table 2 that the proportion of 18:3 linolenic acid in the trehalose encapsulated nanocapsules was better retained in comparison to the alginate encapsulated ones. The glucopyranoside bonding of trehalose makes it resistant to hydrolysis, thus the encapsulated oil remains intact. On the contrary, alginate proved to be less effective than trehalose in protecting the encapsulated oil. However in terms of retarding oxidation of the most unstable fatty acid, both proved to be very effective as encapsulants.
Crystallinity of dried nanocapsules
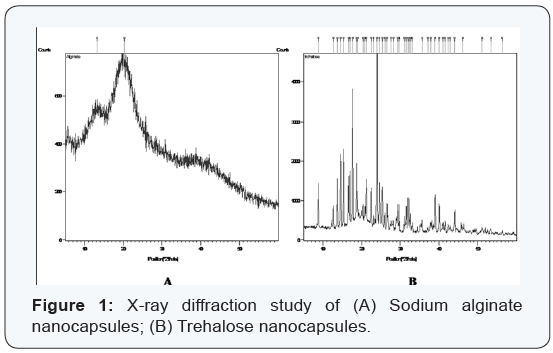
The amorphous nature of sodium alginate nanocapsules and the crystalline nature of the trehalose nanocapsules are well reflected in the X-ray diffraction patterns of the nanocapsules as shown in (Figure 1 A,B) respectively. While XRD-pattern of alginate nanocapsules display a broad peak with two small humps, several sharp high intensity peaks are observed for the XRD pattern of trehalose nanocapsules. The origin of the protruded areas for alginate nanocapsules is unknown. It could be due to the polymeric deformations or the alginate-caseinate linkage that may have formed during the emulsification procedure (Figure 1A). On the other hand, the large Bragg peaks of trehalose nanocapsules demonstrate the presence of organized realms of the crystalline encapsulants covering the core material (Figure 1B).
Release study of dried nanocapsules
From the release data of both types of nanocapsules it was revealed that both formulations presented a similar release profile. Complete release of the encapsulated lipid from trehalose coated nanocapsules occurred within 7 hours, while from the sodium alginate coated nanocapsules, complete release occurred in a longer time period after about 8 hours (Figure 2). In case of the release study a characteristic pattern was observed with an initial fast release up to 40%, followed by a second phase of slow release and finally constant. The initial fast release was typically observed for the trehalose nanocapsules. The hydrolysis of the gel-like polymeric structures of alginate-lecithin complex requires greater time under the experimental conditions than for the crystalline trehalose encapsulants. The second phase of slow release could be attributed to the fact that the affinity of encapsulated lipid for the respective lecithin/encapsulants coatings lead to a slower release rate. Apart from the initial value and before the complete release of lipid, the release after every interval of time from both types of nanocapsules varied significantly from each other. Finally, the release rate assumed a constant pattern when complete release of the encapsulated lipid occurred into the media.
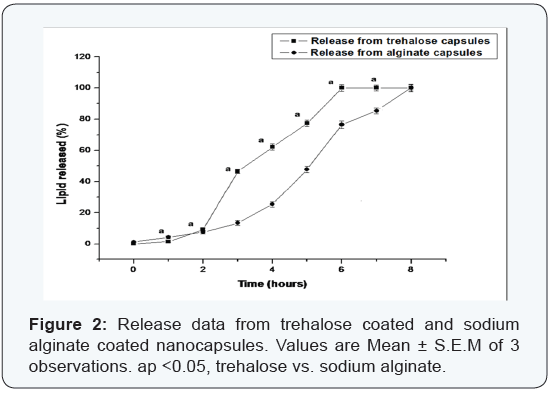
Release kinetics study
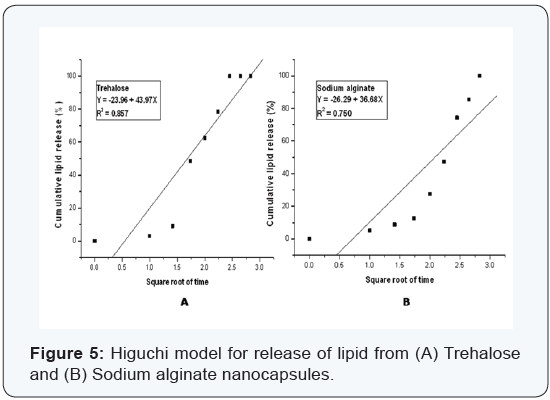
Various kinetic models were used to study the release kinetics of the two types of nanocapsules. In case of zero order rate study [8], the release rate from the nanocapsules was independent of the concentration of the released core material. The first order release rate [9], described the release from the system that occurred in a concentration dependent manner. The Fickian diffusion [10], as described by Higuchi explained the release of lipids as a function of the square root of time. The release from systems results in a change in surface area and diameter of capsules which was illustrated by the Hixson– Crowell cube root law [11]. The Korsmeyer–Peppas model [12] described the lipid release form a polymeric system which was explained by a simple relationship. The plots were based on the release of lipids from nanocapsules. For zero order kinetic model, the cumulative release of lipid (%) versus time was recorded (Figure 3); for first order kinetic model, log cumulative of (%) lipid remaining versus time were recorded (Figure 4); for Higuchi model cumulative lipid release (%) versus square root of time was recorded (Figure 5); for Korsmeyer model log cumulative lipid release (%) versus log time was recorded (Figure 6) and for Hixson-Crowell cube root law, the cube root of lipid (%) remaining in matrix versus time was recorded (Figure 7). Release data from both the nanocapsules were fit in all the different kinetic models discussed above. The results for each of them are given in (Table 3). From (Table 3), ‘n’ value (of Korsmeyer–Peppas model) which is a diffusion component for the kinetic models indicates the lipid release mechanism. When n = 0.5, the lipid is released from the nanocapsules with Quasi– Fickian diffusion process. In cases when n > 0.5, a non-Fickian or zero order release rate is observed [12].
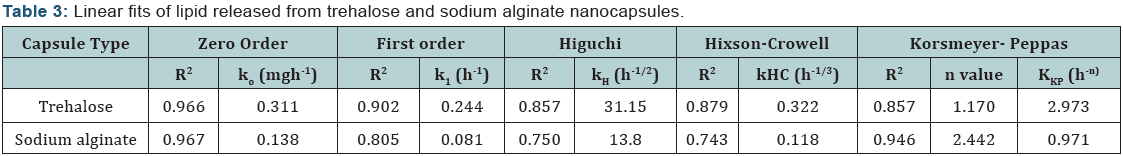
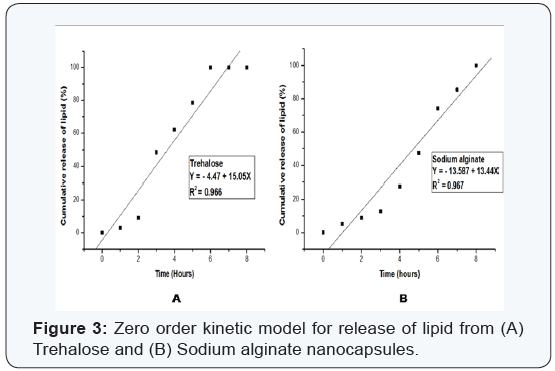
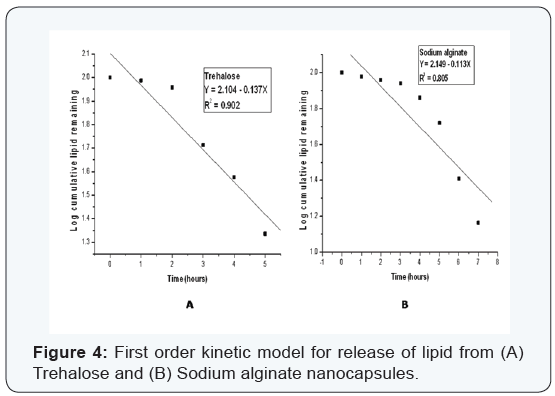
It can be observed from (Figures 3-7) that for release of lipid from trehalose and sodium alginate nanocapsules a linear relationship was observed. In Zero order plot (Figure 3) the R2 value of trehalose was 0.966 and for sodium alginate it was 0.967. Again in first order (Figure 4) plot trehalose showed R2 value of 0.902 and sodium alginate showed 0.805. For the Higuchi model (Figure 5) R2 for trehalose was 0.857 and for sodium alginate was 0.750. Again from Hixson–Crowell cube root law R2 for trehalose was 0.879 and for sodium alginate was 0.743. The different kinetic models described the relationship of release rate with concentration of the lipid. The best linearity for both types of nanocapsules was found for the zero order release rate (R2 = 0.966 for trehalose and R2 = 0.967 for sodium alginate). Hence in both cases a non-Fickian diffusion pattern was observed.
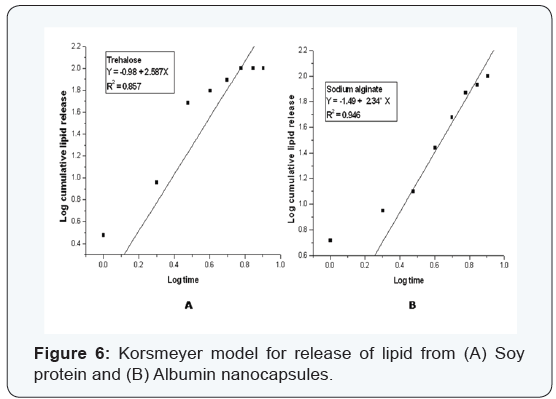
Finally, the Korsmeyer–Peppas model was utilized by incorporating the first 62.3% (4 hours) and 100% (6 hours) of release data for trehalose nanocapsules, and 27.6% (4 hours) and 74.3% (6 hours) for sodium alginate nanocapsules. Here the release mechanism was indicated by the Korsmeyer– Peppas model where n is the release exponent, indicating the mechanism of lipid release [12]. Fickian diffusional release occurred in the usual manner. Here the molecular diffusion mechanism conformed to the case-II relaxation release which is a kind of lipid release mechanism associated with stress factors. The value of the release exponent (n) for sustained release mechanism was found to be 1.1698 for trehalose nanocapsules and 2.4418 for sodium alginate nanocapsules which was greater than the defined limit of 0.5 in both the cases. Hence from the Korsmeyer model power law, both trehalose and sodium alginate nanocapsules followed a non-Fickian order release and both showed a zero-order release rate. From the study it can be concluded that both the nanocapsules released in a considerably delayed fashion in a pH 7.4 medium. Thus it can be concluded that the release rate can be modulated by modifying polysaccharide encapsulants like sodium alginate or trehalose.
Study of storage stability of dried nanocapsules
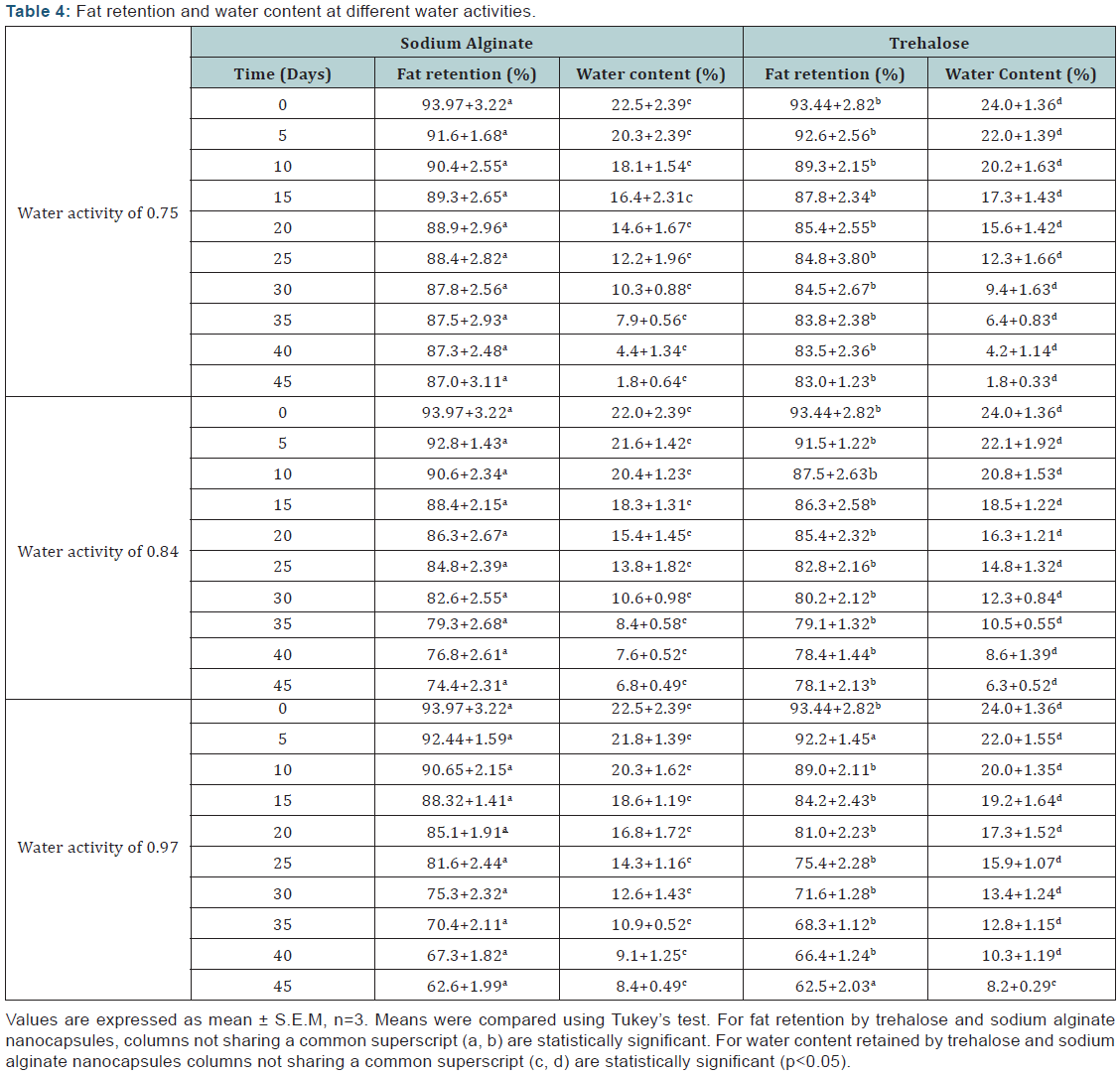
Storage stability of the dried polysaccharide nanocapsules were studied under different humidity conditions. These humidity conditions were prepared in vacuo by subjecting the dried capsules in an environment of saturated salt solutions of water activities 0.75 (NaCl), 0.84 (KCl) and 0.97 (K2SO4) at ambient temperature (25°C) for a period of 45 days. The fat and water content of the nanocapsules were evaluated after definite intervals of time. Greater the amount of moisture present in the system higher was the erosion of the nanocapsules, leading to the degradation of the encapsulated fat. Moisture instigated the hydrolysis of polysaccharides after being in contact with the nanocapsules for prolonged periods. The term “stability” refers to the ability of the nanocapsules to resist changes in the properties of the nanocapsules over time. The nanocapsules may become unstable due to a number of reasons including different physical and chemical processes involved due to the surrounding environment. The instabilities resulted in Dis-organisation of the molecules composing the nanocapsules leading to the creaming or flocculation or coalescence of the emulsions composing the nanocapsules [13]. Best results for fat retention and lowest moisture content were observed when sodium alginate encapsulant was used at an activity of 0.75 even after 45 days, as compared to trehalose coated nanocapsules. In both the cases at lowest water activity best results were observed. But trehalose displayed slightly better results in the long run, due to it’s dehydrate crystalline structure. On the other hand alginate probably got gelled in the presence of moisture when subjected to high water content for a long time in an environment with higher moisture activities (Table 4). The overall oil encapsulation efficiency of the trehalose nanocapsules coincided well with previous studies involving trehalose encapsulants. Previously oil had been encapsulated up to 40% when microencapsulated with trehalose [2]. It was beneficial to form a better understanding of the role of the polysaccharides in encapsulating lipid. The physicochemical properties on the polysaccharide encapsulants influenced their interactions with the encapsulated lipid core material. From the results it was clear that the nanocapsules showed significant environmental-sensitivity. Furthermore they also behaved efficiently in protecting the flaxseed oil, where trehalose showed better response as encapsulants. This fact can be attributed to the smaller size of trehalose molecules which packed themselves more densely in comparison to the larger sized sodium alginate molecules, thus serving as a better protective cover for the encapsulated lipid.
Conclusion
Lipids rich in essential fatty acids are prone to oxidative instability and hence degrade fast. Furthermore, the delivery of these products to the proper site of action in an unscathed form is essential for the complete utilisation of their beneficial properties. Synthesis of nanocapsules rich in such lipids is the only feasible solution to achieve the desired results. The selection of appropriate coating material to attain the best performance as an encapsulants functioning to provide adequate targeted delivery is essential in the synthesis of nanocapsules. From the above study it was observed that the encapsulation technologies can be successfully employed to formulate different lipid based nanocapsules with polysaccharide encapsulants. Trehalose serves as better encapsulants in terms of shelf-life, though the release kinetics study illustrates a similar release phenomenon for both types of nanocapsules. Depending upon the field of application both the encapsulants show immense scope as a suitable coating material. Furthermore, the bioavailability and biocompatibility of both types of encapsulants also ensures the suitability of using any of the two when environmental safety is concerned.
Acknowledgement
The authors would like to acknowledge the ‘Council of Scientific and Industrial Research’ (CSIR) for their financial support.
References
- Shu XZ, Zhu KJ (2002) The release behaviour of brilliant blue from calcium-alginate gel beads coated by chitosan: the preparation method effect. Eur J Pharm Biopharm 53(2): 193-201.
- Cerimedo SMA, Cerdeiro M, Candal JR, Herrera LM (2008) Microencapsulation of a low-trans fat in trehalose as affected by emulsifier type. J Am Oil Chem Soc 85(9): 797-807.
- Sen Gupta S, Ghosh S, Maiti P, Ghosh M (2012) Microencapsulation of conjugated linolenic acid-rich pomegranate seed oil by an emulsion method. Food Sci Technol Int 18(6): 549-558.
- Vega C, Goff HD, Roos YH (2007) Casein molecular assembly affects the properties of milk fat emulsions encapsulated in lactose or trehalose matrices. International Dairy Journal 17(6): 683-695.
- Elder A, Oberdorster G (2005) Translocation and Effects of Ultrafine Particles Outside of the Lung. Clin Occup Env Med 5(4): 785-796.
- Sen Gupta S, Ghosh M (2015) Formulation development and process parameter optimization of lipid nanoemulsions using an alginateprotein stabilizer. J Food Sci Technol 52(5): 2544-2557.
- Tan LH, Chan LW, Heng PW (2005) Effect of oil loading on microspheres produced by spray-drying. J Microencapsul 22(3): 253-259.
- Hadjiioannou TP, Christian GD, Koupparis MA, Macheras PE (1993) Quantitative Calculations in Pharmaceutical Practice and Research, VCH Publishers Inc., New York, USA, pp. 345-348.
- Bourne DWA (2002) Pharmacokinetics. In: GS Banker & CT Rhodes (Eds.), Modern Pharmaceutics. (4th edn), Marcel Dekker Inc., New York, USA, p. 67-92.
- Higuchi T (1963) Mechanism of sustained action medication: Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52: 1145-1149.
- Hixson AW, Crowell JH (1931) Dependence of reaction velocity upon surface and agitation (I) theoretical consideration. Industrial Engineering Chemistry 23(10): 1160-1168.
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA (1983) Mechanisms of solute release from porous hydrophilic polymers. International Journal of Pharmaceutics 15(1): 25-35.
- Mcclements DJ (1999) Food emulsions: Principles, practice and techniques, CRC Press, Florida, Boca Raton.







