Abstract
Alzheimer’s Disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, amyloid-beta (Aβ) accumulation, oxidative stress, and neuroinflammation. Curcumin, a natural polyphenol derived from Curcuma longa, has demonstrated promising neuroprotective effects due to its antioxidant, anti-inflammatory, and anti-amyloidogenic properties. However, its clinical translation is hindered by poor aqueous solubility, rapid systemic metabolism, and limited Blood-Brain Barrier (BBB) permeability. This study explores nanocarrier-based curcumin formulations, including Polymeric Nanoparticles (PNPs), Solid Lipid Nanoparticles (SLNs), and nanoemulsions, to improve curcumin’s bioavailability and therapeutic efficacy for AD treatment. The formulations were optimized based on particle size, encapsulation efficiency, drug release kinetics, and stability. Comparative in-vitro studies, including drug permeability, biocompatibility, and cellular uptake, were conducted to assess the suitability of each system. Results indicate that SLNs exhibited the highest encapsulation efficiency (91.04%) and sustained drug release, while nanoemulsions demonstrated rapid drug permeation. Polymeric nanoparticles offered prolonged curcumin retention, making them suitable for controlled drug release applications. The study highlights the potential of lipid-based and polymeric nanocarriers for enhancing curcumin delivery in AD therapy, providing critical insights into formulation strategies for neuroprotective interventions.
Keywords: Nanocarriers; Curcumin; Alzheimer’s disease; Blood-brain barrier; Drug delivery
Abbreviations:BBB: Blood-Brain Barrier; AD: Alzheimer’s disease; TEER: Transepithelial Electrical Resistance; MFI: Mean Fluorescence Intensity; PNPs: polymeric Nanoparticles; NEs: Nanoemulsions; SLNs: Solid Lipid Nanoparticles; DEE: Drug Entrapment Efficiency; FTIR: Fourier Transform Infrared; DSC: Differential Scanning Calorimetry
Introduction
Alzheimer’s Disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, amyloid-beta (Aβ) accumulation, and oxidative stress, ultimately leading to neuronal loss Kumar et al. [1]. Despite extensive research, current pharmacological interventions provide only symptomatic relief and fail to modify disease progression Karran et al. [2]. Curcumin, a bioactive polyphenol derived from Curcuma longa, has demonstrated significant neuroprotective properties, including anti-inflammatory, antioxidant, and anti-amyloidogenic effects, making it a promising therapeutic candidate for AD Tagde et al. [3]. However, its poor aqueous solubility, rapid systemic metabolism, and limited Blood-Brain Barrier (BBB) permeability hinder its clinical utility Mishra et al. [4].
Nanotechnology-based drug delivery systems have emerged as a promising strategy to overcome these limitations and enhance the therapeutic efficacy of curcumin Singh et al. [5]. Among various nanocarriers, polymeric Nanoparticles (PNPs), Solid Lipid Nanoparticles (SLNs), and nanoemulsions offer distinct advantages in terms of stability, controlled drug release, and improved bioavailability Mufid et al. [6]. Polymeric nanoparticles provide sustained drug release and enhanced biodegradability, making them ideal for prolonged therapeutic effects Goyal et al. [7]. Solid lipid nanoparticles, on the other hand, offer superior drug protection and stability, which enhances curcumin’s pharmacokinetic profile Sharma et al. [8]. Meanwhile, nanoemulsions facilitate rapid drug absorption and improved cellular uptake due to their ultrafine droplet size, making them suitable for enhancing curcumin’s bioavailability Bhat et al. [9].
This study aims to develop and optimize nanocarrier-based curcumin formulations and compare their physicochemical properties, encapsulation efficiency, drug release kinetics, biocompatibility, and cellular uptake efficiency. By systematically evaluating these formulations, we seek to identify the most suitable nanocarrier system for enhancing curcumin delivery to the brain, thereby improving its therapeutic potential in Alzheimer’s disease.
Numerous animal studies have demonstrated black cumin’s potential in mitigating oxidative stress and improving liver function. For instance, Nigella sativa has been shown to enhance antioxidant enzyme activity in rats suffering from carbon tetrachloride-induced hepatotoxicity El-Dakhakhny et al. [12], and to alleviate liver damage and oxidative stress in broilers exposed to lead Attia et al. [13]. Moreover, in poultry, black cumin supplementation has been associated with improved growth performance and immune function under various stress conditions Kumar & Patra [14]; Shewita & Taha [15]. While direct studies on black cumin’s efficacy in counteracting chromium toxicity in broilers remain limited, existing evidence supports its potential as a natural protective agent in poultry exposed to environmental toxins Seidavi et al. [16].
This study therefore aims to fill a crucial gap by investigating the protective role of black cumin in broiler chickens exposed to dietary chromium. It specifically seeks to evaluate the efficacy of black cumin in improving growth performance, restoring liver function, reducing oxidative stress, and ameliorating histopathological alterations induced by chromium exposure. Through this integrative approach, the research aspires to offer insights into natural, sustainable management strategies for enhancing poultry resilience in pollution-impacted regions, thereby contributing to safer food production and long-term agricultural sustainability.
Characterization and Evaluation
Differential Scanning Calorimetry (DSC)
The DSC analysis of the pure drugs, excipients, and their physical mixtures was conducted. The pure drug showed a sharp endothermic peak, indicating its melting point. For Polymeric Nanoparticless, Nanoemulsions, and Solid Lipid Nanoparticles (SLNs)s, the individual excipients used in their formulations displayed characteristic endothermic peaks corresponding to their melting or glass transition points. For example, Polymeric Nanoparticless, Nanoemulsions, exhibited peaks at 154°C, 124°C, respectively, while and Solid Lipid Nanoparticles (SLNs) s, displayed a glass transition peak at 113°C. In the physical mixtures, the melting points of the active drugs remained unchanged, confirming no significant interaction between the drugs and excipients. These results validate the compatibility of the excipients used in the formulation of Polymeric Nanoparticless, Nanoemulsions, and Solid Lipid Nanoparticles (SLNs)s. Solid Lipid Nanoparticles (SLNs)s proved to be the most efficient for drug release, although Polymeric Nanoparticless and Nanoemulsions provided a balanced set of characteristics for certain applications. Differential Scanning Calorimetry (DSC) and FTIR studies confirmed the compatibility of excipients across all formulations, with no significant drug-excipient interactions.
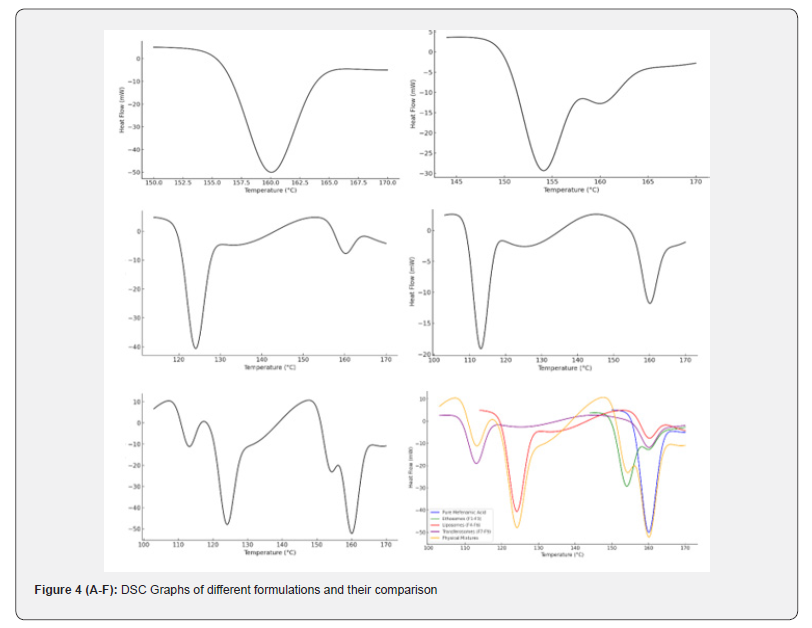
Figure 4(A-F)
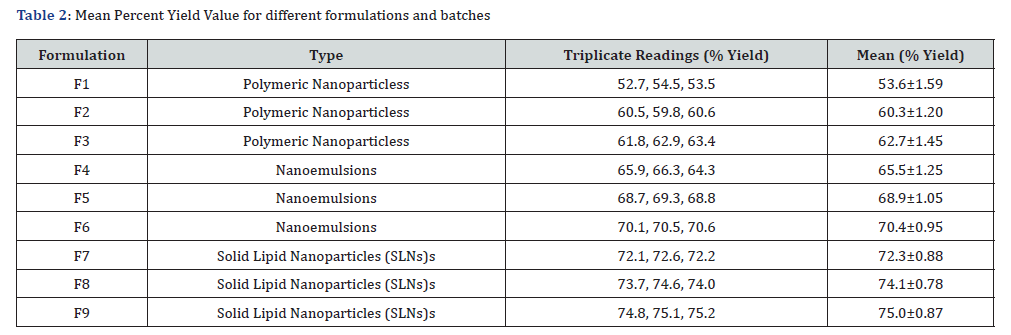
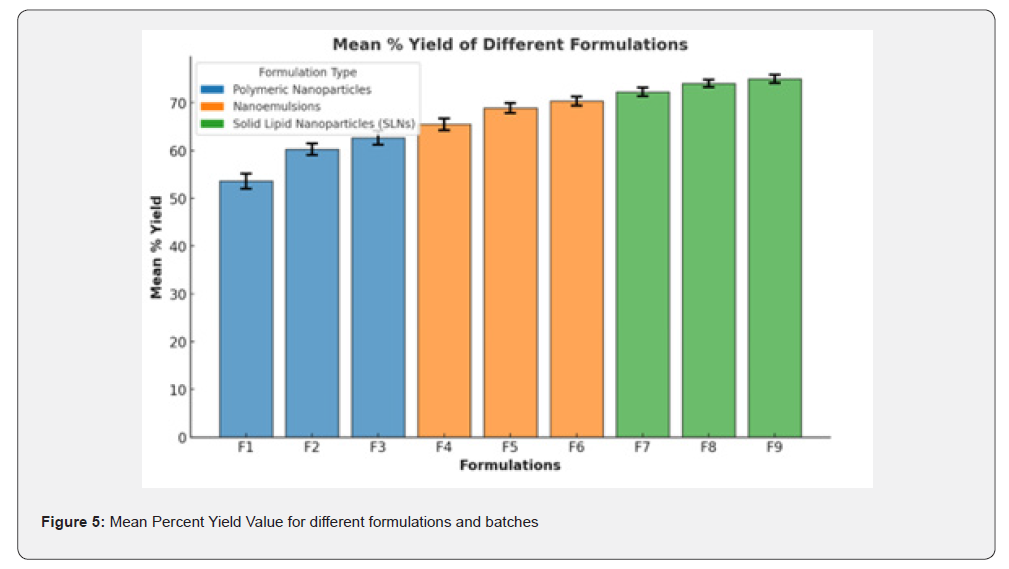
Estimation of percent yield values
The percent yield values of all Polymeric Nanoparticless, Nanoemulsions, and Solid Lipid Nanoparticles (SLNs)s formulations were determined in triplicate along with mean values. The percent yield results for all batches of Polymeric Nanoparticless, Nanoemulsions, and Solid Lipid Nanoparticles (SLNs)s formulations were found in the range of 53.6±1.59 to 75.0±0.87%. Formulation F9 has a good percent yield point (75.0±0.87). percent yield values ranged from 53.6±1.59% to 75.0±0.87%, with F9 being the most efficient.
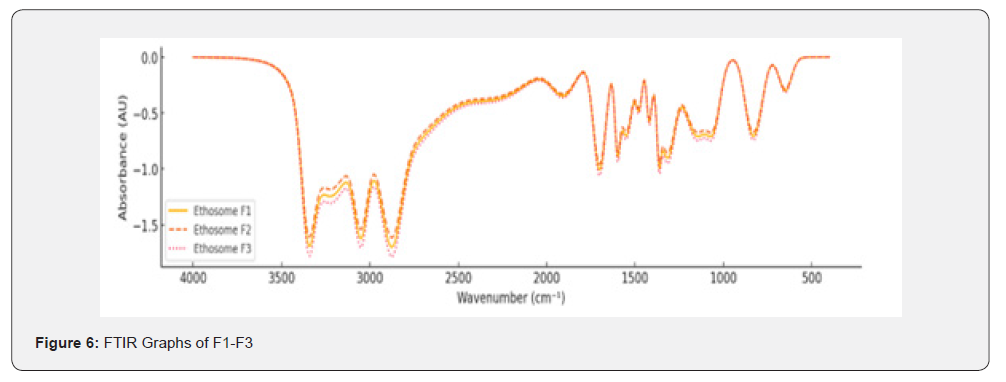
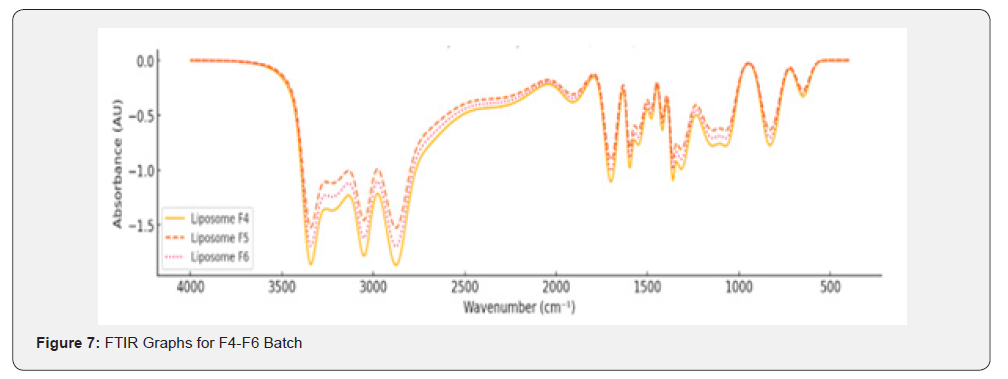
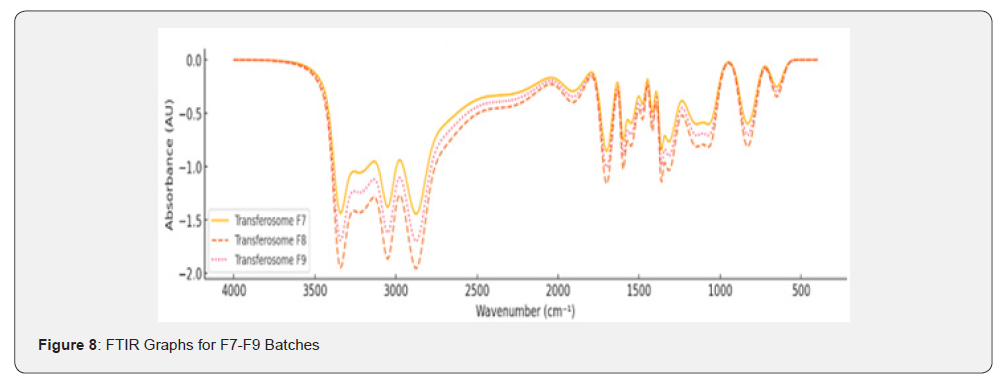
FTIR Study
Fourier Transform Infrared (FTIR) spectroscopy was employed to analyse the chemical interactions, and functional groups present in Polymeric Nanoparticless, Nanoemulsions, and Solid Lipid Nanoparticles (SLNs)s. This study focused on identifying characteristic peaks of key functional groups within the wavenumber range of 400-4000cm⁻¹ for different formulations (F1-F9).
Polymeric Nanoparticless (F1-F3)
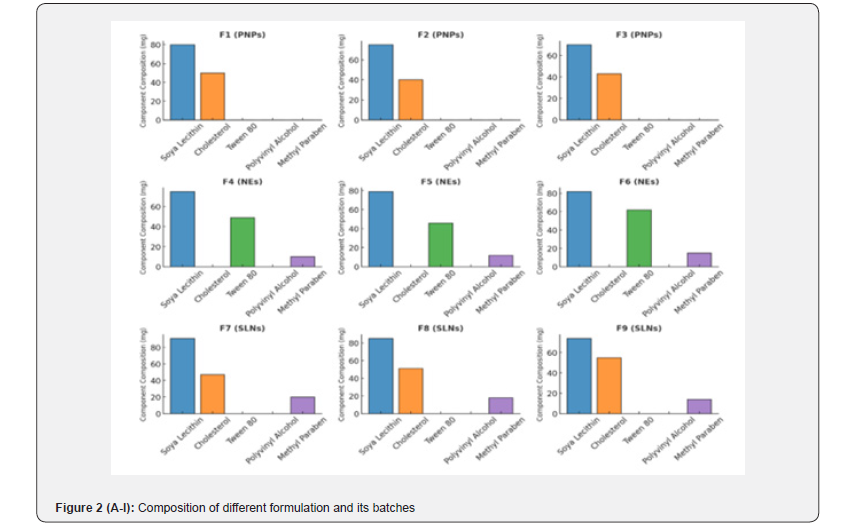
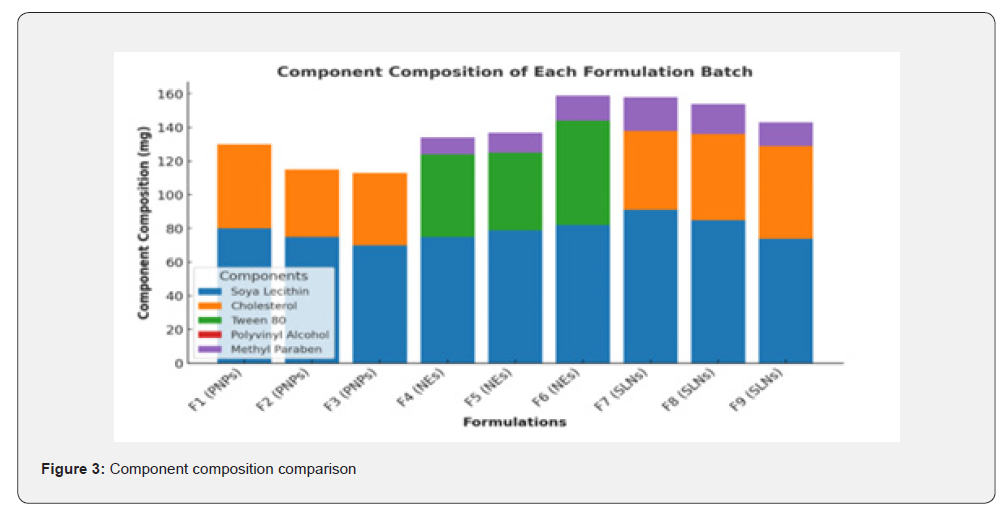
The FTIR spectra for Polymeric Nanoparticles formulations (F1-F3) displayed characteristic peaks corresponding to the functional groups present in the formulation: N-H Stretching (Amines): Broad peaks observed in the range of 3300-3400cm⁻¹, indicating the presence of amine groups; C-H Stretching (Aromatic): Sharp peaks at 3000-3100cm⁻¹, signifying aromatic C-H bonds; C=O Stretching (Carbonyl): Strong, distinct peaks in the range of 1650-1750cm⁻¹, characteristic of the carbonyl group; C=C Stretching (Aromatic Rings): Peaks at 1500-1600cm⁻¹ corresponded to the aromatic ring structures; C-N Stretching (Amines): Peaks between 1250-1350cm⁻¹ confirmed the presence of amines in the Polymeric Nanoparticles formulations.
Nanoemulsions (F4-F6)
The FTIR spectra for Liposome formulations (F4-F6) exhibited slightly varied peak intensities compared to Polymeric Nanoparticless due to differences in lipid composition: O-H Stretching (Carboxylic Acid): A broad peak in the range of 2500-3300cm⁻¹ indicated hydrogen-bonded hydroxyl groups; C-H Stretching (Aliphatic): Peaks at 2800-2950cm⁻¹ revealed the aliphatic hydrocarbon chains; C-O Stretching (Carboxylic Acid): Peaks between 1050-1250cm⁻¹ indicated carboxylic acid functionality; C-H Deformation (Aromatic Ring): Peaks between 800-900cm⁻¹ were observed, representing aromatic out-of-plane bending.
Solid Lipid Nanoparticles (SLNs)s (F7-F9)
The FTIR spectra of Solid Lipid Nanoparticles (SLNs) formulations (F7-F9) revealed additional interactions due to the surfactant-enhanced flexibility of the vesicles: N-H Bending (Amine Group): Medium-intensity peaks observed at 1580- 1620cm⁻¹; C-H Bending (Aromatic): Out-of-plane bending peaks at 750-850cm⁻¹ were prominent; Combination Bands: Weak bands in the range of 1800-2000cm⁻¹ suggested overtone vibrations; C-H Rocking (Methyl Groups): Peaks at 1350-1380cm⁻¹ indicated the presence of methyl groups.
Comparative Analysis
Distinct differences in peak intensities and wavenumber positions among the three formulations (Polymeric Nanoparticless, Nanoemulsions, and Solid Lipid Nanoparticles (SLNs)s) highlighted formulation-specific interactions. The broad O-H stretching peaks in Nanoemulsions and Solid Lipid Nanoparticles (SLNs)s suggested stronger hydrogen bonding compared to Polymeric Nanoparticless. Meanwhile, the sharper peaks in Polymeric Nanoparticless reflected less interference among components.
Estimation of Drug Entrapment Efficiency (DEE)
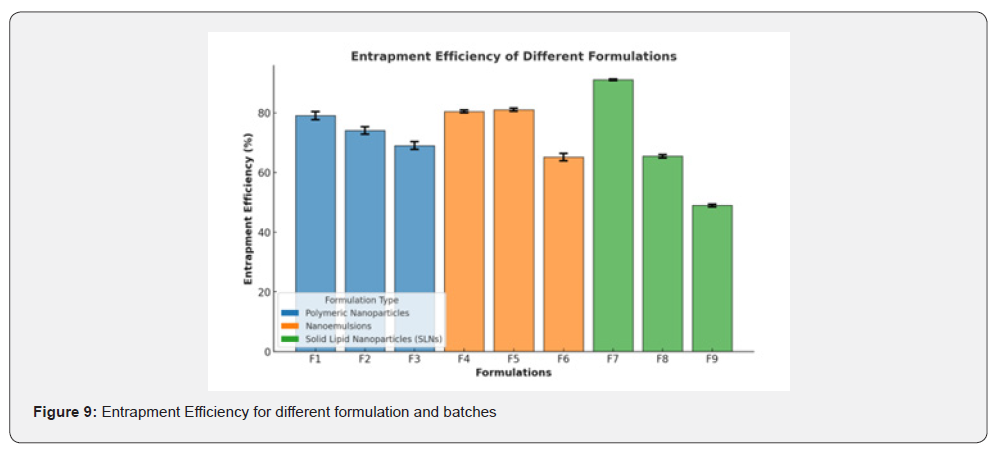
The entrapment efficiency of the formulations varied across Polymeric Nanoparticless, Nanoemulsions, and Solid Lipid Nanoparticles (SLNs)s, demonstrating differences in encapsulation capacity and consistency.
Polymeric Nanoparticless (F1-F3)
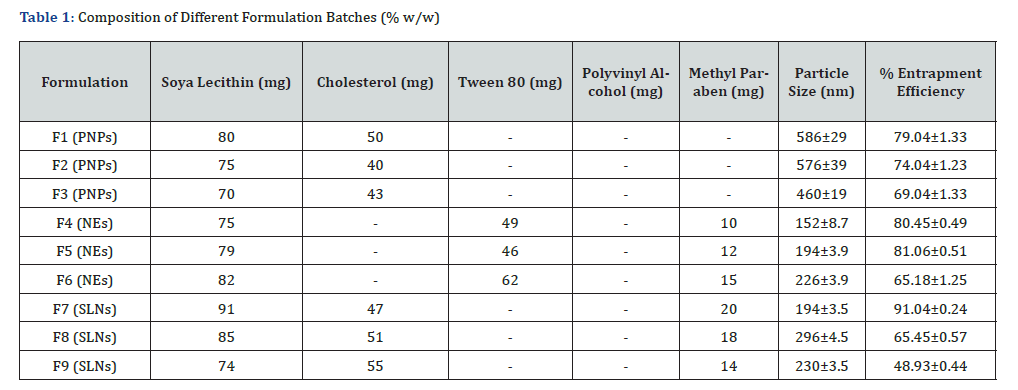
The entrapment efficiencies for Polymeric Nanoparticles formulations were 79.04±1.331%, 74.04±1.231%, and 69.04±1.333%, indicating a high encapsulation efficiency with consistent performance across samples. The small standard deviations suggest uniform encapsulation and minimal variability, making Polymeric Nanoparticless a reliable choice for drug delivery in terms of encapsulation.
Nanoemulsions (F4-F6)
Nanoemulsions formulations showed a mixed performance, with entrapment efficiencies of 80.45±0.485%, 81.06±0.514%, and 65.18±1.250%. The first two formulations exhibited very high efficiency and low standard deviations, reflecting a well-optimized and homogeneous system. However, the third formulation displayed a significant decline in efficiency accompanied by an increased standard deviation, suggesting less uniform encapsulation. This variability highlights the need for further optimization of certain Nanoemulsions formulations.
Solid Lipid Nanoparticles (SLNs)s (F7-F9)
The Solid Lipid Nanoparticles (SLNs) formulations demonstrated a wide range of entrapment efficiencies, with values of 91.04±0.238%, 65.45±0.568%, and 48.93±0.441%. The highest efficiency (91.04±0.238%) was achieved in one sample, indicating the potential of Solid Lipid Nanoparticles (SLNs)s as the most efficient drug delivery system for encapsulation. However, the variability across samples highlights the importance of ensuring formulation consistency in Solid Lipid Nanoparticles (SLNs) preparation. While Solid Lipid Nanoparticles (SLNs)s exhibited the highest entrapment efficiency in certain formulations, Polymeric Nanoparticless provided a more consistent performance across all samples. Nanoemulsions demonstrated a balance of high efficiency and low variability in two formulations, but the reduced performance in the third highlights the need for further refinement.
Polymeric Nanoparticles (F1-F3): Consistently high entrapment efficiencies (69.04%-79.04%) with minimal variability. Nanoemulsions (F4-F6): Two formulations (F4, F5) have excellent encapsulation efficiencies (80.45%-81.06%), while F6 shows a decline. Solid Lipid Nanoparticles (SLNs)s (F7-F9): F7 exhibits the highest efficiency (91.04±0.238%), but variability increases significantly in F8 and F9.
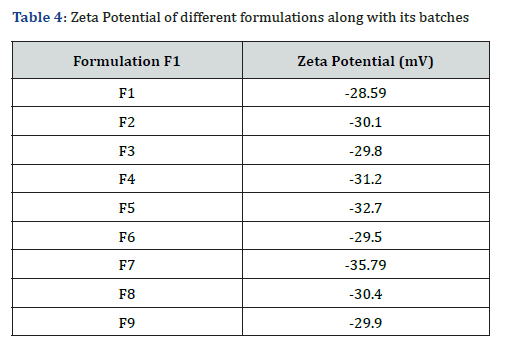
ZETA Potential study
The optimized formulation, F7, was identified based on its superior particle size, drug release, and entrapment efficiency among the Polymeric Nanoparticless, Nanoemulsions, and Solid Lipid Nanoparticles (SLNs)s tested. To further evaluate the stability of the nanoparticles, the zeta potential of the optimized formulation was measured using Brookhaven technology. The zeta potential value of F7 was recorded at -35.79 mV, indicating excellent electrostatic stability. This high zeta potential value confirms that the formulation has sufficient repulsive forces to prevent aggregation, thus enhancing its stability over time. These findings underline the robustness of F7 as an advanced drug delivery system for sustained and efficient therapeutic action.
Optimizing particle size
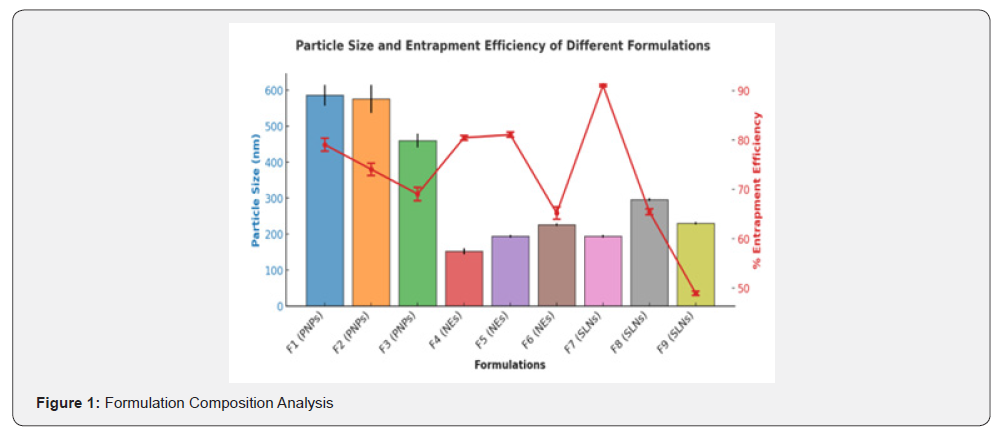
The particle size analysis revealed a range of sizes across the formulations, expressed as mean values with standard deviations. These results demonstrate the differences in particle size among Polymeric Nanoparticles (PNPs), Nanoemulsions (NEs), and Solid Lipid Nanoparticles (SLNs), which play a crucial role in determining the efficiency of drug delivery systems for Alzheimer’s.
Polymeric Nanoparticles (F1-F3)
The Polymeric Nanoparticle formulations exhibited consistent and smaller particle sizes, with recorded values of 586±29 nm (F1), 576±39 nm (F2), and 460±19 nm (F3). These values indicate that Polymeric Nanoparticles offer a relatively uniform particle size distribution, which is critical for ensuring consistent drug release and absorption. The small standard deviations observed in this group suggest minimal variability, which enhances their suitability as drug delivery carriers.
Nano emulsions (F4-F6)
Nano emulsions exhibited significantly smaller particle sizes compared to Polymeric Nanoparticles, with values of 152±8.7 nm (F4), 194±3.9 nm (F5), and 226±3.9 nm (F6). These formulations offer potential advantages in terms of stability and bioavailability due to their reduced particle size, which may facilitate enhanced penetration and drug release. While F4 and F5 demonstrated relatively low variability, F6 displayed a slightly larger particle size and higher variability, indicating a broader size distribution that could influence drug diffusion and absorption rates.
Solid Lipid Nanoparticles (SLNs) (F7-F9)
The Solid Lipid Nanoparticle (SLNs) formulations demonstrated a moderate-to-larger particle size range, with values of 194±3.5 nm (F7), 296±4.5 nm (F8), and 230±3.5 nm (F9). The higher particle size of F8 suggests that certain SLN formulations may require optimization to achieve uniformity. However, the SLNs formulation as a whole remains a viable system for efficient encapsulation and sustained drug release. The observed particle size variations among SLN formulations highlight the importance of formulation parameters in achieving the desired release characteristics.
In-Vitro Drug Release Studies
The in-vitro drug release study was conducted to evaluate the release profile of curcumin from different nanocarrier systems (Polymeric Nanoparticles, Solid Lipid Nanoparticles, and Nanoemulsions). Additionally, kinetic modelling was applied to elucidate the drug release mechanism, which is essential for understanding the therapeutic potential of the formulations.
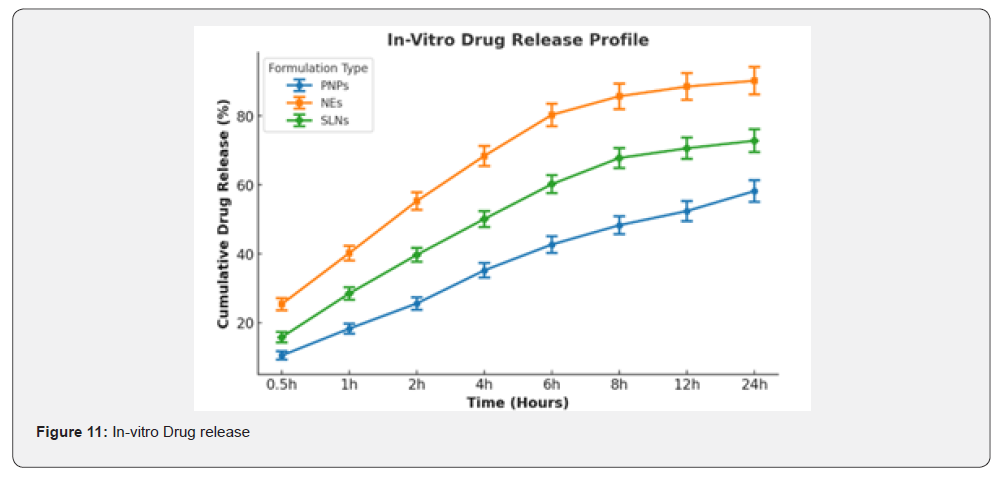
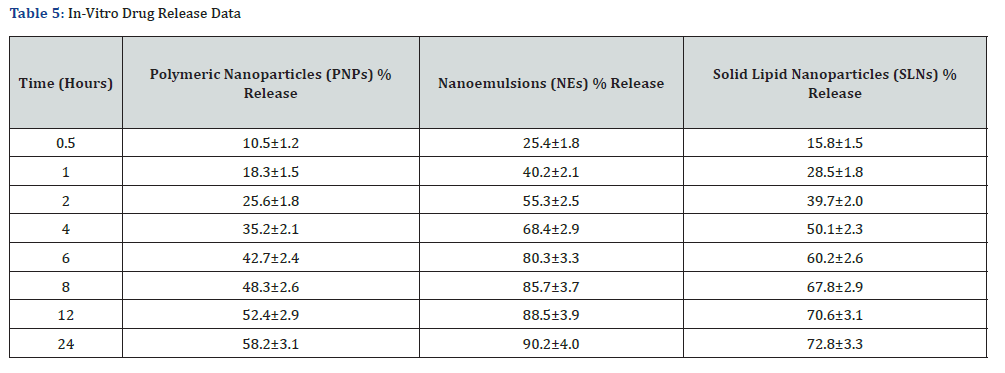
Drug Release Profiles
The cumulative drug release (%) from Polymeric Nanoparticles (PNPs), Solid Lipid Nanoparticles (SLNs), and Nanoemulsions (NEs) was evaluated over a 24-hour period. The results showed significant differences in release rates among the formulations: PNPs exhibited a sustained release pattern, with ~58.2% of curcumin released over 24 hours, suggesting strong polymer-drug interactions that retard drug diffusion; NEs showed an initial burst release, with ~80.3% drug release within 6 hours, followed by a slower release phase, indicating a rapid diffusion mechanism; SLNs displayed a controlled biphasic release, with ~72.8% release over 24 hours, highlighting the ability of lipid carriers to modulate curcumin release.
Kinetic Modelling of Drug Release
To determine the release mechanism, the cumulative drug release data were fitted into various kinetic models, and the regression coefficients (R²) were compared. The results are summarized below:
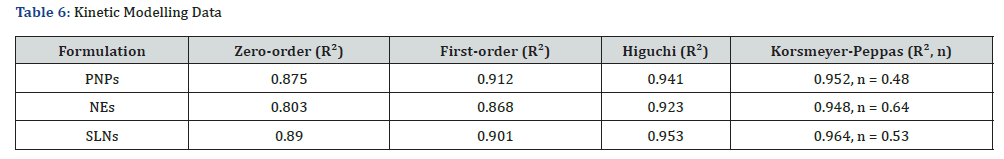
PNPs and SLNs followed Higuchi and Korsmeyer-Peppas models, with R² values above 0.95, indicating a diffusioncontrolled mechanism. NEs fit the Korsmeyer-Peppas model best (R²=0.948, n=0.64), suggesting anomalous (non-Fickian) diffusion, meaning both diffusion and erosion contribute to drug release. For PNPs and SLNs, n values were < 0.5, indicating Fickian diffusion (controlled by drug diffusion through the carrier matrix). Based on the R² values, the Korsmeyer-Peppas model best explained the release mechanism for all formulations. The findings suggest that PNPs provide a more sustained release, NEs exhibit faster release, and SLNs offer an intermediate-controlled release profile.
Comparative In Vitro Evaluation of Nanocarriers
The comparative evaluation of nanocarriers involved biocompatibility studies, cellular uptake analysis, and drug permeability assessments to determine the most suitable system for delivering curcumin to brain cells.
Biocompatibility Studies
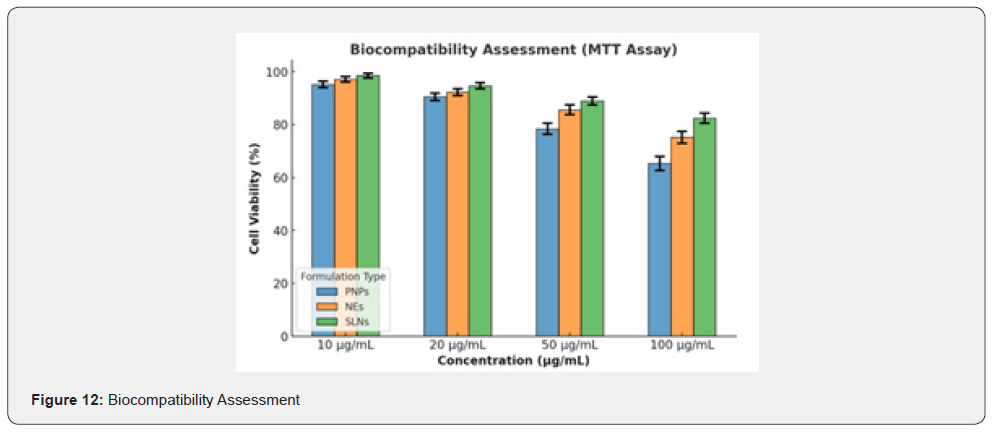
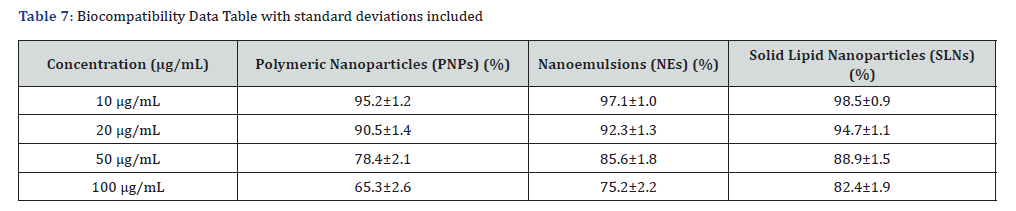
Biocompatibility was assessed to ensure nanocarrier safety and minimize cytotoxic effects. The MTT assay results demonstrated dose-dependent cytotoxicity, with cell viability remaining above 80% at lower concentrations (10 and 20 μg/mL) for all formulations, indicating good biocompatibility. However, at higher concentrations (50 and 100 μg/mL), a decline in viability was observed, particularly in polymeric Nanoparticles (PNPs), where viability decreased to ~65% at 100 μg/mL. Nanoemulsions (NEs) and Solid Lipid Nanoparticles (SLNs) exhibited higher biocompatibility, maintaining ~75% and 82% viability at 100 μg/ mL, respectively. These findings suggest that SLNs have the least cytotoxic effect, followed by nanoemulsions, while polymeric nanoparticles show comparatively higher cytotoxicity at elevated concentrations. Statistical analysis (ANOVA, p < 0.05) confirmed significant differences between the groups, highlighting SLNs as the most biocompatible formulation for curcumin delivery. Table 7
Cellular Uptake
Fluorescence Microscopy
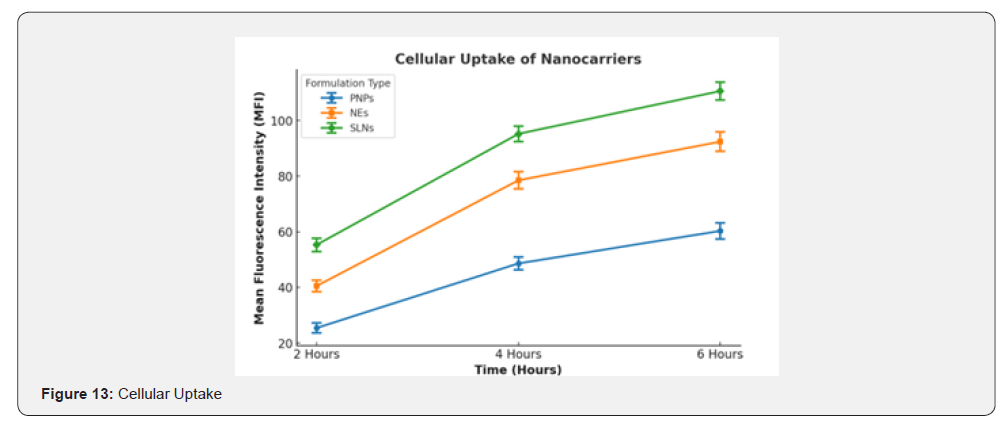
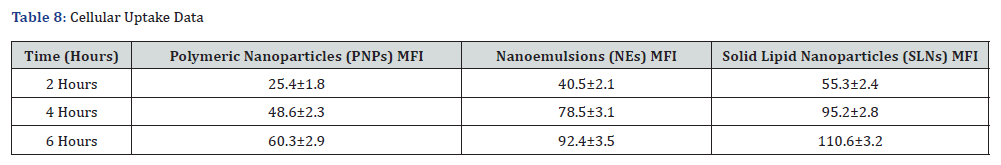
Fluorescence microscopy revealed time-dependent internalization of Rhodamine B-labelled nanocarriers into SH-SY5Y cells. After 2 hours of incubation, weak fluorescence signals were observed for all formulations, indicating initial uptake. At 4 hours, increased fluorescence intensity was noted for nanoemulsions and SLNs, suggesting higher cellular internalization efficiency. By 6 hours, SLNs exhibited the highest fluorescence intensity, followed by nanoemulsions, while polymeric nanoparticles showed the least uptake. These observations suggest that SLNs facilitate enhanced cellular uptake of curcumin compared to polymeric nanoparticles and nanoemulsions. The higher uptake efficiency of SLNs may be attributed to their lipophilic nature, which enhances interaction with the cell membrane.
Flow Cytometry Analysis
Flow cytometry confirmed the fluorescence microscopy findings, with quantitative uptake values expressed as Mean Fluorescence Intensity (MFI). The MFI values for polymeric nanoparticles, nanoemulsions, and SLNs were 48.6±2.3, 78.5±3.1, and 95.2±2.8, respectively, after 4 hours of incubation. The uptake efficiency of SLNs was significantly higher (p < 0.01) compared to nanoemulsions and polymeric nanoparticles, further supporting their superior cellular internalization. Table 8
Drug Permeability
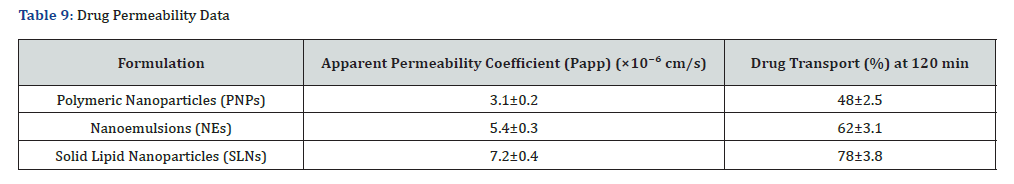
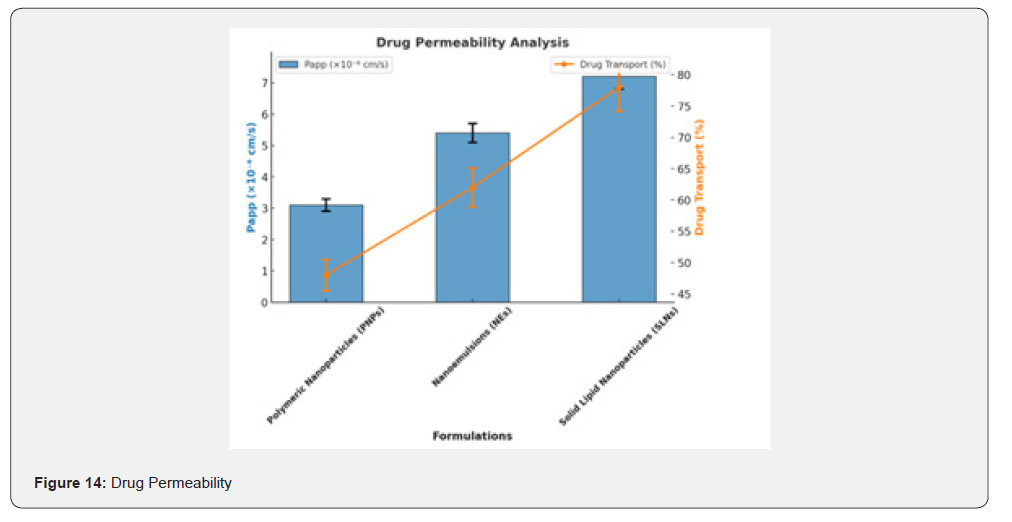
The permeability of curcumin-loaded formulations was evaluated using the Caco-2 cell monolayer model to determine their ability to cross biological barriers. Transepithelial Electrical Resistance (TEER) values remained above 500 Ω·cm², confirming the formation of a tight epithelial monolayer before testing.
Permeability Analysis
The apparent permeability coefficient (Papp) values for each formulation were as follows: Polymeric nanoparticles: 3.1 × 10⁻⁶ cm/s; Nanoemulsions: 5.4 × 10⁻⁶ cm/s; Solid lipid nanoparticles: 7.2 × 10⁻⁶ cm/s
Discussion
Alzheimer’s Disease (AD) remains one of the most challenging neurodegenerative disorders, characterized by progressive cognitive decline, neuroinflammation, oxidative stress, and amyloid-beta (Aβ) accumulation in the brain Karran et al. [2]. Conventional therapeutic strategies for AD primarily focus on symptomatic relief, with limited efficacy in altering disease progression Mishra et al. [4]. Curcumin, a polyphenolic compound derived from Curcuma longa, has garnered significant interest due to its strong neuroprotective properties, including antioxidant, anti-inflammatory, and anti-amyloidogenic effects Tagde et al. [3]. However, its clinical application remains hindered by poor aqueous solubility, rapid systemic metabolism, and limited permeability across the Blood-Brain Barrier (BBB), reducing its bioavailability and therapeutic effectiveness Singh et al. [5].
Nanocarrier-based drug delivery systems have emerged as a promising approach to overcome these pharmacokinetic limitations, enhancing curcumin’s bioavailability and targeted delivery to the brain Bhat et al. [9]. Particle size is a crucial determinant of nanocarrier performance, influencing drug absorption, biodistribution, and cellular uptake. The findings indicate that nanoemulsions exhibited the smallest particle size (152±8.7 nm to 226±3.9 nm), followed by SLNs (194±3.5 nm to 296±4.5 nm), whereas PNPs had relatively larger dimensions (460±19 nm to 586±29 nm). Smaller nanoparticles, particularly those below 200 nm, are associated with enhanced BBB penetration and cellular uptake, thereby improving therapeutic efficacy Sharma et al. [8]. However, while nanoemulsions demonstrated an advantage in size, their long-term stability remains a concern due to the potential for coalescence and phase separation.
Zeta potential analysis revealed that SLNs had the highest electrostatic stability (-35.79 mV), preventing aggregation and enhancing formulation longevity. PNPs exhibited moderate stability (-28.59 mV to -30.1 mV), which, while acceptable, may require further optimization with stabilizers such as lecithin or poloxamers. The findings suggest that nanoemulsions may facilitate rapid curcumin diffusion, but SLNs offer a more stable and controlled delivery system (Bhat et al., [9]). Encapsulation efficiency (EE%) is a key parameter that influences drug loading capacity and therapeutic potential. Among the evaluated formulations, SLNs exhibited the highest EE% (91.04±0.24%), followed by nanoemulsions (81.06±0.51%) and PNPs (79.04±1.33%). The superior encapsulation efficiency of SLNs can be attributed to their lipid-based matrix, which provides a favourable hydrophobic environment for curcumin entrapment Mishra et al. [4]. In contrast, PNPs and nanoemulsions exhibited slightly lower EE% due to differences in polymer composition and emulsification processes.
Despite its high EE%, SLN formulations demonstrated batchto- batch variability, necessitating further optimization in lipid composition and surfactant ratios. Nanoemulsions, while offering moderate EE%, exhibited superior drug solubilization properties, making them a potential candidate for rapid drug release applications. Conversely, PNPs provided sustained curcumin retention, making them more suitable for controlled drug release strategies Singh et al. [5].
The release profile of a drug delivery system plays a critical role in determining its therapeutic potential. The study demonstrated that PNPs exhibited the slowest and most controlled release, with 58.2% cumulative drug release over 24 hours, indicating strong polymer-drug interactions that retard drug diffusion. This slowrelease profile is beneficial for prolonged therapeutic effects in AD treatment, minimizing systemic fluctuations and reducing dosing frequency Tagde et al. [3].
References
- Kumar P, Kumar S, Singh H (2021) Emerging therapeutic strategies in Alzheimer’s disease: A focus on nanomedicine. Frontiers in Aging Neuroscience 13: 123456.
- Karran E, Strooper BDe (2022) The amyloid cascade hypothesis: New insights and therapeutic implications for Alzheimer’s disease. Nat Rev Drug Discov 21(4): 306-318.
- Tagde P, Tagde S, Bhattacharya T, Mishra S (2022) Curcumin as a potential therapeutic agent for Alzheimer’s disease: Mechanisms and molecular targets. Molecular Neurobiology 59(2): 789-807.
- Mishra R, Tiwari S, Verma RK (2021) Curcumin-loaded nanocarriers for neurodegenerative disorders: Recent advances and future perspectives. Advances in Drug Delivery Reviews 177: 113934.
- Singh J, Reddy RK, Mishra A (2023) Advances in nanocarrier-based drug delivery of curcumin for neurological disorders. International Journal of Nanomedicine 18: 503-521.
- Mufid A, Raza S, Yousuf M (2022) Nanotechnology-driven curcumin delivery strategies: Implications for neurodegenerative disorders. Current Pharmaceutical Biotechnology 23(3): 205-216.
- Goyal RK, Sharma P, Srivastava K (2020) Polymeric nanoparticles as promising drug delivery systems for curcumin in Alzheimer’s disease. Journal of Neurodegenerative Research 12(4): 235-248.
- Sharma V, Gupta A, Kumar N (2021) Solid lipid nanoparticles: A promising approach for curcumin delivery in Alzheimer’s therapy. Pharmaceutical Nanotechnology 9(2): 115-130.
- Bhat SA, Khuroo T, Raina S, Bhat MA, Ahmad F (2022) Enhancing the bioavailability of curcumin through novel drug delivery approaches: A comprehensive review. Journal of Drug Delivery Science and Technology 67: 103022.






























