The Prognostic Significance of Preoperative CA 19-9, Neutrophil / Lymphosit And Platelet / Lymphosit Ratio in Patients with Pancreatic Ductal Adenocarcinoma
Niyazi Gambarli1, Kursat Dikmen*2, Hasan Bostanci1, Ahmet Cagri Buyukkasap1, Mustafa Kerem1 and Ferit Taneri1
1Azerbaycan Onkoloji Hastanesi, Azerbaijan
2Department of General Surgery, Turkey
Submission: November 24, 2018;Published: December 11, 2018
*Corresponding author: Kursat Dikmen, Department of General Surgery, Turkey
How to cite this article: Niyazi G, Kursat D, Hasan B, Ahmet C B, Mustafa K, etc all. The Prognostic Significance of Preoperative CA 19-9, Neutrophil / Lymphosit And Platelet / Lymphosit Ratio in Patients with Pancreatic Ductal Adenocarcinoma. J of Pharmacol & Clin Res. 2018; 6(4): 555692. DOI: 10.19080/JPCR.2018.06.555692
Abstract
Background: It has been demonstrated that pro-inflammatory markers like neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) are prognostic factors for many cancers. In the study presented here our aim is to investigate the prognostic significance of NLR, PLR and CA 19-9 in patients with pancreatic ductal adenocarcinoma (PDAC).
Patients and Methods: Data from 118 patients diagnosed with PDAC between September 2011 and September 2016 were examined retrospectively. In the whole blood count of all patients in the preoperative period; neutrophil, lymphocyte, platelet counts, and CA19-9 levels were recorded. Demographic data about the patients, tumour location, tumour diameter, T and N stage, stage, perineural invasion, number of metastatic lymph nodes, surgical margins and survival times were examined. Log-rank test, Cox proportional hazards test and Kaplan Meier method were used for univariate, multivariate and survival analysis respectively.
Results: According to univariate analysis we have determined that 4 histopathological and 3 preoperative serum parameters have prognostic value for decreased survival times. Degree of poor differentiation (p=0.012), presence of perineural invasion (p=0.016), lymph node metastasis (p=0.019), surgical margin positivity (p=0.017), high NLR (p=0.001), high PLR (p=0.006) and low lymphocyte count (p=0.031) were associated with decreased survival times. According to multivariate analysis, we have found that poor differentiation (p=0.001), >2 cm tumour diameter (p=0.025), surgical margin positivity (p=0.014), high NLR (p=0.015) and high PLR (p=0.030) were independent risk factors for poor prognosis.
Conclusion: Higher NLR and PLR values are associated with decreased survival times in patients undergoing resection for PDAC and are independent prognostic parameters.
Keywords: Pancreatic Ductal Adenocarcinoma; Neutrophil/Lymphocyteratio; Platelet/Lymphocyte Ratio; CA19-9 Prognosis
Introduction
Pancreas cancers rank fourth among deaths due to cancer because of their high malignancy potential and inability to be diagnosed at an early stage [1]. Currently, although the only curative option is surgery only 20% of these tumours can be resected at the time of diagnosis [2]. In addition, 5year survival rates of even the operated patients are around 20-25% despite standard treatments due to a high risk of relapse [3,4]. Determining the prognosis of a tumour with such a high malignant potential is quite important. Clinicopathological factors like tumour diameter and differentiation, lymph node metastasis, presence of lymphovascular and perineural invasion and surgical margin status are being frequently used in determining the prognosis [5-7]. Yet, almost all these parameters used in determining the prognosis are known only after resection. Therefore, they cannot be used in estimating the prognosis before treatment. Thanks to advances in molecular pathology, although there are numerous studies showing that markers investigated to estimate the prognosis of pancreas cancer are successful, they cannot be routinely used because of their high cost [8-10]. Investigations for easy to use and reliable markers in determining pancreatic cancer prognosis particularly in the preoperative period are still continuing.
There are studies reporting that tumour progression can be predetermined through systemic inflammatory activation used by cancer cells, cancer proliferation and metastasis or regulation of angiogenesis [11,12]. Among these systemic inflammatory marker’s parameters like NLR, PLR, C-reactive protein or CA19-9 have been the subject of important studies [13-15]. While higher neutrophil infiltration around the tumour is associated with poor prognosis, lymphocyte infiltration around the tumour has been associated with good prognosis and thus the view that NLR is a reflection of systemic inflammation has gained acceptance [16]. NLR, PLR and CA19-9 are simple and widely used parameters utilized in estimating the prognosis of many cancers [17,18]. However, cut-off values of the markers evaluated in these studies display variability. Inability to establish an international common value for this reason limits their use. Moreover, CA19-9 is a tumour marker widely used for tumours of the gastrointestinal canal, but its low specificity as a tumour marker diminishes its value. Its elevation even in benign pathologies of the pancreatobiliary system limits its value as a single factor for diagnostic purposes or as an independent factor in estimating the prognosis. In this study our aim is to determine the importance CA19-9, NLR and PLR values in estimating the prognosis of disease in the preoperative period in patients diagnosed with pancreatic ductal adenocarcinoma.
Patients and Methods
Patients
Data from 196 patients histologically diagnosed with pancreatic ductal adenocarcinoma in Gazi University Medical Faculty General Surgery Clinic between September 2011 and September 2016 were retrospectively analysed. Patients with metastatic disease, distal bile duct tumours, ampulla vateri and duodenum tumours, with other malignancies of the pancreas like intraductal papillary mucinous cystic neoplasm and mucinous cystic adenocarcinoma, patients with clinical signs of infection at time of diagnosis, patients receiving neoadjuvant chemotherapy or radiotherapy, patients with history of malignancy and patients with a mortal course in the first 30 days postoperatively were excluded from the study. Finally 118 patients meeting the study criteria were included in the study. Pathological assessment of the resected specimens was performed according to the AJCC 7th edition guideline [19]. The period between the date the patients were diagnosed and their date of death or their date of last followup was determined as the survival time.
Data collection
Demographic details, hematologic parameters, operation type, histopathological features and survival data were collected by review of patients’ records, retrospectively. Peripheral blood was obtained at the time of diagnosis before surgery. Baseline NLR was calculated as neutrophil count divided by lymphocyte count and PLR was calculated as platelet count divided by lymphocyte count. The patients were categorised at the median value of NLR, PLR and CA19-9. NLR, PLR and CA19-9 values of 3.0, 140 and 122 were used as median cutoff values, respectively. In addition, clinicopathological features like patients’ age, sex, tumour localisation, tumour diameter, T and N stage, disease stage, presence of lymph vascular and perineural invasion, number of metastatic lymph nodes, surgical margin status and overall survival times were recorded.
Surgical Procedure
Preoperative assessment of all patients was performed by standart triphasic thin slice computed tomography examination. Patients with distant organ metastasis, tumour invasion to hepatic artery and/or superior mesenteric artery, and tumour thrombus in the superior mesenteric vein and portal vein were accepted as unresectable. Standard pancreaticoduodenectomy together with standard lymph node dissection were performed for tumours localised in the head of the pancreas or uncinate process. Postresection reconstruction procedure was performed by pancreatic jejunostomy, hepaticojejunostomy and gastrojejunostomy over the same bowel loop. In case the tumour was in the body or tail of the pancreas, distal pancreatectomy and/or splenectomy procedure was performed.
Postoperative Follow-Up
The patients were admitted to the surgical ward after 1night post-op follow-up in the intensive care unit. All patients were administered adjuvant chemotherapy and/or radiotherapy after discharge. The patients were followed up with thoracoabdominal computed tomography or PET CT exam, whole blood count and tumour markers (CEA and CA19-9) every 3 months in the first 2 years, at 6 monthly intervals in the following 3 years and once a year afterwards.
Statistical analysis
All statistical analyses were performed with IBM SPSS version 20.0 (IBM Co., Armonk, NY, USA). Continuous data were analysed using mean, median, standard deviation and 95% confidence interval (CI). Chi-square test or Fisher exact test were used for comparative analyses of categorical data. Log-rank test was used for univariate analysis and Cox proportional hazards test was used for multivariate analysis. Kaplan-Meier survival analysis method was used for analysis of survival data. P<0.05 values were accepted as statistically significant.
Results
The study group consisted of 118 patients with potentially curative resected PDAC (74 men and 44 women). The mean age at diagnosis was 63.4 years (median, 65 years; range, 29–90 years). We found a mean WBC count of 7.9 ± 3.1 106/ml, median neutrophil count of 5.3 (0.9-20) 106/ml, median lymphocyte count of 1.8 (0.3-3.9) 106/ml, median NLR of 3.0 (0.25-50.6), median PLR of 140.4 (5.5-460), and median CA19-9 of 122 (0.6-20000) IU/ ml. According to patient records, 99 pancreaticoduodenectomy (Whipple procedure) and 19 distalpancreatectomy were performed. Demographic and hematological data of all patients are shown in Table 1.
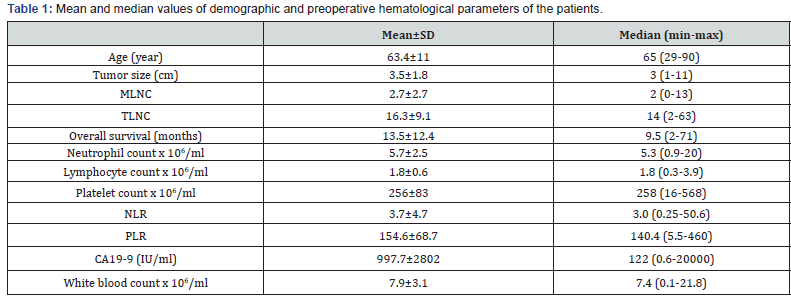
MLNC: Metastatic lymph node count; TLNS: Total lymph node count; NLR: Neutrophil/Lymphocyte ratio; PLO: Platelet/Lymphocyte ratio

1-, 3- and 5- year survival rates for all patients were 56.5%, 18.9% and 4.7% respectively. In total 21 out of 118 patients (17.7%) lived for more than 24 months. Median survival time for all patients was 20.4 months. Survival times were found higher in patients with lymphocyte count measured as 1.8 x 106/ml and above than in patients with a count below this value (P=0.031), but upon comparison of the groups according to higher and lower platelet and neutrophil counts the difference observed was not found to be statistically significant (P=0.154 and P=0.063, respectively). Survival times of patients with NLR<3 were found to be higher than of patients with NLR≥3 (P=0.001). Survival times of patients with PLR<140 were found to be higher than of patients with PLR≥140 (P=0.006). No statistical difference was found among other hematological serum parameters Table 2.
Survival times of patients with poorly differentiated tumour, presence of perineural invasion and lymph node metastasis were found to be statistically significantly lower (p=0.012, p=0.016 and p=0.019 respectively). Survival times of patients with positive post-resection surgical margin (R1) were also lower than of patients with negative surgical margin (R0) (p=0.017). Survival times of patients with NLO<3 and PLO<140 were found to be longer and this difference was statistically significant (p=0.001 and p=0.006 respectively) (Figure 1). Survival times of patients with lymphocyte count <1.8 x 106/ml were found to be statistically significantly lower compared to patients with lymphocyte count ≥1.8 x106/ml (p=0.031). Differences between patients’ survival times with respect to factors like age, sex, tumour localisation, perineural invasion, T stage, CA 19-9 level, and neutrophil and platelet counts were not statistically significant Table 3.

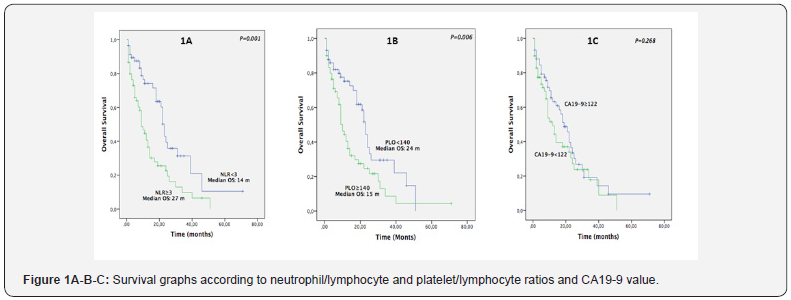
According to univariate analysis results we determined that 4 histopathological and 3 preoperative serum parameters had prognostic value for decreased survival times. Poor degree of differentiation, presence of perineural invasion, lymph node metastasis, surgical margin positivity, high NLR (≥3), high PLR (<140) and low lymphocyte count (<1.8 x 106/ml) were associated with poorer prognosis (Table 3). According to multivariate analysis results, we found that poor degree of differentiation, ≥ 2 cm tumour diameter, positivity of surgical margin after resection (R1), high NLR (≥3) and high PLR (≥140) were independent risk factors for poor prognosis (Table 3).
Discussion
Although the history of the relationship between cancer and systemic inflammation dates back nearly 100 years, number of studies endeavouring to establish the connection between tumour inflammation and prognosis and to understand tumour biology have further increased in recent years [20]. The relationship between cancer cells and systemic inflammatory response has been studied in many different cancer types. There are numerous studies investigating the role of inflammation on prognosis of pancreatic ductal adenocarcinomas in particular in whose aetiology chronic inflammation has been implicated [13- 22]. Systemic inflammatory response plays an important role in cancer progression [12]. Increased neutrophil infiltration and decreased lymphocyte infiltration around the tumour have been associated with poor prognosis. Especially in advanced stage pancreas cancers lymphocyte infiltration around the tumour is less than that in early stage pancreas cancers and this condition is associated with decreased survival times [23]. Moreover, it is known that lymphocytes induce cytotoxic cell death and inhibit tumour cell proliferation and tumour migration which play a significant role in the tumour defence mechanism [24].
Inflammatory process plays a critical role in phases of tumour development like invasion and metastasis. Cancer cells produce the myeloid growth factor which increase the production of neutrophils. It has been reported that increase in neutrophil count is associated with decreased survival especially in metastatic melanoma and lung cancers [25]. Thus, it has been investigated whether NLR can be a potential prognostic factor for pancreas cancer. In addition, it has been reported that platelet / lymphocyte ratio can also have a prognostic significance in pancreatic ductal adenocarcinomas and other cancer types. Since CA19-9 is a predictive marker that is easy to assess in the preoperative period like NLR and PLR and directly indicates tumour malignity and provides information about tumour grade and resectability, its relationship with pancreatic cancer has also been frequently investigated [26,27]. Although there are studies reporting positive results for all these parameters, no clear cutoff values have been determined for NLR, PLR and CA19-9. Due to limitations like the small number of patients in these studies, lack of a determined cut-off value and/or the heterogeneity of the data included in the study, they are not frequently used routinely. In order to clarify these uncertainties and to add to reports from various centres around the world and to contribute to the existing literature, in our study evaluating data from 118 patients undergoing resection for pancreatic ductal adenocarcinoma, the relationship of clinicopathological features and preoperative serum hematological parameters with survival has been assessed.
According to multivariable analysis results in our study survival times of patients with NLR ≥3.0 were found to be statistically significantly lower than those with NLR <3.0. Moreover, NLR was found to be inversely related to tumour diameter, degree of tumour differentiation, lymph node metastasis and resection margin positivity. Almost all parameters of prognostic significance for ductal adenocarcinomas of the pancreas are known after resection. Therefore, in pancreas cancers where the only curative option is surgery we think that NLR that can be easily obtained from routinely used serum measurements especially in the preoperative period is a simple method that can be used to assess tumour resectability and to predict post-resection survival. There are important limitations both in our study and in other studies investigating the prognostic significance of NLR [28,29]. Especially the lack of a clear cut-off value for NLR and data assessment without taking treatment strategies into consideration are among its limitations. In our study a high NLR was associated with poor prognosis just as in other studies. Contrary to studies reporting these positive results, Clark et al. [30] and Sanjay et al. [31] reported that high NLR did not have a prognostic significance. Yet, the number of patients in these studies was 44 and 51 respectively which was a serious disadvantage. Moreover, the data in these studies was heterogeneous, different statistical methods were employed and there were differences in inclusion and exclusion criteria. In the study conducted by Ben et al. all values from 1 to 6 were tried for NLR cutoff value and the most powerful difference was noted for 2 as an NLR cutoff value [32]. In the study by Ασαοκα ετ αλ. [33] positive results were reported for the mean NLR value of 2.7 [33].
A high NLR is possible with an increase in neutrophil count and/or a decrease in lymphocyte count. In our study while the median survival time was 26 months in patients with low neutrophil count (<5.3 x 106/ml), it was 16 months in patients with high neutrophil count (≥5.3 x 106/ml). But this difference was not statistically significant. Survival times of patients with a lymphocyte count of <1.8 x 106/ml were worse than those of patients with a lymphocyte count of ≥1.8 x 106/ml (16 and 25 months, respectively). Our study supports other studies showing that survival times are improved with tumour lymphocyte invasion or an increase in lymphocyte count in the circulation [30- 34]. Cancer cells are reported to decrease lymphocyte functions via production of immunological cytokines like IL-10 and transforming growth factor β [35]. Upon comparison of pancreatic ductal adenocarcinomas with other gastrointestinal tumours, the association between lymphocytopenia and survival has been found more significant in pancreatic ductal adenocarcinomas [36]. In our study the decrease in lymphocyte count rather than the increase in neutrophil count was held responsible for the elevated NLR. Since there are conflicting studies about the relationship between the changes in neutrophil or lymphocyte count alone and survival, we believe that NLR is a more significant parameter.
Higher preoperative CA19-9 levels may reflect the biologically aggressive behaviour of the tumour and presence of distant organ metastasis. There are studies reporting that CA19-9 is a direct predictive marker for tumour malignancy, stage and respectability. In our study the survival times of patients with a CA19-9 value < 122 U/ml and ≥122 U/ml were 22 and 17 months respectively but the difference was not statistically significant. Moreover, no significant difference was detected between stage and T stage and CA19-9. The reason for this is that the large majority of patients included in our study were at stage 2 (93.1%) and T3 (93.2%) and thus did not display a homogeneous distribution. In addition, elevation of CA19-9 values even in benign pathologies like obstructive jaundice, acute cholecystitis and tobacco use is a serious handicap.
The crucial roles of platelet activation through regulation of angiogenesis, degradation of extracellular matrix and release of adhesion molecules and some growth factors are well known in cancer development [37]. The interaction between tumour cell and platelet is a process that starts well before the adhesion of tumour cells to endothelial cells in areas far from the tumorous tissue. Platelet adhesion to tumour cells enables the release of vascular endothelial growth factor that induces vascular permeability, extravasation of cancer cells and new vessel formation [38]. Platelets increase matrix metalloproteinase-9 secretion in pancreatic adenocarcinoma cells and thus contribute to increasing the invasion capacity of tumour cells [39]. Also, in this study it was reported that drugs with anti-platelet properties inhibit the invasion capacity of tumour cells through decreased matrix metalloproteinase-9 secretion [39]. Due to this inhibition tumour cell invasion is suppressed. Moreover, proinflammatory cytokines like IL-1, IL-3 and IL-6 stimulate the proliferation of megakaryocytes known to be responsible for the production of platelets [40]. There are numerous studies investigating the prognostic significance of PLR in many cancer types [41]. Smith et al. [42] reported PLR to be an independent prognostic factor for pancreatic ductal adenocarcinoma. In that study for every unit increase in PLR the risk of death was increased 0.004-fold and they adopted 400 x 106/ml as the cut-off value for platelet count and 1.0 x 106/ml as the cut-off value for lymphocyte count. In another study conducted on 205 patients PLR cutoff value was adopted as 300 and no differences were reported with respect to survival results [43]. In our study survival times of patients with PLR ≥140 were found to be statistically significantly lower than those of patients with PLR <140.
The primary limitations of our study were its being a retrospective study and the small number of patients. Second, patient stratification according to treatment modalities was not performed in the patients included in our study. A third limitation might be the lack of a clear cut off value just as in other studies and making an assessment by applying the determined cut off value for NLR in a narrow range like 2.9 or 3.1 to different groups. Also, even if we do not include patients with active infection or another pathology expected to trigger inflammation in the study, changeability of NLR due to a non-specific reason such as stress renders the data in our study heterogeneous. In conclusion, among preoperative serum hematological parameters higher NLR and PLR values are associated with decreased survival in patients undergoing resection for pancreatic ductal adenocarcinoma and they are independent prognostic parameters. Preoperative NLR and PLR values are simple and easy to apply parameters that can be used in estimating disease prognosis in patients with PDAC. However, to be able to use them as more widespread and reliable parameters studies involving larger number of patients and having data determined according to more homogeneous criteria are warranted.
References
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2): 74-108.
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, et al. (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. The New England journal of medicine 350(12): 1200-1210.
- Moon HJ, An JY, Heo JS, Choi SH, Joh JW, et al. (2006) Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas 32(1): 37-43.
- Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, et al. (2004) Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. The British journal of surgery 91(5): 586-594.
- Kure S, Kaneko T, Takeda S, Inoue S, Nakao A,et al. (2005) Analysis of long-term survivors after surgical resection for invasive pancreatic cancer. HPB: the official journal of the International Hepato Pancreato Biliary Association 7(2): 129-134.
- Sierzega M, Popiela T, Kulig J, Nowak K (2006) The ratio of metastatic/ resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas 33(3): 240-245.
- Ueda M, Endo I, Nakashima M, Minami Y, Takeda K, et al. (2009) Prognostic factors after resection of pancreatic cancer. World journal of surgery 33(1): 104-110.
- Liu R, Chen X, Du Y, Yao W, Shen L, et al. (2012) Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clinical chemistry 58(3): 610-618.
- Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, et al. (2009) A compendium of potential biomarkers of pancreatic cancer. PLOS medicine 6(4): e1000046.
- Chung HW, Lim JB (2014) Clinical significance of elevated serum soluble CD40 ligand levels as a diagnostic and prognostic tumor marker for pancreatic ductal adenocarcinoma. Journal of translational medicine 12: 102.
- Hainaut P, Plymoth A (2013) Targeting the hallmarks of cancer: towards a rational approach to next-generation cancer therapy. Current opinion in oncology 25(1): 50-51.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5): 646-674.
- Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, et al. (2009) Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. American journal of surgery 197(4): 466-72.
- Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, et al. (2014) Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. British journal of cancer 110(1): 183-188.
- Wei Y, Jiang YZ, Qian WH (2014) Prognostic role of NLR in urinary cancers: a meta-analysis. PloS one 9(3): e92079.
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, et al. (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell 16(3): 183-194.
- Cho H, Hur HW, Kim SW, Kim SH, Kim JH, et al. (2009) Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer immunology, Immunotherapy CII 58(1): 15-23.
- Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, et al. (2014) Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. International journal of cancer 134(10): 2403-13.
- Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, (2010) AJCC Cancer Staging Manual. 7th ed editors. New York, USA.
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917): 860-867.
- Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ, et al. (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. Journal of surgical oncology 91(3): 181-184.
- Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y et al. (2007) The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 73(3-4): 215-220.
- Yamashita J, Ogawa M, Shirakusa T (1995) Free-form neutrophil elastase is an independent marker predicting recurrence in primary breast cancer. Journal of leukocyte biology 57(3): 375-378.
- Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, et al. (2012) Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and outlook. Cancer journal (Sudbury, Mass) 18(2): 160-175.
- Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, et al. (2009) Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. European journal of cancer 45(11): 1950- 1958.
- Dong Q, Yang XH, Zhang Y, Jing W, Zheng LQ, et al. (2014) Elevated serum CA19-9 level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: a pilot study. World journal of surgical oncology 12: 171.
- El Nakeeb A, El Shobary M, El Dosoky M, Nabeh A, El Sorogy M, et al. (2014) Prognostic factors affecting survival after pancreaticoduodenectomy for pancreatic adenocarcinoma (single center experience). Hepato-gastroenterology 61(133): 1426-1438.
- Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI et al. (2010) Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. American journal of surgery 200(2): 197-203.
- Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, et al. (2013) Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. British journal of cancer 109(2): 416-421
- Clark EJ, Connor S, Taylor MA, Madhavan KK, Garden OJ, et al.(2007) Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB: the official journal of the International Hepato Pancreato Biliary Association 9(6): 456-460.
- Sanjay P, de Figueiredo RS, Leaver H, Ogston S, Kulli C, et al. (2012) Preoperative serum C-reactive protein levels and post-operative lymph node ratio are important predictors of survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. Jop 13(2): 199-204.
- Ben Q, An W, Wang L, Wang W, Yu L, et al. (2015) Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas 44(3): 471-477.
- Asaoka T, Miyamoto A, Maeda S, Tsujie M, Hama N, et al. (2016) Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al] 16(3): 434-440.
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, et al. (2004) CD8+ tumor-infiltrating lymphocytes together with CD4+ tumorinfiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 28(1): e26-e31.
- Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, et al. (1999) Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. The American journal of pathology 155(2): 537- 547.
- Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, et al. (2006) Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas 32(1): 22-28.
- Egan K, Crowley D, Smyth P, O’Toole S, Spillane C, et al. (2011) Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PloS one 6(10): e26125.
- Sierko E, Wojtukiewicz MZ (2004) Platelets and angiogenesis in malignancy. Seminars in thrombosis and hemostasis 30(1): 95-108.
- Suzuki K, Aiura K, Ueda M, Kitajima M (2004) The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas 29(2): 132-140.
- Alexandrakis MG, Passam FH, Moschandrea IA, Christophoridou AV, Pappa CA, et al. (2003) Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. American journal of clinical oncology 26(2): 135-140.
- Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T (2012) Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. Journal of gynecologic oncology 23(4): 265-273.
- Dominguez I, Fernandez del Castillo C (2012) Preoperative plateletlymphocyte ratio in resected pancreatic ductal carcinoma: is it meaningful? American journal of surgery 203(3): 412.
- Dominguez I, Crippa S, Thayer SP, Hung YP, Ferrone CR, et al. (2008) Preoperative platelet count and survival prognosis in resected pancreatic ductal adenocarcinoma. World journal of surgery 32(6): 1051-1056.






























