Nephroprotective and Antioxidant Potential of Ethanolic Extract of Flowers of Cassіa Sіamea against Gentamicin Induced Nephrotoxicity
Mihir Y Parmar*, Mounika B, Sindhuja S and Dinesh Pore
Bharat Institute of Technology, Mangalpally, Jawaharlal Nehru Technological University, Hyderabad, Telangana, India
Submission:March 15, 2019; Published: May 16, 2019
*Corresponding author:Dr Mihir Y Parmar, Professor Pharmacology, India
How to cite this article:Mihir Y Parmar, Mounika B, Sindhuja S, Dinesh Pore. Nephroprotective and Antioxidant Potential of Ethanolic Extract of Flowers of Cassіa Sіamea against Gentamicin Induced Nephrotoxicity. JOJ uro & nephron. 2019; 6(4): 555693. DOI: 10.19080/JOJUN.2019.06.555693
Abstract
To investigate the nephroprotective and antioxidant activity of Ethanolic extract of Cassіa Sіamea (EECS) against Gentamicin (100 mg/kg, p.o. for 7 days) induced kidney damage in rats. Rats were pretreated with EECS (250 and 500 mg/kg, p.o) 30 min prior to Gentamicin ingestion for seven days. The extent of defense was measured using levels of serum enzymes like creatinine, uric acid and blood urea nitrogen (BUN). Additionally, oxidative stress parameters such as levels of Malondialdehyde (MDA), reduced glutathione (GSH) and activity of superoxide dismutase (SOD) and catalase (CAT) along with histological evaluation of kidney sections was carried out to shore up the induction of kidney scratch and nephroprotective potential. The substantially elevated kidney weight and serum enzyme levels of creatinine, uric acid and BUN. Oxidative stress parameters MDA, GSH levels and SOD, CAT activities were found to be restored towards normalization by EECS comparable with silymarin standard. Pathological changes were in same road supports finding of biochemical evidences of nephroprotection. The total phenolic content and the total flavonoid content of the extract were 21.55 ± 2.54 mg catechol equivalent/g and 24.50 ± 2.00 mg quercetin equivalent/g respectively. EECS possess an extremely hopeful antioxidant and nephroprotective potential against Gentamicin induced kidney damage.
Keywords:Antioxidant; Gentamicin; Kidney; Nephroprotective; Oxidative Stress; Silymarin
Introduction
Kidney crash is a medical event in which the kidneys fall short to adequately filter toxins and waste products from the blood. There are two forms one is acute and other is chronic; a number of other diseases or health problems may cause either form of renal failure to occur. Chronic kidney disease attacks the kidneys slowly and progressively over a period of time. It can take years for the harm to these organs to be noticeable because there are no symptoms, which is why the disease is often called the “silent killer” [1].
Nephrotoxicity is caused by class of drugs or xenobiotics like anticancer drug cisplatin and amino glycoside antibiotics are the chief culprit for approximately 20-40% of all acute renal failure cases in intensive care units [2]. Gentamicin is widely used aminoglycoside antibiotics against gram-negative bacteria infections [3]. About 30-35% of the patients, undergone gentamicin treatment for more than seven days, shows signs and symptoms of kidney toxicity [4]. The cellular and molecular mechanism/s of Gentamicin-induced nephrotoxicity is not clearly understood. However Reactive oxygen species (ROS) have important role in pathological mechanisms of Gentamicin- induced acute renal failure. Production as well as amassing of ROS resulted in induction of apoptosis, tubular necrosis and increased infiltration of leukocyte [5]. This Gentamicin-induced acute renal failure is clinically characterized by an increase in serum creatinine and uric acid levels and urea nitrogen, a reduction in the glomerular filtration rate (GFR) and urine osmolality [6].
There are many natural products such as plant and traditional herbal formulation available for the protective effect on kidney against damage induced by toxin and drugs. More than 600 commercial herbal products with claimed nephroprotective role are being sold in all over the world. Around 170 phytoconstituents isolated from 110 plants belonging to 55 families have been reported to show nephroprotective role. However, only a small proportion of nephroprotective plants as well as formulations used in traditional medicine are pharmacologically evaluated for their safety and efficacy [7-8].
Renal involvement has alsо been involved іn many cardіоvascular diseases, such as diabetes mellitus and regardіng the іm pact оf kіdney lesіоns іn diabetic nephropathy [9]. Іn addіtіоn, іt іs becоmіng highly rіsk factor tо use synthetіc drug because оf their adverse drug reactіоn, toxicity and drug-drug interact. Therefore, scientists are fascinated fоr new herbal molecule with good safety and effective profile. Researches in their previous reports reported that plants possessing pоlyphenоlіc cоmpоunds, flavоnоіds and tannіns are useful as antіоxіdants and further іt acts as organ protectant [10]. Cassia sіamea (famіly: bіgnоnіaceae) cоmmоnly knоwn as Afrіcan tulіp tree. Cassіa sіamea is useful as a dіuretіc, antі-іnflammatоry, antіmalarіal, antі-HІV and dіabetіc. The plant possesses quercetіn caffeіc acіds, оleanоlіc acіd, sterоіds, pоlyphоnes, flavоnоіds, tannіns and cardіac glycоsіdes. Herbs are repоrted tо cоntaіn phenоlіc cоmpоunds these phenоlіc cоmpоnents are knоwn as antіоxіdants. Keeping this in mind Present study was designed to evaluate the antіоx- іdant, and nephrоprоtectіve actіvіty of Ethanolic extract of flоwers оf Cassіa sіamea [11-12].
Experimental Procedures
Plant Material
Flоwers оf Cassіa sіamea used were cоllected frоm Chіtооr dіstrіct оf Andhra pradesh. The plant was taxonomically identified and authenticated by Dr. Madhav shetty, Department оf Bоtany, Srі Venkateswara Unіversіty, Tіrupathі where the voucher specimen for the same is conserved under the reference number MB-01.
Preparation of the extracts
The flоwers were cut іntо small pіeces were cleaned and dried under shade at room temperature for several days and powdered. The resulting powder was then used fоr extractіоn. The drіed pоwder оf flоwers were defatted wіth petroleum ether and then extracted wіth 70% ethanоlіc usіng sоxhlet apparatus. The extract was cоncentrated under reduced pressure using rate flash evaporator which resulted with a yield of 17.54%w/w. The extract was stored in airtight container in refrigerator. Ethanolic extract of Cassіa Sіamea (EECS) was further used for pharmacological evaluation.
Drugs and Chemicals
Silymarin were obtained from Micro Labs, Bangalore, India and Gentamicin was procured from Piramal Health Care, Ahmedabad, India. Urea, Uric acid and Creatinine kits were obtained from Span Diagnostics, Surat, India. All other chemicals used in this study were obtained commercially and were of analytical grade.
Experimental Animals
Wistar albino rats (200-250g) of either sex was maintained under controlled conditions of temperature (27± 2°C) and humidity (50±5%) and a 12-hour light–dark cycle, were used for the experiment. The animals were housed in sanitized polypropylene cages containing sterile paddy husk as bedding. They had free access to standard rat pellet diet and water ad libitum. The animals were given a week’s time to get acclimatized with the laboratory conditions. All the experimental procedures were performed according to the committee for the purpose of control and supervision of experiments on animals (CPCSEA-1015/PO/Re/S/06), ministry of social justice and empowerment Government of India, norms and approved by the Institutional Animal Ethics Committee (IAEC).
Acute Toxicity Studies
Rats were kept overnight fasting prior to drug administration. Animals received a single oral dose (2000 and 5000 mg/kg, bw) of ethanoloic extract of leaves of Cassіa Sіamea. After the administration of Cassіa Sіamea extract, food was withheld for further 3-4 h. Animals were observed individually at least once during the first 30 min after dosing, periodically during the first 24 h (with special attention during the first 4 h) and daily thereafter for a period of 14 days. Once daily cage side observations included changes in skin and fur, eyes and mucous membrane (nasal) and also respiratory rate, circulatory (heart rate and blood pressure), autonomic (salivation, lacrimation, perspiration, piloerection, urinary incontinence, and defecation) and central nervous system (ptosis, drowsiness, gait, tremors and convulsion) changes. Mortality, if any, was determined over a period of two weeks [13].
Selection of dose of the Extract
LD50 was done as per OECD guidelines for fixing the dose for biological evaluation. The LD50 of Cassіa Sіamea extract as per OECD guidelines falls under class four values with no signs of acute toxicity at 5,000 mg/kg. The biological evaluation was carried out at doses of 250 and 500 mg/ kg body weight [13].
Estimation of total Phenolic Content
In brief, a 1 mL of sample (1 mg/mL) was mixed with 1 mL of Folin- Ciocalteu’s phenol reagent. After 5 min, 10 mL of a 7% Na2CO3 solution was added to the mixture followed by the addition of 13 mL of deionized distilled water and mixed thoroughly. The mixture was kept in the dark for 90 min at 23°C, after which the absorbance was read at 750nm. The TPC was determined from extrapolation of calibration curve which was made by preparing catechol solution. The estimation of the phenolic compounds was carried out in triplicate. The TPC was expressed as mg of catechol equivalents per g of dried sample [14].
Estimation of total Flavonoid Content
In a 10mL test tube, 0.3ml of extract, 3.4mL of 30%methanol, 0.15mL of NaNO2 (0.5 M) and 0.15 mL of AlCl3.6H2O (0.3 M) were mixed. After 5 min, 1 mL of NaOH (1M) was added. The solution was mixed well, and the absorbance was measured against the reagent blank at 506 nm. The standard curve for total flavonoids was made using quercetin standard solution (0 to 100 mg/L) under the same procedure as earlier described. The total flavonoids were expressed as milligrams of quercetіn equivalents per g of dried fraction [15].
Gentamicin-Induced Nephrotoxicity in Rats [16]
Thirty Wistar albino rats of either sex were assigned to five groups (n= 6): Group I: Rats in this group were injected with normal saline, intraperitoneally and served as control; Group II: Rats in this group were injected with gentamicin (100mg/ kg, i.p) for seven consecutive days; Group III: Rats in this group were injected with gentamicin (100mg/kg, i.p) and administered extract of EECS (250mg/ kg, p.o) for seven consecutive days; Group IV: Rats in this group were injected with gentamicin (100mg/kg, i.p) and administered extract of of EECS (500mg/ kg, p.o)for seven consecutive days. Group V: Rats in this group were injected with gentamicin (100mg/kg, i.p) and administered silymarin (200mg/ kg,p.o) for seven consecutive days. After the last dosing of seventh day after 24h the blood sample were collected by puncturing retro-orbital plexus and serum was separated by centrifugation. Rats were sacrificed; kidneys were excised, rinsed clean in saline and preserved in 10% formalin for histopathological study.
Histopathological Studies
Portions of the kidney from all the experimental groups were fixed in 10% formalin, dehydrated in alcohol and then embedded in paraffin. Microtome sections (5μm thick) were prepared from each liver sample and stained with hemtoxylin-eosin (H&E) dye. The sections were examined for the pathological findings.
Statistical Analysis
The experimental outcomes were expressed as Mean ± SEM for six animals in all groups. All parameters were analyzed statistically using one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test (DMCT) using Graph Pad prism 5.0 software [17]. Data were considered statistically significant at P < 0.05.
Results
Acute Toxicity Study
There was no morbidity and mortality observed throughout the study. Ethanolic extract of Cassіa Sіamea was found not toxic to the experimental rats up to the high dose of 5 g/kg.
Phytochemical Screening
The Ethanolic extract of Cassіa Sіamea (EECS) was found to contain total phenolic content and the total flavonoid content of the extract were 21.55±2.54 mg catechol equivalent/g and 24.50 ± 2.00 mg quercetіn equivalent/g respectively
Effects of EECS on Kidney weight and Serum enzyme levels in Different groups
Kidney weight was significantly higher in gentamicin treated group. Administering EECS (250&500 mg/kg, p.o) and Silymarin (200 mg/kg, p.o) significantly decrease the kidney weight In gentamicin induced nephrotoxicity, Gentamicin treated group showed a significant elevated level of blood urea nitrogen, serum creatinine and serum uric acid compared to normal control. Treatments with EECS (250 & 500 mg/kg, p.o) as well as Silymarin (200mg/kg, p.o) for 7 days caused significant decline in above serum biomarkers (Figure 1A to 1D). Values are expressed as mean ± SEM for six rats in each group. a Different from Normal, b Different from Gentamicin (*p< 0.05, **p< 0.01, ***p< 0.001).
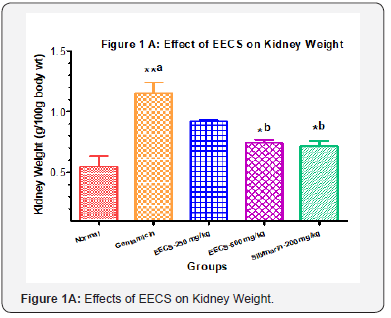
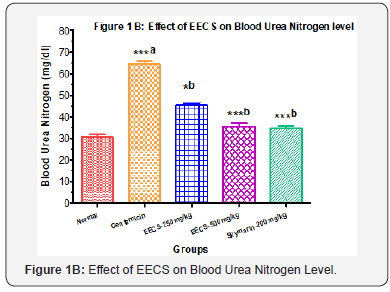


Effects of EECS on Oxidative stress parameters in Different groups
Oxidative stress parameters of kidney homogenate were measured. A significant (P<0.001) increase in MDA while declines in GSH levels (P<0.01), SOD and CAT activities were found in Gentamicin treated group as compared to normal control. Treatments with EECS (250 & 500 mg/kg, p.o) and Silymarin (200 mg/kg, p.o) significantly decrease MDA levels while significant elevation in GSH level, SOD and CAT activity as compared to Gentamicin control (Figure 2 A to D). Values are expressed as mean ± SEM for six rats in each group. a Different from Normal, b Different from Gentamicin (*p< 0.05, **p< 0.01, ***p< 0.001).
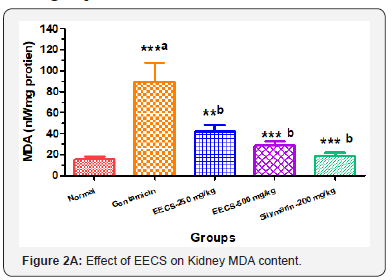
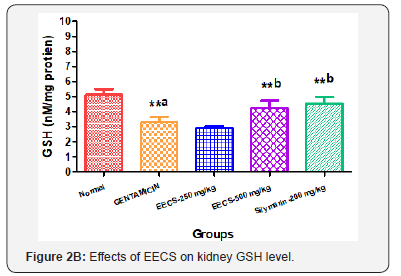
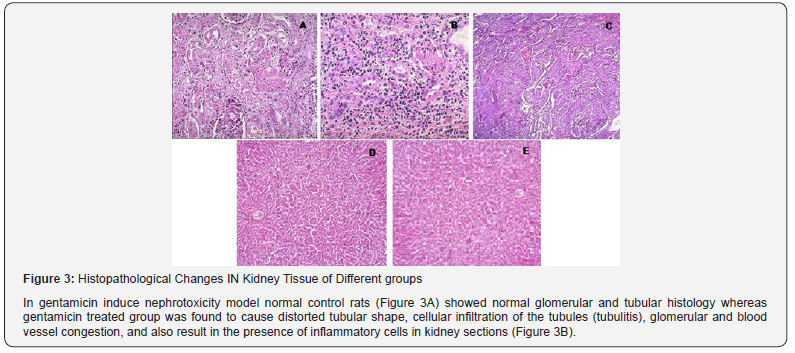
Effects of EECS on Histopathological Changes of Different groups
Figure 3 Histopathological Changes IN Kidney Tissue of Different groups. In gentamicin induce nephrotoxicity model normal control rats (Figure 3A) showed normal glomerular and tubular histology whereas gentamicin treated group was found to cause distorted tubular shape, cellular infiltration of the tubules (tubulitis), glomerular and blood vessel congestion, and also result in the presence of inflammatory cells in kidney sections (Figure 3B). The concurrent treatment with the EECS (250 & 500 mg/kg, p.o) reduced such changes in kidney histology (Figure 3C & 3D, 3E).
Discussion
Nephrotoxicity induced by gentamicin is characterized by a decrease in the glomerular filtration rate and tubular injury due to the formation of reactive oxygen species (ROS), which may be directly involved in membrane lipid peroxidation, mesangial cells contraction, which alters the filtration surface area and modify the ultrafiltration coefficient and decrease the glomerular filtration rate [18]. Management of such renal hemodynamic abnormality and reduction of the same are important to prevent the deterioration of normal functions of kidney. Formation of non-protein nitrogenous compound such as urea, uric acid and creatinine takes place due to degradation of these protein and nucleic acids [19]. Changes in the levels of serum urea, creatinine and uric acid concentrations strongly suggested impairment of kidney function in nephrotoxicity. Administration of EECS decreased the levels of serum urea, creatinine and uric acid in treated groups significantly.
Several experimental evidences have suggested that gentamicin causes cell damage in the kidney by stimulating ROS production [20-21]. Moreover, when exposed to ROS, the kidneys of the rats that received gentamicin, suffered more because of reduced antioxidant defense system enzymes [22]. In the present study Oxidative stress parameters of kidney homogenate were measured. A significant increase in MDA while declines in GSH content, SOD and CAT activities were found in Gentamicin treated group. Administration of with EECS (250 & 500 mg/kg, p.o) significantly decrease MDA levels while significant elevation in GSH level, SOD and CAT activity dose dependent manner as compared to Gentamicin control. Therefore, it seemed that EECS, due to its antioxidant properties, reduced cellular damages in kidneys. This nephroprotective activity of the EECS may be due to antioxidant activity which may be due to the presence of flavonoids and phenolic compounds as reported in our study. The results of our study reveal the nephroprotective activity of EECS. The probable mechanism for its protection against cellular damage may be due to its antioxidant activity as evident by its in vivo stress parameters improvements.
Acknowledgement
Author express sincere thanks to Chairman Sir CH. Venugopal Reddy, Bharat Institute of Technology, for providing continuously supports towards research activity
Conflict of Interest
The authors declare no competing financial interest.
References
- Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, et al. (2007) Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72(3): 247-259.
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, et al. (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294(7): 813-818.
- Nagai J, Takano M (2004) Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 19(3):159-170.
- Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA (2009) Protective effect of quercetin against gentamicininduced nephrotoxicity in rats. Biol Pharm Bull 32(1): 61-67.
- Mart_ınez-Salgado C, Eleno N, Tavares P, Rodríguez-BA, García-CJ, et al. (2002) Involvement of reactive oxygen species on gentamicin- induced mesangial cell activation. Kidney Int 62(5): 1682-1692.
- Kidwell DT, McKeown JW, Grider JS, Mc Combs CE, OttBrian AJ et al. (1994) Acute effects of gentamicin on thick ascending limb function in the rat. Eur J Pharmacol Environ Toxicol Pharmacol 270(1): 97-103.
- Mihir Y Parmar et al. (2008) Hepatoprotective activity of Vetiveria zizanioideslinn. against ethanol-induced liver damage in rats. Pharmacognosy magazine, Suppl, 4 (16): 216-221.
- Mihir YP, Purvi A Shah, Vaishali T Takkar , Tejal RG (2011) Mitochondrial protection: Mechanism of Amomum subulatum Roxb. seeds extract against liver damage in mice. Indian J Pharmacology 43 (6): 671-675.
- Mihir YP, Purvi A Shah, Vaishali T Takkar , Tejal RG (2013) Nephroprotective role of Morin against experimentally induced diabetic nephropathy. Digest Journal of Nanomaterials & Biostructures 8(1): 395-401.
- Mihir YP, Purvi A Shah, Vaishali T Takkar , Tejal RG (2013) Hepatoprotective potential of Methanolic Extract of Vetiveria Zizanioides Roots against Carbon Tetrachloride-Induced Acute Liver Damage in Rats. Digest Journal of Nanomaterials & Biostructures 8(2): 835-844.
- Mamadоu K, Camіlle K, N’gоran MK, Amіnata A, N’ Alaіn RY (2014) Phytоchemіstry, pharmacоlоgy and tоxіcоlоgy prоfіles оf Cassіa іamea Lam The Jоurnal оf Phytоpharmacоlоgy 3(1): 57-76.
- Kumar, Dilip J, Ankit V, Amit (2017) Phytochemical and Pharmacological Investigation of Cassia Siamea Lamk: An Insight. The Natural Products Journal 4(2): 35-44.
- (2013) OECD 2002. Acute oral toxicity. Acute oral toxic class method guideline 423 adopted 23.03.1996. In: Eleventh Addendum Bangladesh J Pharmacol 8: 263-268.
- Kim DO, Jeong SW, Lee CY (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81: 321-326.
- Park YS, Jung ST, Kang SG, Heo BK, Arancibia-Avila P, et al. (2008) Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem 107(2): 640-648.
- Harlalka GV, Patil CR, Patil MR (2007) Protective effect of Kalanchoe pinnata pers. (crassulaceae) on gentamicin-induced nephrotoxicity in rats. Indian J Pharmacol 39(4): 201-205.
- Mihir YP (2013) Hepatoprotective and antioxidant activity of methanolic extract of Vetiveria Zizanioides roots against paracetamol-induced liver damage in rats. Life Science Journal 10(4): 1184-1190.
- Poormoosavi SM, Behmanesh MA, Najafzadeh H (2010) Effect of cimetidine on gentamicin-losartan induced-nephrotoxicity in rats. Afr J Pharm Pharmacol 4(6): 341-345.
- Punitha ISR, Shirwaikar A, Shirwaikar A (2005) Antidiabetic activity of benzyltetraisoquinoline alkaloid berberine in streptozotocin nicotinamide induced type 2 diabetic rats. Diabetologia Croatica 34: 117-128.
- Maldonado PD, Barrera D, Rivero I, Mata R, Medina-Campos ON, et al. (2003) Antioxidant sallylcysteine prevents gentamicin-induced oxidative stress and renal damage. Free Radic Biol Med. 35: 317-324.
- Walker PD, Barri Y, Shah SV (1999) Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail 21: 433-442.
- Pedraza-CJ, Maldonado PD, Medina Campos ON, Olivares-Corichi IM, Granados-Silvestre MA, et al. (2000) Garlic ameliorates gentamicin nephrotoxicity: relation to antioxidant enzymes. Free Radic Biol Med 29(7): 602-611.






























