Illusive Dynamic Nadirs and Masks of Postoperative Hyponatraemia and the TURP Syndrome: Volumetric overload over time (VO/T) concept for resolving its puzzle
Ahmed N Ghanem1*, Salma A Ghanem2, Khalid A Ghanem3, Nisha Pindoria4 and Yasmina Saad Elsayed5
1Jasmine Tower, President Mubarak Street, Mansoura 35511, Egypt
2Barts & The Royal London NHS Trust, Whitechapel, London
3Mansoura University Hospital, Mansoura 35511, Egypt
4 North Middlesex University Hospital, Sterling Way, London
5Mansoura University Hospital, Mansoura 35511, Egypt
Submission: November 3, 2018; Published: March 05, 2019
*Corresponding author:Ahmed N Ghanem MD, Retired Consultant Urological Surgeon, FRCS, Mansoura University, Egypt
How to cite this article:Ahmed N G, Salma A G, Khalid A G, Nisha P, Yasmina S E. Illusive Dynamic Nadirs and Masks of Postoperative Hyponatraemia and the TURP Syndrome: Volumetric overload over time (VO/T) concept for resolving its puzzle. JOJ uro & nephron. 2019; 6(4): 555691. DOI: 10.19080/JOJUN.2019.06.555691
Abstract
Introduction and objective: Postoperative hyponatraemia (HN) cause serious morbidity and mortality, of which the transurethral resection of the prostate (TURP) syndrome is a unique model. Clinical presentation is circulatory shock and multiple vital organ dysfunction/ failure (MVOD/F) or death. All severe cases were reported retrospectively and attributed to multiple toxic/ dilution hypotheses interchangeably with recognized clinical conditions. The overlooked VO/T causes dynamic HN nadirs and masks making it a complex clinical and biochemical jigsaw puzzle. The objective here is to resolve this puzzle.
Patients and methods: Investigations based on clinical observations, critical literature analysis, physics-physiological and clinical prospective studies done over the past 32 years. Observations and deductive analysis identified volumetric overload over time (VO/T) insult, HN nadirs, clinical paradoxical masks of shock and MVOD/F. Prospective studies verified and quantified VO/T insult causing secondary, tertiary HN nadirs and osmotic gaps. Severity depends on VO/T and fluid type of sodium-free (VO1) and sodium-based (VO2) fluids.
Results: “VO/T” causes the biochemical and clinical features of HN and the TURP syndrome. Dilution HN “shock” and “VO” concepts were reported but VO insult remained invisible. The puzzle was resolved after unraveling the dynamic role of T in HN nadirs. 3.5L of VO1 infused in 1h causes HN shock and MVOD/F syndrome. The immediate postoperative secondary HN nadir is proportional to VO1 and clinical severity but shock mask is confused with haemorrhage or sepsis shocks. The late tertiary HN nadir is disproportional to both as osmotic fluid shift fluid into cells “Missing VO”. Cell oedema and necrosis confuses VO1 with cerebral or cardiac infarction. Inappropriate therapeutic response with “aggressive vascular expansion” erases HN, makes shock irreversible and establishes MVOD/F.
Conclusion: The concept of “VO/T’ insult explains the aetiology of HN and the TURP syndrome and pathophysiology of HN nadirs unveiling its paradoxical presentation masks and refuting dilutional and toxic hypotheses. It exposes the biochemical, clinical and therapeutic illusions, resolving HN puzzle and has paved the way to identify “VO shocks”, “optimize” fluid therapy and precise life-saving hypertonic sodium therapy. The new capillary-interstitial hypothesis based on G tube dynamic makes resolving MVOD/F and ARDS puzzle neither difficult nor distant.
Keywords: Hyponatraemia; The transurethral resection of the prostate (TURP) syndrome; The adult respiratory distress syndrome (ARDS); The multiple vital organ dysfunction/failure (MVOD/F) syndrome; Volumetric overload shocks (VOS); Hypertonic sodium therapy
Abbreviations: HN: Hyponatraemia; TURP: The Trans-urethral Resection of the Prostate Syndrome; HST: Hypertonic Sodium Therapy; VO: Volumetric Overload; VOS1: VO Shock of Sodium-Free fluids or ‘Type1’; VOS2: VO Shock of sodium-based fluids or ‘Type2’; T: Time; MVOD/F: The Multiple Vital Organ Dysfunction/ Failure syndrome; SSC: Serum Sodium Concentration; IVI: Intravenously Infused Fluids; ARF: Acute Renal Failure; ECF: Extra-Cellular Fluid Compartment; IVF: Intra-Vascular Fluid Compartment; ICF: Intracellular Fluid Compartment; ECF: Extra-Cellular Fluid; BW: Body Weight (BW) Percent BW; H: Hour (h) L: Litre; SC: Symptomatic Cases; NSC: None Symptomatic Cases; A, B, C, D Times Refer to Hospital Admission, Preoperative, immediate 1h and 24h Postoperative, respectively; CVP: Central Venous Pressure
Introduction
This report concerns hospital-induced hyponatraemia (HN) particularly the postoperative of which that complicating the transurethral resection of the prostate (TURP) is well known syndrome. Of historical interest, in 1913, Rowan tree [1], reported acute water intoxication. In 1946, Danawiski et al. [2], reported HN shock and its hypertonic sodium therapy (HST) in dogs. In 1948, Creevy [3] reported post TURP reaction induced by the absorption of water irrigant causing haemolysis. Since the introduction of osmotic electrolyte-free irrigant, haemolysis has no longer been seen in clinical practice. In 1956, Harrison et al. [4], first reported the TURP syndrome as dilutional HN shock and the successful use of HST.
The absorption of 1.5%Glycine irrigant is well known to induce the TURP syndrome but was not quantified and rarely reported. All cases of hospital postoperative HN are likewise induced by a sodium-free fluid such as 5%Glucose but rarely incriminated. In 1987, Arieff [5] reported a unique article, alluding to 8 liters of gained 5% Dextrose. In 1990, Ghanem and Ward [6], identified and quantified the precise volumetric overload (VO), contributed by both irrigant absorption and intravenously infused (ivi) fluids, as the real insult. The concept of VO was unimaginable cause of shock previously as it contradicted received concepts based on faulty physiological law that dictates the rules on fluid therapy. New reported discovery of hydrodynamic phenomenon of porous orifice (G) tube addressed this issue with reference to capillary and circulatory haemodynamic under physiological and pathological conditions [7].
Hyponatraemia is the most common biochemical abnormality in clinical practice causing serious morbidity and mortality among men [4,6,8], women [5,9,10] and children [11,12]. ‘The incidence of postoperative HN is about 1%, or 250,000 cases among the roughly 25 million inpatient operations that are performed each year, in USA [8]. It is of great surgical and medical interest that causes much international concern and anxiety. It lacks a definition. pathos-physiological etiology is unknown, therapy is disputed, and prognosis is poor [8-12]. The diagnosis is extremely difficult and differential diagnosis is enormous. It is huge biochemical and clinical jigsaw puzzle with false and missing pieces. Resolving such puzzle required identification of causative insult and factors making it illusive.
It is of note that severe to lethal cases of HN [5,8-12] and TURP syndrome [4,6,13] were reported retrospectively. Serum sodium concentration (SSC) measurement introduced into clinical practice after World War II [14] coinciding with the wide clinical use of ivi fluid therapy [15,16]. HN became a clinical diagnosis soon later [17]. It is <70 years old. During this period few studies have added key issues, hundreds of authors contributed most puzzle pieces while thousands of prospective studies repeatedly added little but fueling the debates.
In order to understand the disease and resolve debates on patho-physiology and management much more than analyzing hundreds prospective studies and doing some was required. Critical literature review, deductive analysis and drawing analogy kept the main picture in focus while fitting correct pieces and identifying the stepping-stones. The false and missing pieces had to be segregated then rejected and discovered, respectively. Clinical observations identified the insult and its paradoxes, illusive nadirs and presentation masks, before precise quantification using prospective clinical [6] and scientific physics studies [7].
The TURP syndrome is generally thought ‘well known, rare, obscure and limited to urology’ but it was foreseen as unique model for resolving its own puzzle and that of postoperative HN. The bottom line for understanding the condition concerns: how much volume, of what type and during what time a fluid gained access into vascular system, and what is immediate haemodynamic effects and delayed clinical masks? The TURP syndrome is unique because all quantifiable VO occurs during surgery of less than one hour (1h) [6,13].
The stepping-stones of ‘shock, HST [2,4] and anoxia [5]’ led through the difficulties to new understanding of “VO” as the real causative insult, were reported [4,6] years ago. However, the puzzle has not been resolved yet. Multiple 21 dilutional and toxic hypotheses [13] used interchangeably, and in combinations with recognized clinical conditions [5,8-12], for pathological explanation [6] testify that HN has remained illusive. The concept of shock [2,4] was affirmed not to be due to any of the unduly incriminated haemorrhage [18,19] or sepsis [20] shock. Vascular shock and acute renal failure (ARF) were observed paradoxical effects of VO and the analogy with MVOD/F was reported [6]. MVOD/F was originally reported as adult respiratory distress syndrome (ARDS) [21]. Other authors [13] affirmed our data on incidence, quantity of absorbed irrigant and blood loss in the absence of sepsis, hypothermia and hypoxaemia. However, the concept of “VO/T” has remained invisible so has the debateresolving new advances on the subjects [6,7].
The role of VO and its type of sodium-free (VO1) and sodiumbased fluid (VO2) in inducing the secondary and tertiary HN nadirs and illusive clinical masks, taking time (T) into account, was a scientific challenge that took years to unravel. Presenting with paradoxical hypotension shock and ARF to surgeons and encephalopathy coma [5,8-12] to physicians are a few among many bizarre illusive clinical masks of HN, based on which analogy to MVOD/F was made [6]. The objective of this report is resolving the puzzle of HN and TURP syndrome, highlighting the invisible “VO/T” insult and identifying dynamic HN nadirs and its illusive clinical paradoxes and presentation masks that may also help to resolve the puzzle of MVOD/F or ARDS.
Methods
Investigations included clinical observations, critical literature review with deductive analysis plus physics and clinical prospective studies done over the past 33 years. The majority of puzzle pieces contributed by many authors and stepping-stones that led to new understanding were gathered and aptly fitted, segregating false and missing pieces for rejection and discovery. Clinical observations, analysis and drawing analogy identified the real insult and factors causing paradoxes, nadirs and masks of HN.
Prospective clinical study quantified the “VO” insult, its biochemical and clinical effects but it took years to unravel the dynamic role of T causing HN to be so illusive. Physics studies discovered a hydrodynamic phenomenon that challenges the law on capillary-ISF transfer. The studies were continuous process of going back and forth between mentioned methods, using appropriate statistics tools and mathematics to achieve the objective imposed by the scientific challenge. The data is based on MD Theses accepted at the Institute of Urology, Mansoura University, Egypt, 1988. Overlapping with previous reports is minimal unavoidable necessity for clear understanding required for resolving the puzzle of HN and the TURP syndrome.
Clinical Observations
The most important clinical observations were: First, accepting that shock and annuria are real clinical features of VO though paradoxical to received concepts. Second, despite giving the standard shock therapy of ‘aggressive vascular expansion’, using VO2 fluids that elevated SSC and corrected HN, the vascular shock persisted becoming irreversible with full blown picture of MVOD/F” or death! Third, the received concept of elevating central venous (CVP) pressure to a level of 18 cm water or higher during the management of hypotension shock is inconsistent with its normal value of around 0 with a range of -7 to +7 cm water. Fourth, if arterial blood pressure is truly responsible for capillary-ISF filtration, as the current law indicates, why is oedema never seen clinically to complicate the most common arterial hypertension, and why does massive extra-vascular fluid shift occur during shock therapy despite hypotension? This is of vital relevance to the observed shock and anuria of “VO/T” insult, causing cell oedema and MVOD/F.
Hyponatraemic Nadirs
HN nadir is the lowest drop of SSC induced by a given sodiumfree VO1. A single bolus of VO1 induces a dynamic dilutional HN nadir that depends on the diluted body fluid compartment and T, inducing 3 different values: the primary, secondary and tertiary. The primary HN nadir refers to a situation when all gained VO1 dilutes IVF only. VO1 infusion done and SSC measured within minutes makes it is so rapidly transient and practically impossible to detect with massive VO to be of any practical clinical significance. The secondary HN nadir is that occurring in the immediate postoperative period that defines HN of the TURP syndrome. It occurs when all gained VO1 pervades extra-cellular fluid (ECF), causing the lowest SSC, measured within <2h of VO. The tertiary HN nadir is the most frequently detected drop of SSC at 24h or later that also reflects ECF dilution. Being T dependent, it is most dynamic and illusive, revealing only part of the gained VO1, after osmotic fluid shift into cells or intracellular fluid (ICF). The interaction of factors inducing and defining dynamic HN nadirs and illusive clinical masks are presented.
Results
Per-operative VO includes by both absorbed irrigant and ivy fluids, of which VO1 induced by 1.5% Glycine irrigant and 5%Glucose fluids while VO2 is induced by saline-based fluids.
VO Insult
Data of prospectively studied 100 TURP patients showed direct proportional relationship of VO1 to the immediate postoperative drop of SSC (Figure 1). VO was the most significant factor to clinical signs (P=0.0007). Summary of VO according to fluid type is shown in (Figure 2). Hypo-osmolality was less significant (p=0.0212) and HN was not (p=0.0597), the latter became significant only after removing VO from the multiple regression analysis. Mean VO of symptomatic cases was 3.5l, of which one liter was saline and 2.5l were VO1 fluids, 0.5l was 5% Dextrose and 2l was 1.5% Glycine gained during the TURP surgery of 1h duration.
Figure 3 demonstrates the diluted serum solute contents shown as % of normal preoperative value at the immediate postoperative period (C). It demonstrates diluted serum contents are common affecting symptomatic cases (SC) of 10% of patients and none symptomatic (NSC) cases. Secondary HN nadir is not only proportional to VO1 but also clinical severity at C time. It is of note that the drop of hemoglobin and albumin is dilutional, not due to blood loss. Precise blood loss was measured, and sepsis was excluded by negative urine and blood cultures in our prospective study. All diluted serum solutes were unduly incriminated in dilutional hypotheses while VO1 has remained invisible. The rise of SSC elevating HN nadir at 24h is either physiological due to urine and insensible water loss or pathological due to osmotic fluid shift into cells. Hence, the tertiary HN nadir may be misleading at D time as it does not reflect the real massive VO1, making HN appear disproportional to the clinical severity. The real VO1 and clinical severity remain to match the earlier secondary HN at C.
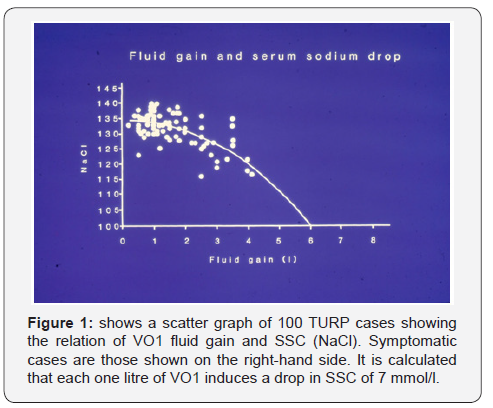
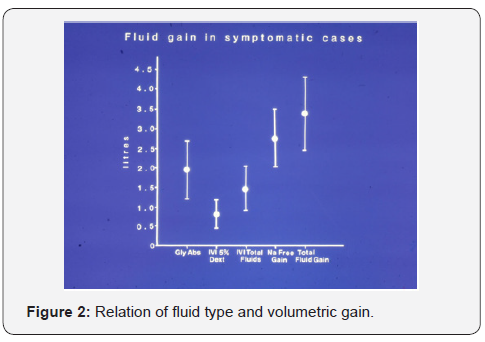
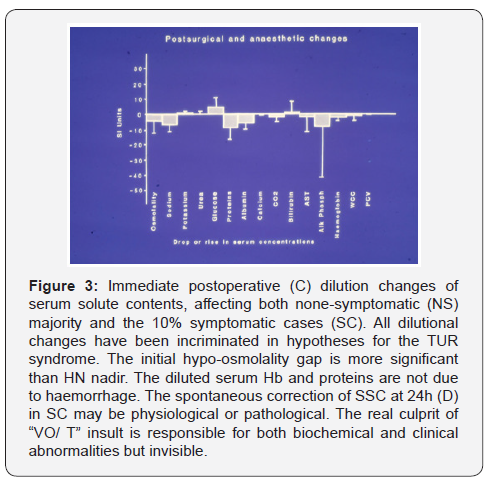
Figure 4 demonstrate the rise of serum glycine, reflecting VO1 of the absorbed 1.5% Glycine gained per-operatively. The rise of serum Glycine at C returned spontaneously to normal within 24h at D time without any specific therapy. All serum solute changes whether drop or rise seemed to move towards normal at 24h but it is difficult to tell whether such dynamic change is physiological or pathological correction.
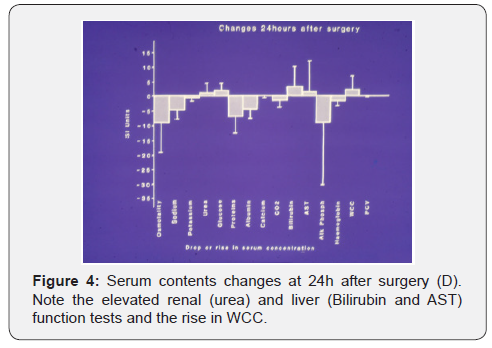

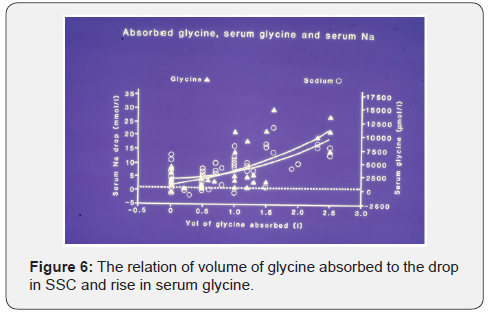
The rise of renal and hepatic function tests, and white cell count, affecting only SC occurred most prominently at D, reversing the initial dilutional drop affecting all patients at C. The rise of SSC from secondary to tertiary HN nadir is thus pathological in the presence of annuria of ARF. Glycine rise (Figure 4) and its metabolites of ammonia and oxalates were incriminated as toxic causes of TURP syndrome. Ammonia may be detected only when hepatic dysfunction becomes failure as part of MVOD/F at D. Cardiac, cerebral, renal, hepatic and respiratory dysfunction or failure has its own clinical methods of detection. Figure 5 shows the serum solutes changes at D. Note the rise in renal and liver function tests and WBC. Figure 6 correlates the drop in SSC and rise in serum glycine to the volume of Glycine absorbed.
The most important factors affecting dynamic HN nadirs and its clinical masks are observed. Each litre of VO1 causes a drop of 7 mmol/l in SSC causing HN nadir at C (Figure 1). The secondary HN nadir at C is based on mean SSC. The tertiary HN nadir at D and the “Missing VO” that shifted into ICF during the adaptation period are observed. The matching clinical severity is segregated into haemodynamic disturbance, MVOD/F of cardiac, cerebral and respiratory organs, and renal response. Any additional VO2 fluids may erase HN nadirs but worsen VO and establishes MVOD/F. A secondary HN nadir that becomes a tertiary one after 24h, neither reflects the real VO1 nor correlates with clinical severity. The dynamic effect of T is now considered.
The Role of T
Not only VO1 (quantity and type) but also T is important for defining HN nadirs. VO1 gained in 1h (the induction period) induces the secondary HN nadir that accurately represents both the drop of SSC diluting ECF Figures 1,5 and correlates with clinical severity, However, the precise dynamic relationship of VO to clinical severity is T dependent, more specifically severity is dependent on the induction and adaptation periods of VO.
Induction Period
The importance of the induction period of T lies in the fact that when a given VO1 such as 3.5l (5%BW) is infused to an adult in 1h it is pathological and may be lethal. However, when given over a period of 24h it is about normal daily fluid intake. HN is thus inversely proportional to T of the induction period.
Toxicity of Fluid Type
When VO quantity and induction period remain constant, fluid type affects the severity of VO toxicity. The same VO of 3.5l (5% BW ivi in 1h) of distilled water is probably lethal, of 1.5%Glycine is critical, of 5%Glucose is serious, of 3% Mannitol is severe and of VO2 is perhaps of moderate severity. The evidence here is essentially based on common sense clinical observations as was derived from multiple reported studies on ivi fluids in animals that match NH nadirs and clinical severity of the TURP syndrome cases.
VO/T Insult
The above data demonstrates that “VO/T” is the insult causing the biochemical and clinical severity of secondary HN nadir at the immediate postoperative T. The most illusive effect of “VO /T” continues to operate invisibly during the tertiary HN nadir.
The Tertiary HN
The tertiary HN nadir is the most commonly measured drop of SSC postoperatively at 24h or later, typically at the morning after surgery (D). It is so dynamic that it misleadingly leads to multiple interpretations and conclusions. It may represent a spontaneous physiological rise of a previous secondary HN nadir if the renal function is maintained and insensible loss is taken into account. This is seen with VO1 of <2L when renal function allows dieresis. However, anuria of ARF is common with large VO1, during which rise of SSC also occurs due to osmotic fluid shift into ICF causing cell oedema of MVOD/F. The severity of MVOD/F is disproportional to the apparently mild HN nadir of rising SSC. The adaptation period of T is an overlooked factor causing tertiary HN with diagnosis of illusive clinical masks.
The “Missing VO”
The apparent rise of SSC occurring over 24-48h after massive VO1 and anuria is pathological due to internal osmotic water shift from ECF into ICF. The fluid that moves and lodges inside cells is the “Missing VO”, which is mathematically and clinically in excess of 2/3 of gained VO1. During the adaptation period of T, not only the “Missing” part of VO1 goes into cells but also fluids flooding ECF shift into the potential body cavities of pleura and peritoneum, evident at postmortem examination, due to the limited vascular system capacitance.
Furthermore, the elapsed adaptation period of T allows various interpretation of tertiary HN. Unawareness of a previous SSC drop confuses the secondary with tertiary HN nadir and overlooks the “Missing VO”. The spontaneous rises of SSC make the tertiary HN appears of lesser magnitude or insignificant as SSC may revert to normal or above normal, particularly with saline infusion, giving a false sense of improvement. It is impossible to tell if the rise of SSC is physiological improvement due to urine excretion and insensible loss or pathological due to the osmotic water shift into ICF and VO2 fluid infusions. Anuria is evident clinically, but pitting is not a feature of cellular edema to reflect osmotic fluid shift into ICF.
The tertiary HN nadir neither represents all gained VO1 nor reflects the severity of its clinical features. The latter remain to match the earlier secondary HN nadir and its VO1. The part of VO1 that remains in ECF causing the tertiary HN is about 1/3 of the real total VO1. The remaining 2/3 is the “Missing VO1” causing cellular oedema and MVOD/F. A massive VO1 of 3.5l/h causing secondary HN nadir of 107 mmol/l . It presents with paradoxical vascular hemodynamic shock and anuria of ARF that has a tertiary nadir of 126 mmol/l at 24h when MVOD/F is of critical severity. In the absence of an accurate per-operative fluid balance chart, only the increase in body weight (BW) can reveal the real total VO insult, its “Missing VO” part and any excess VO2 fluids in ECF and potential body cavities.
Osmolality Changes
In addition to “VO/T”, there are other factors that affect the tertiary HN, contributing to biochemical, clinical and therapeutic conspiracy of confusion and illusion. Saline or any ivi VO2 fluids, may erase remaining evidence on HN and totally mask the real
Osmolality Gaps
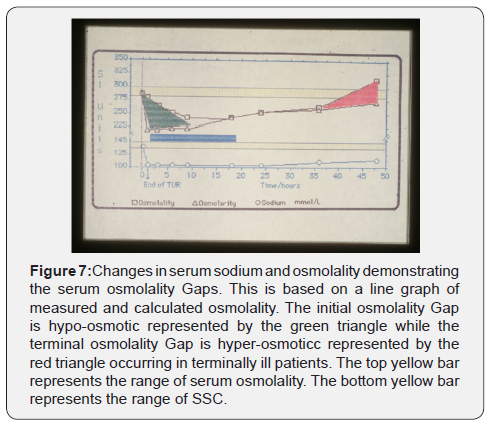
There are two osmolality gaps corresponding to the secondary and tertiary HN: the initial hypo-osmotic and late hyper-osmotic gaps, respectively (Figure 7). Being dependent on SSC, the gap of VO1 of metabolizable glucose and glycine is hypoosmolality but isotonic 3%Mannitol induces iso-osmolality HN. Likewise, the tertiary HN being dependent on SSC, T and therapy, late gap is dynamic and elusive. It may be hypo-, iso or hyper- osmolality gap. The latter gap occurs in established MVOD/F cases, has poor prognostic value and is shown in Figure 7. The late osmolality gap may rebound to hyper-osmolality with inappropriate therapy in patients with established MVOD/F.
Discussion
The presented evidence demonstrates that “VO/T” is the invisible insult causing all serum solute changes and grades, nadirs, paradoxes and masks of HN and TURP syndrome. Biochemical, clinical and therapeutic illusions have caused misinterpretation of data and the multidimensional puzzle. Neither bleeding, sepsis, chemical toxins, hypothermia, vascular obstruction nor hypoxaemia play a primary role in pathophysiology of HN and the TURP syndrome [6]. The clinical features from prodromal symptoms to severe signs and sudden death though it presents with masks of recognized conditions, have a different patho-aetiology.
Severity Grades and Presentation Masks
The observed analogy with MVOD/F and severity grade of HN is evident from presented data and analyzed literature reports. Hypotension shock and combination of MVOD/F is the main illusive presentation at a surgical setting [6]. Vascular shock is documented though usually incorrectly attributed to haemorrhage [18,19] or sepsis [20] because the paradoxical shock of “VO/T” was previously inconceivable. Heart failure, infarction or arrest and sudden death [22-24], and respiratory distress or pulmonary oedema [25,26] may cause a dilemma of diagnosis during or immediately after surgery. Those aware of the TURP syndrome consider dilutional and toxic hypotheses [13], while sudden death is attributed to recognized medical conditions, such as myocardial cerebral infarctions, each of which is an acceptable cause of death for different pathological causes. The real cause may be overlooked at postmortem examination unless the pathologist is fully aware of the condition [24]. Respiratory distress and pulmonary oedema have also been reported [25,26].
Encephalopathy coma of postoperative [4-6,10,27-32] and medical [8,9,11,12,33-36] HN cases is common presentation to physicians, which with convulsions and paralysis indicate cerebral oedema and infarction. The real culprit of VO1 insult, being invisible, was unmentioned in all but one retrospective [5] and prospective [9] report. Of noteworthy, HN coma complicates any surgery and is not limited to TURP. The predisposing factors and route of VO1 gain, through prostate or peripheral vein or peritoneum is of minor significance to patho-etiology but relevant to prevention of irrigant absorption. Other electrolyte changes [33,34], renal [35] and hepatic dysfunctions [36,37], coagulopathies [38,39] and gastro-intestinal disorders may occur. Combinations are common but one or two MVOD/F may prevail. Figure 4 demonstrates the abnormal renal and hepatic function tests at D time as part of MVOD/F.
The Clinical Illusion
With such bizarre features and wide range of severity, the masks of clinical presentations of HN and TURP syndrome are many. The differential diagnosis is enormous and extremely hard. Prodromal symptoms may be attributed to hypothermia [40] or to anesthesia, drugs and glycine toxicity [41] while severe cases may be mistaken for known medical conditions of shock or cerebral and cardiac failure or infarction, respiratory, renal or hepatic failure [22-37]. This adds to the dilemma of dilutional and toxic hypotheses of TURP syndrome [13]. Postmortem examination may show oedema and necrosis of brain or heart that may be overlooked being mistaken for arterial occlusion but organ necrosis of VO1 occurs in absence of arterial ischaemia and hypoxaemia. The cause of death is missed unless VO1 is proved by low SSC in the vitreous body sample taken from the eye [24]. Verifying cerebral and coronary arteries for obstructive atheroma or clots should be done.
Clinically, the condition has the same haemodynamic disturbance of haemorrhage or septic shock, but hypovolaemia is not synonymous with hypotension. It mimics MVOD/F of any single vital organ, but the insult is generalized due to shock and cell oedema or necrosis (infarction) of VO1. The elevated renal and hepatic function tests and leucocytosis figure 4 occurring during the adaptation period of T, at 24h or later are effects of VO. Brain, heart and lungs having special grading and assessment methods are grouped as MVOD/F. In severe cases of VO1, annuria of ARF is resistant to loop diuretics of up to the triple normal dose and VO2 therapy worsens VO. Biochemical and clinical severity of the tertiary HN nadir being disproportional cause misdiagnosis and therapeutic illusion.
Therapeutic Illusion
The fundamental difference between surgical and medical presentations is the therapeutic response it evokes. Presenting with profound vascular hypotension shock in theatre or recovery room at the time of secondary HN nadir contradicts preconceived concepts on shock and fluid therapy. “Aggressive vascular expansion” executed frantically and indiscriminately in falsebelief that haemorrhage, hypovolaemia or sepsis is at operation. The misguided attempt of massive VO2 infusion erases HN, makes shock irreversible, worsens VO and establishes MVOD/F. Other authors affirmed our clinical observations on prodromal to severe manifestations of HN of VO1 but unfortunately was attributed to hypothermia [40] or glycine toxicity [9,41]. The same manifestations, however, have been reported with glucose and mannitol fluids in the absence of hypothermia, glycine and ammonia toxicity [13].
Capacitance of the cardiovascular system is limited to 7l and CVP is around 0 (+7 to -7) cm water [42]. Aggressive vascular expansion exceeding these limits, even during genuine hypovolaemia or sepsis shock therapy, spills over excess fluids to ISF whether it corrects arterial hypotension or not. The osmotic pressure of plasma proteins thought to be the force to return fluid back into IVF does not exist [10]. The capillaries have pores that allow easy passage of protein molecules. Guyton and Coleman [43] elegant subcutaneous chamber demonstrated this and proved that ISF pressure is -7cm water. Increased venous pressure is well known common cause of oedema but high arterial pressure has no such effect. This should evoke thoughts about Starling’s law and received concepts on fluid therapy. The newly reported hydrodynamic phenomenon of the narrow orifice (G) tube [10] demonstrated its significance to capillary and vascular haemodynamic under both physiological and pathological conditions. VO causes hypotension shock indicating that acute vascular volumetric changes of hyper- or hypovelamina cause shock at capillary-ISF level, causing cell anoxia [5] despite full oxygen saturation of arterial blood.
Presenting to physicians later with encephalopathy coma, at the time of tertiary HN nadir, is an illusion too. Most gained VO1 has already gone missing from IVF and ECF into cells, hence rarely reported. The recommended therapy in such situation has been conservative with fluid restriction, loop diuretics and cardio-respiratory supportive measures while HST was thought contraindicated. For elevating SSC, ether isotonic saline or plasma VO2 fluids was liberally used [33], or HST of low concentration was slowly infused [8]. This is contradictory to fluid restriction. Despite debating this view when the available evidence was observations or anecdotal cases and recently reporting hard evidence [6,7], the general view on therapy of HN remains unchanged [5,8,10-12], save a welcome recent report by World authorities on HN [9].
Hypothesis and Disputes
Irrigant absorption is well known to induced HN of TURP syndrome, attributed to multiple dilutional HN [4-13,22-41], hypo-osmolality [6,44-50], low albumin [33,49] and calcium [51] or toxic hypotheses (ammonia, glycine, serum K+ and acid phosphatase). Hemoglobin (Hb) dilution was always thought due to haemorrhage in treating shock. All serum changes used, interchangeably and in combinations with mentioned medical conditions, for explaining the pathophysiology of TURP syndrome and its bizarre clinical presentation masks.
The long-standing dispute between hypo-osmolality and HN [43-49] as causes of severe symptoms is based on the observation that marked hyponatraemia may occur without severe matching signs. This may be resolved on understanding of the presented data on tertiary HN nadirs and osmolality gaps. Figures (1-4) demonstrate the bases of most dilutional and toxic hypotheses. The “VO/T” insult is so obvious that is invisible. All previous studies neither quantified irrigant absorption nor considered ivi fluids.
Data Interpretation
Correlating data on VO1 induced by both the absorbed irrigant and ivi fluids to the signs of HN of the TURP syndrome makes its bizarre features easier to understand, detect and explain. The above results demonstrate that both hypo-osmolality and HN of VO1 occur with glycine and dextrose fluids being metabilazable [6,44-50] but mannitol causes HN without hypo-osmolality [28]. Hence mannitol appears less toxic than glucose or glycine and HN may be disproportional to signs [44]. Hypo-osmolality is short lived and may be undetectable [13]. Both VO2 and/or cellulysis with liberation of cell osmols may revert serum osmolality to normal or above normal, causing late osmolality gap reported in MVOD/F patients with poor prognosis [52]. Patients may have iso-, hyper- or hypo-osmolality at any time. The late hyperosmolality gap is a rebound of the initial hypo-osmolality gap. It has great therapeutic potential in preventing occurrence of late gap, if HST is timely and rapidly given. Likewise, HN has a longer T scale. Hence both hypo-osmolality and HN of VO1 play vital but transient secondary role in pathophysiology, lasting hours and days, respectively [9]- when remained unclouded by VO2 infusions.
It has been demonstrated that ivi of one litre of 1.5% Glycine or 5% Dextrose, gained in 1h, caused a drop-in serum sodium of 7 mmol/l and induced prodromal symptoms in healthy volunteers [42]. The lowest reported serum sodium level compatible with life was 98 mmol/l occurring with 3% Mannitol [28] and 101 mmol/l with 1.5% Glycine [22-30]. The corresponding lethal dose of pure VO1 is 5.6l. The mean VO1 for HN of <120 mmol/l of middle severity range was 3.5l (5%BW), including 1l of ivi VO2 fluids [9]. These data are the bases of using HN nadirs and mean SSC drop, respectively, in relation to subjective clinical severity grade.
Figure 1 shows that 10-14 mmol/l drop of SSC indicates VO1 of <2l that may or may not be self-correctable. A drop of 15-20 mmol/l indicates VO1 of 2.5L. An extra ivi one litre of VO2 fluid brings the total VO mean to 3.5L (5%BW) causing severe signs. An adult patient may accommodate an extra 1-2L of VO2 fluids but recovery from MVOD/F is the exception. A combination of VO1 and VO2 of up to 10% BW or larger volume of pure VO2 is common after major surgery and trauma resuscitation [17,35]. Massive VO2 induces critical MVOD/F that may not be lethal but is rarely reported or incriminated, except in the unique original report on the adult respiratory distress syndrome (ARDS) [21].
Saline infusions cause apparent rise of SSC to normal or above normal but obviously worsens VO. This may explain the delayed TURP syndrome presentation with features mimicking cerebral or cardiac infarction, in which HN and hypo-osmolality though detectable [9] is easily erasable. A delay of appropriate therapy causes cell lyses liberating toxic contents into IVF that also erase hypo-osmolality, rebounding to hyper-osmolality gap. Giving VO2 erases both HN and hypo-osmolality. Liberated toxic cell contents into circulation have confused the findings with sepsis and misguided research on MVOD/F syndrome, particularly in the presence of leucocytosis demonstrated here as a response of VO insult (Figure 4). Sepsis is perhaps over-incriminated in MVOD/F. The fact that one or two litres of normal saline are better tolerated than that of metabolizable hypotonic 1.5% Glycine or an isotonic 5% Glucose fluids, does not mean a direct proportional relationship between VO2 quantity and safety.
The reported haemodynamic evidence on cardiac stress during TURP surgery [40,41] occurs in the absence of hypothermia, hypoxaemia and sepsis in all cases of the TURP syndrome. Also, most severe cases of HN and TURP syndrome suffer from coma in the absence of ammonia toxicity that is only rarely detected in established hepatic failure as part of MVOD/F. The condition also occurs with 3%Mannitol [30] and Sorbitol in the absence of elevated serum glycine. All dilutional and toxic hypotheses are equally incorrect, leaving the real culprit of “VO/T’ insult invisible.
Severe to lethal HN and TURP syndrome cases occur rarely during prospective studies and only two Eastbourne studies documented such cases [6,51], while all HN cases were reported retrospectively. An incidence of 10% causing moderate to severe morbidity [6] with mortality of 0.5-1.5% [50] is the currently accepted figures [13]. Other authors affirmed the accuracy of our data on incidence, per-operative blood loss and fluid balance [13]. The incidence applies to postoperative, if not all hospitalinduced HN [33-37] affecting men, women and children of medical and surgical specialties [4-13], whether reported as HN or with a specialty label of the TURP syndrome or identical twins [33,37]. Tertiary HN nadir and the “Missing VO” induce a triple biochemical, clinical and therapeutic illusions.
The Biochemical Illusion
“VO/T” insult is not only overlooked but also the contribution of ivi fluids is usually ignored, being contradictory to received concepts on fluid therapy of shock and monitoring critically ill patients. Osmotic shift of the water component of VO1 fluids from ECF into ICF (Missing VO) causes apparent elevation of SSC and wide cell oedema with dysfunction of vital and non-vital organs, explaining the illusion of tertiary HN nadir. Cell oedema, necrosis and lyses complete the picture of shock and MVOD/F. Cellular, unlike the interstitial venous or cardiac, oedema is not pitting. It manifests clinically with the bizarre features of MVOD/F. VO2 fluids may correct low SSC but masks HN marker, confuses diagnosis, worsens VO and causes internal drowning, and above all does not correct cell oedema by mobilizing the “Missing VO” out of cells and patient’s body. Capillary dysfunction causes shock and cell anoxia in the absence of hypoxaemia, as has recently been proved histo-pathologically [13].
The total fluid balance and net VO with reference to fluid type at the time of diagnosis, particularly of cases that suffer from cerebral or cardiac ischaemic episode or infarction and/ or respiratory distress in the postoperative period, should be carefully analyzed. SSC and osmolality changes on a time scale reveal valuable evidence [6]. Though not easy, the tertiary HN nadir or normal SSC postoperatively, must be carefully analyzed with reference to “VO/T” taking fluid type into account. In cases of ARF with high urine output, the serious attempt for spontaneous recovery by the kidneys may be defeated by the “output-input fluid chase”, in which every urine output is thoughtlessly replaced by equal quantity of ivi fluids. At many times, quantifying VO can only be detected by the increase in BW
Understanding the above discussed issues on VO/T has allowed the discovery of volumetric overload shocks in the patho-etiology of HN and TURP syndrome [53,54]. It also allowed the discovery of the hydrodynamics of a porous orifice tube that provides the correct replacement for the wrong Starling’s law for the capillary-interstitial fluid transfer [55]. Resolving the puzzle of MVOD/F syndrome and ARDS is pending.
Conclusion
The concept of “VO/T’ insult explains the aetiology of HN and TURP syndrome and pathophysiology of HN nadirs unveiling its paradoxical illusive presentation masks and refuting dilutional and toxic hypotheses. Irrigant absorption of 1.5% Glycine induces the TURP syndrome but was neither quantified nor reported, likewise 5% Dextrose induces all postoperative HN cases though VO1 is rarely reported. Secondary HN nadir of VO1 is proportional to clinical severity but presentation with vascular shock contradicts received concepts and initiates the inappropriate therapy of ‘aggressive vascular expansion’. Likewise, tertiary HN nadir causes biochemical, clinical and therapeutic illusions. Osmotic fluid shift into ICF causes apparent rise of SSC but the “Missing VO” causes cell oedema or necrosis of vital organs. The bizarre features of MVOD/F cause difficulty in diagnosis and differential diagnosis of HN when presenting with masks of cerebral or cardiac ischaemia or infarction. It is important to differentiate infarction of arterial obstruction from that due to cell oedema and necrosis as delayed presentation masks of HN and the TUR syndrome.
The real unique value of understanding the exact pathoetiology of the ‘rare, obscure and well known’ but unique TURP syndrome lies not only in resolving its own puzzle but also that of identical twins and conditions characterized with HN. This has paved the way to identify new “VO shocks”, correct rules to “optimize” fluid therapy in shock and report conditions for successful life-saving HST of severe inadvertent HN cases. In the light of the new hypothesis for capillary-ISF dynamics, resolving another most illusive puzzle of MVOD/F syndrome or ARDS should not be too difficult or distant.
References
- Rowntree LO (1923) Water Intoxication. A.M.A . Arch. Int. Med 32: 157.
- Danowski TS, Winkler AW, Elkington JR (1946) The treatment of shock due to salt depression; comparison of isotonic, of hypertonic saline and of isotonic glucose solutions. J Clin Invest 25: 130-138.
- Creevy CD (1947) Haemolytic reactions during transurethral prostatic resection. J Uro 58: 125-131.
- Harrison RH, Boren JS, Robinson JR (1956) Dilutional hyponatraemic shock: another concept of the transurethral prostatic reaction. J Uro 75 (1): 95-110.
- Arieff AI (1986) Hyponatraemia, convulsion, respiratory arrest and permanent brain damage after elective surgery in healthy women. N Engl J Med 314 (24): 1529-1534.
- Ghanem AN, Ward JP (1990) Osmotic and metabolic sequelae of volumetric overload in relation to the TURP syndrome. Br J Uro 66(1): 71-78.
- Ghanem AN (2001) Magnetic field-like fluid circulation of a porous orifice tube and its relevance to the capillary-interstitial fluid circulation: preliminary report. Medical Hypotheses 56(3): 325-334.
- Arieff AI (1993) Management of hyponatraemia. Br Med Jour 307: 305-308.
- Ayus JC, Arieff AI (1999) Chronic Hyponatraemic Encephalopathy in Postmenopausal Women: Association of Therapies with Morbidity and Mortality. JAMA Middle East 9(10): 58-63.
- Lane N, Allen K (1999) Hyponatraemia after orthopaedic surgery. Editorial. Br Med Jour 318: 1363-1364.
- Arieff AI (1992) Hyponatraemia and death or permanent brain damage in healthy children. Br Med Jour 304: 1218-1222.
- Halberthal M, Halperin ML, Bohn D (2001) Acute hyponatraemia in children admitted to hospital: retrospective analysis of factors contributing to its development and resolution. Br Med Jour 322: 780-782.
- Hahn RG (1997) Irrigating fluids in endoscopic surgery. Br J Uri 79(5): 669-680.
- Gamble JL (1949) Chemical Anatomy, Physiology and Pathology of Extra-cellular Fluid. Cambridge, Mass. Harvard University Press.
- Darrow DC, Pratt EL (1950) Fluid Therapy. JAMA 143: 365.
- Moyer CA (1953) Fluid Balance. Year Book Publishers, Chicago.
- Chassin JL (1954) Postoperative electrolyte disturbances. Surg. Clin. N. Amer 34(2): 323-342.
- Spencer Hoyt H, Goebel J L, Lee HI, Schoenbrod J (1958) Types of shock reaction during transurethral resection and relation to acute renal failure. J Uro 79: 500-507.
- Logie JRC, Keenan RA, Whiting PH, Steyn JH (1980) Fluid absorption during prostatectomy. Br J Uro 52(6): 526-528.
- Bertrand J, Gambini A, Cazalaa JB (1981) Le syndrome de resection de la prostate (TURP) syndrome, mythe oy realite? Jour d’ Urologie 87: 1-4
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967) Acute respiratory distress in adults. Lancet 12(7511): 319-323.
- Charlton AJ (1980) Cardiac arrest during transurethral surgery after absorption of 1.5% glycine. Anaesth 35: 804-807.
- Osborn DE, Rao PN, Greene MJ, Barnard RJ (1980) Fluid absorption during transurethral surgery. Br Med Jour 28: 1549-1550.
- Lessels AM, Honan RP, Haboubi NY, Ali HH, Greene MJ (1982) Death during prostatectomy. J Clin Path 35(1): 117.
- Jacobson J (1965) Prolonged respiratory inadequacy following Transurethral Resection of the Prostate. Anaesth 20(3): 329-333.
- Heytens L, Camu F (1984) Pulmonary edema during caesarean section related to the use of oxytocin drugs. Acta Anaesthesiologica Belgica 35(2): 155-164.
- Henderson DJ, Middleton RG (1980) Coma from hyponatraemia of the transurethral resection of prostate. Urology 15(3): 267-271.
- Kirshenbaum MA (1979) Sever mannitol induced hyponatraemia complicating transurethral prostatic resection J Uro 121(5): 686-688.
- Istre O, Bjoennes J, Naes R (1994) Postoperative cerebral oedema after Transcervical Endometrial Resection and Uterine Irrigation with 1.5% Glycine. Lancet 344(8931): 1187-1189.
- Arieff AI, Ayus JC (1993) Endometrial ablation complicated by fatal hyponatraemic encephalopathy. JAMA 270(10): 1230-1232.
- Whitfield HN, Mills VA (1985) Percutaneous nephrolithotomy. Br J Uro: 603-604.
- Kabalin JN (1993) Laser surgery performed with right angle firing neodymium: YAG laser fibre at 40 watts power setting. J Uro 95-99.
- Dandonna P, Fonseca V and Baron (1985) Hypoalbuminaemic hyponatraemia: a new syndrome? Br Med Jour 291(6504): 1253-1255.
- Batuman V, Dreisbach A, Maesaka JK, Rothkopf M Ross E (1984) Renal and electrolyte effects of total parentral nutrition. Jour of Parentral and Entral Nutrition 8(5): 546-551.
- Mayer CA (1950) Acute temporary changes in renal function associated with major surgical procedures. Surgery 27(2): 198-207.
- Watters DAK, Chamroonkul MA, Eastwood MA (1984) Changes in liver function associated with parentral nutrition. J Roy Coll Surg Edin 29: 339-344.
- Thompson PD, Gledhill RF, Quin NP, Rossor MN, Stainly P, et al. (1986) Neurological complications associated with parentral treatment: central pontine myelinolysis and Wernicke’s encephalopathy. Br Med Jour 292(6521): 684-685.
- Bird D, Slade N, Feneley RCL (1982) Intravascular complications of transurethral resection of the prostate. Br J Uro 54(5): 564-565.
- Friedman NJ, Hoag MS, Robinson AJ, Aggeler PM (1969) Haemorrhagic syndromes following transurethral resection for benign adenoma. Arch Intern Med 124(3): 341-349.
- Evans JWH, Singer M, Chapple CR, Macartney N, Walker JM, et al. (1992) Haemodynamic evidence for cardiac stress during transurethral surgery. Br Med Jour 304(6828): 666-671.
- Nilsson A, Randmaa I, Hahn RG (1996) Haemodynamic effects of irrigating fluids studied by Doppler ultrasonography in volunteers. Br J Urol 77(4): 541-546
- Guyton AC (1986) Textbook of Medical Physiology. (7th edn) Philadelphia. An HBJ International Edition. WB Saunders Company. Chapters: 36, 19, 21 and 26.
- Guyton AC, Coleman TG (1968) Regulation of interstitial fluid volume and pressure. Annals New York Academy of Sciences 150(3): 537-547.
- Wright HK, Gann DS (1962) Severe postoperative hyponatraemia without symptoms of water intoxication. Surg Gyn & Obst 98(5): 553-556.
- Berg G, Fedor EJ, Fisher B (1962) Physiologic observations related to the transurethral resection reaction. J Uro 87: 4596-4600.
- Beirne GN, Madsen PO, Burns RO (1965) Serum electrolyte and osmolality changes following transurethral resection of the prostate. Br Jour Uro 93: 83-86.
- Desmond J (1970) Serum osmolality and plasma electrolytes in patients who develop dilutional hyponatraemia during transurethral resection. Can Jour Surg 13(2): 116-121.
- Wakim KG (1971) The pathophysiologic basis for the clinical manifestations and complications of transurethral prostatic resection. J Uro 106(5): 719-728.
- Norris HT, Aashem GM, Sherrard DJ and Tremann JA (1973) Symptomatology, pathophysiology and treatment of the transurethral resection of the prostate syndrome. Br J Uro 45(4): 420-427.
- Sellevold O, Brevic H, Tveter K (1983) Changes in oncotic pressure, osmolality and electrolytes following transurethral resection of the prostate using glycine as irrigant. Scand J Uro Nephrol 17: 31-36.
- Rhymer JC, Bell TJ, Perry KC, Ward JP (1985) Hyponatraemia following transurethral resection of the prostate. Br J Uro 57: 450-452.
- Inaba H, Hirasawa H, Mizuguchi T (1987) Serum osmolality gap in postoperative patients in intensive care. Lancet 1(8546): 1331-1335.
- Ghanem SA, Ghanem KA, Ghanem A N (2017) Volumetric Overload Shocks in the Patho-Etiology of the Transurethral Resection of the Prostate (TURP) Syndrome and Acute Dilution Hyponatraemia: The Clinical Evidence Based on Prospective Clinical Study of 100 Consecutive TURP Patients. Surg Med Open Access J 7(5): 1-7.
- Ghanem KA and Ghanem AN (2017) Volumetric overload shocks in the patho-etiology of the transurethral resection prostatectomy syndrome and acute dilution hyponatraemia: The clinical evidence based on 23 case series. Basic Research Journal of Medicine and Clinical Sciences ISSN 2315-6864 6(4): 35-43.
- Ghanem KA and Ghanem AN (2017) The proof and reasons that Starling’s law for the capillary-interstitial fluid transfer is wrong, advancing the hydrodynamics of a porous orifice (G) tube as the real mechanism. Blood, Heart and Circ 1(1): 1-7.






























