Pseudosarcomatous Myofibroblastic Proliferation of the Urinary Bladder
Ktari Kamel1,2*
Department of Urology, Hospital FattoumaBourguiba, Tunisia
Department of Pathology, Hospital Fattouma Bourguiba, Tunisia
Submission: June 08, 2018; Published: July 02, 2018
*Corresponding author: Ktari Kamel, epartment of Urology, Hospital FattoumaBourguiba, Tunisia, Email: ktari-kamel@hotmail.fr
How to cite this article: Ktari Kamel. PseudosarcomatousMyofibroblasticProliferation of the Urinary Bladder. JOJ uro & nephron. 2018; 5(5): 555672. DOI: 10.19080/JOJUN.2018.05.555672
Summary
Myofibroblastic proliferations of the bladder in adults are unusual lesions with a benign course. These proliferations, whether spontaneous or secondary to instrumentation, have identical morphology and behaviour. Histologically, similar lesions have been reported in the literature using different names, such as inflammatory pseudotumour, pseudosarcomatousfibromyxoidtumour, nodular fasciitis, postoperative spindle cell nodule. Recently, some authors proposed that these lesions are similar enough to be considered the same entity, designated as “pseudosarcomatousmyofibroblastic proliferation” and insisted on the necessity to distinguish them from the inflammatory myofibroblastictumour of the childhood. The latter, recently recognized as tumour, has a malignant potential and is capable of giving metastases.
We describe the case of a 68-year-old woman who presented a vesical mass. The histopathological study concluded to a pseudosarcomatousmyofibroblastic proliferation.
Keywords: Urinary bladder;Pseudotumour;Pseudosarcomatous;Myofibroblastic proliferation; Inflammatory pseudotumour
Introduction
Pseudosarcomatousmyofibroblastic proliferation (PMP) of the bladder has been described by many. Roth [1] first described the lesion as a reactive pseudosarcomatous response in 1980. In the past, these lesions have often been initially misdiagnosed as malignancies such as sarcomatoid urothelial carcinoma, leiomyosarcoma, and rhabdomyosarcoma. The patient with PMP presents with symptoms that include urgency, urinary frequency, dysuria and hematuria, urinary obstruction, and pelvic pain[2]. These tumors can occur at any age[3,4]. PMP usually appears as a polypoid or nodular, sometimes ulcerated, exophytic mass with broad attachment to the bladder wall[2,4]. Treatment usually consists of transurethral resection or partial cystectomy[4]. PMP can grow extensively through the muscularis propria to invade the perivesicular adipose tissues, peritoneum, and omentum[4]. Recurrence has recently been reported in 3 cases[4], but it has not been reported to metastasize [4,5].
Case Report
A 68-year-old woman was admitted because of gross hematuria. Seven days prior to admission, she noted dark blood in her urine with dysuria. But she reported neither urgency, frequency, burning nor fever or chills. She had no history of urinary tract infection, instrumentation, trauma or other urological problems. She was a nonsmoker and was otherwise in good health.
Physical findings on admission were essentially unremarkable.
An ultrasound study of the kidneys and the urinary bladder revealed a broad-based polypoid mass (4×3cm) which was located in the right posterolateral wall of the bladder. Cystoscopy showed a large ovoid tumor on the right lateral wall of the urinary bladder near the left ureteral orifice, Transurethral resection was performed, and pathology disclosed a picture of spindle cell proliferation in the submucosal and mucosal layers of the urinary bladder. The cellular proliferation was associated with mild nuclear atypia and eosinophilic cytoplasm within a fibrillary or myxoid background. It was compatible with pseudosarcomatousmyofibroblastic lesion of the urinary bladder.
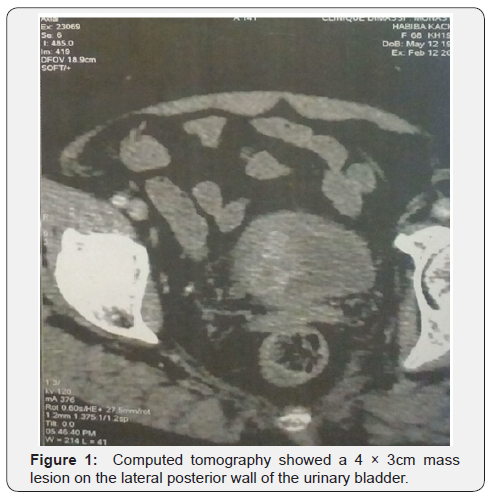
Then, thoracic/abdominal/pelvic computed tomography was arranged and disclosed a 5×4cm mass lesion on the right posterior wall of the urinary bladder with muscle layer invasion, but no lymph node lesion and no evidence of extension or metastasis was noted. Bone scan is normal.
A radical cystectomy was done.
Histologic evaluation of the mass showed infiltration of the bladder wall by a cellular population composed of oval, spindled, and stellate myofibroblastic cells arranged in a loose edematous and myxoid stroma with numerous small blood vessels and inflammatory cell infiltrate including, lymphocytes, plasma cells, and eosinophils. The cells were haphazardly arranged; however, rare foci of fascicles were also noted. In the high-power field microscopy, the tumor cells showed mild to moderate nuclear atypia, prominent nucleoli, and eosinophilic cytoplasm. Mitotic figures were present. However, no abnormal figures were noted. The lesion-elicited areas of focal necrosis associated with mucosal ulceration and invaded the muscularis propria. In the immunohistochemical study, the tumor cells were positive for anaplastic lymphoma kinase (ALK). A limited population of tumor cells was immunoreactive desmine et actine. Based on the previously mentioned morphologic and immunohistochemical characteristics, the diagnosis of pseudosarcomatousmyofibroblastic proliferation was finally posed.
The post-operative course was uneventful. The patient did not receive any adjuvant treatment. She is currently free of disease without evidence of tumor recurrence or metastasis by 1-year follow-up (Figure 1).
Discussion
PMP of the urinary bladder was first reported by Roth in 1980[1]. He described a 32-year-old woman with recurrent cystitis and hematuria associated with an ulcerated bladder lesion. Unique histologic features included tumor-like, atypical spindle cell proliferation. Histology and etiology were not defined in Roth’s report, although chronic cystitis was characteristic in the history.
In subsequent years, many descriptive names have been used to describe histologically similar lesions, including inflammatory pseudotumor, nodular fasciitis, postoperative spindle cell nodule, pseudosarcomatousfibromyxoid tumor, PMP, and inflammatory myofibroblastic tumor. Unfortunately, no consensus on nomenclature seems imminent[4].
Recent reports show a male predominance (3:1), with a mean patient age range at presentation of 47 to 54 years (range, 3-89 years)[4,6]. Hematuria is the most common presenting symptom (60%). The following symptoms, pelvic pain (7%), mass lesion (7%), obstructive symptoms (4%) and urinary tract infection (4%) were often noted. Most cases are limited to the bladder, although concurrent involvement of the prostate has been reported[4,6]. Lesions range in size from 1cm to 12cm, and are polypoid or nodular, with variable degrees of mucosal hemorrhage and ulceration. The cut surface is graywhite to tan-pink and often gelatinous. Microscopically, the lesions vary from highly myxoid to highly cellular, and are composed of bland, spindle-shaped myofibroblastic cells, with an associated interlacing vascular network and a variable but polymorphous inflammatory infiltrate. Mitotic rates range from 0 to 20 per 10 high-power field. Invasion into the muscularis mucosa and muscularis propria is common, and infrequently the process may involve perivesical adipose tissue, peritoneum or even omentum. Necrosis, if present, is usually focal and associated with surface ulceration or deep muscularis propria invasion. Immunohistochemically, the spindle cells have expression for epithelial markers such as cytokeratin AE1/3 (94%), and smooth muscle markers such as smooth muscle actin (68%) and desmin (60%)[4,6]. Transurethral resection or partial cystectomy has been reported as the treatment of choice[7]. Whether these lesions are reactive or neoplastic is unresolved. It has been shown recently that a t(2;5) involving the Alk-1 gene can be demonstrated by fluorescent in situ hybridization in a substantial number of cases with expression of Alk-1protein by immunohistochemistry, supporting the notion that a significant proportion of these histologically similar lesions are truly neoplastic, while the remainder may be either reactive or harbor a different genetic derangement [6].
Most lesions can be managed by transurethral resection, although more extensive resection is sometimes required. Cases regarded as “typical” occasionally recur locally but do not metastasize[4,6]. Iczkowski et al. [5] reported the death of 1 patient who was not a candidate for definitive tumor ablation after transurethral resection of a 13cm tumor. The tumor grew to 37.5cm, resulting in urinary obstruction and urosepsis. Sandhu and Iacovou7 reported the case of a patient with fullthickness bladder invasion and tumor fixation to the rectus sheath who was treated with 4 months of oral antibiotics. They saw resolution of the lesion at 9 months of follow-up, and the patient was free of recurrence after 3 years[7].) PMP of the urinary bladder has unique clinical and pathologic features that allow their distinction from primary bladder sarcoma and sarcomatoid carcinoma [8]. The definition of PMP and inflammatory myofibroblastic tumor of the urinary bladder is still controversial because of the malignant potential[9]. Transurethral resection or partial cystectomy is sufficient to eradicate
References
- Roth JA (1980) Reactive pseudosarcomatous response in urinary bladder. Urology 16(6): 635-637.
- Lundgren L, Aldenborg F, Angervall L, Kindblom LG (1994) Pseudomalignant spindle cell proliferations of the urinary bladder. Hum Pathol 25(2): 181-191.
- Ricchiuti DJ, Ricchiuti VS, Ricchiuti RR, Qadri AM, Resnick MI (2000) Fibrous inflammatory pseudotumor of the bladder. Rev Urol 2(4): 232- 235.
- Harik LR, Merino C, Coindre JM, Amin MB (2006) Pseudosarcomatous myofibroblastic proliferations of the bladder. Am J Surg Pathol 30(7): 787-794.
- Iczkowski KA, Shanks JH, Gadaleanu V, Cheng L, Jones EC, et al. (2001) Inflammatory pseudotumor and sarcoma of the urinary bladder: differential diagnosis and outcome in thirty-eight spindle cell neoplasms. Mod Pathol 14(10): 1043-1051.
- Montgomery EA, Shuster DD, Burkart AL, Esteban JM, Sgrignoli A, et al. (2006) Inflammatory myofibroblastic tumors of the urinary tract. Am J Surg Pathol 30(12): 1502-1512.
- Sandhu SS, Iacovou JW (1997) Pseudotumor of the bladder. J R Soc Med 90: 46-47.
- Spiess PE, Tuziak T, Tibbs RF, Bassett R, Tamboli P, et al. (2007) Pseudosarcomatous and sarcomatous proliferations of the bladder. Hum Pathol 38(5): 753-761.
- Houben CH (2007) Pseudosarcomatous myofibroblastic proliferations of the bladder: a clinicopathologic study of 42 cases. Am J Surg Pathol 31: 642.






























