Transplantation of Restored Kidneys from Living Related Donors after Small Renal Tumor Resection: A Prospective Clinical Pilot Study
Yoshihide Ogawa1*, Shinyu Shiroma2, Keimei Kojima2, Rensuke Mannami2, Makoto Mannami2, Naoki Mitsuhata2, Mitsuo Nishi3 and Hisaaki Afuso4
1 Department of Urology, Tokyo-West Tokushukai Hospital, Japan
2 Department of Urology, Uwajima Tokushukai Hospital, Japan
3 Department of Urology, Saint Martin’s Hospital, Japan
4 Department of Urology, Okinawa Chubu Tokushukai Hospital, Japan
Submission: May 29, 2018; Published:June 12, 2018
*Corresponding author: Yoshihide Ogawa, Department of Urology, Tokyo-West Tokushukai Hospital, Japan, Email: yoshihide.ogawa@tokushukai.jp
How to cite this article:Ogawa Y, Shiroma S, Kojima K, Mannami R, Mannami M, et al. Transplantation of Restored Kidneys from Living Related Donors after Small Renal Tumor Resection: A Prospective Clinical Pilot Study. JOJ uro & nephron. 2018; 5(4): 555669. DOI: 10.19080/JOJUN.2018.05.555669
Abstract
We launched a clinical trial that aimed to study the utility and safety of restored kidney transplantation between family members using therapeutic donated kidneys. Incidental small renal tumors were identified during the examination of two men and three women undergoing preoperative donor workups for living related kidney transplantation. Living related kidney transplantation was performed using the donor kidneys, each of which was partially nephrectomized and restored, in three ABO-compatible cases and two ABO-incompatible cases. The small renal tumors were identified as renal cell carcinoma in three cases and as angiomyolipoma in two cases. The first recipient with a functioning graft died suddenly of cardiac arrest 2.5 months after surgery. The four remaining recipients (two of whom underwent simultaneous bilateral nephrectomy for polycystic kidneys and one of whom underwent simultaneous nephrolithotomy) have experienced a satisfactory quality of life. Their most recent serum creatinine levels ranged from 0.56 to 1.36 mg/dl, without tumor recurrence at 1.3 to 5.10 years after surgery.
In conclusion, potential donors with a therapeutic kidney often must make difficult decisions of whether to donate to their relatives; however, successful restored kidney transplantation using kidneys with small tumors, despite the limited use of this approach, may provide novel options for living related kidney transplantation.
Keywords: Restored kidney transplantation; Related donor; Small renal tumor; High-risk recipient; Cancer transmission
Abbreviations: RCC: Renal Cell Carcinoma; CKD: Chronic Kidney Disease; PN: Partial Nephrectomy; RN: Radical Nephrectomy; AML: Angiomyolipoma; ACKD: Acquired Cystic Kidney Disease
Introduction
The transplantation of the nephrectomized, restored kidney with small renal cell carcinoma (RCC) has been sporadically reported previously, but its frequency has gradually increased. Yu et al. summarized 97 cases [1], including 8 and 10 cases described in articles by our group [2,3]. The WHO and UNOS accept therapeutic kidney donations, including donations of kidneys with small renal tumors [4]. In Japan, clinical transplantation by utilizing therapeutic kidneys was empirically practiced without any ethical conflict up to 2007 [4]. An organ trafficking problem connected with the use of therapeutic kidneys occurred in 2006, and the Japanese government (Ministry of Health, Labor, and Welfare) issued an official notice in 2007 banning kidney transplantation using donors with therapeutic kidneys. After learning that a large number of usable therapeutic kidneys (e.g., with small renal tumors) were discarded every year [5], our medical group launched a restored kidney transplantation team to address the problem of saving “unrescuable” dialysis patients who are eager to undergo transplantation but do not have a suitable donor because of the limited donor supply for traditional kidney transplantation. Another official notification that was subsequently issued from the Ministry of Health, Labor and Welfare announced that for cases in clinical trials only, there is no limitation on using any kind of therapeutic kidney for restored kidney transplantation. This notification resulted in the motivation to initiate a clinical trial of restored kidney transplantation (2008). After the relevant literature and empirical reports were reviewed [3,6], a protocol for living renal transplantation with restored kidneys betweenThe transplantation of the nephrectomized, restored kidney with small renal cell carcinoma (RCC) has been sporadically reported previously, but its frequency has gradually increased. Yu et al. summarized 97 cases [1], including 8 and 10 cases described in articles by our group [2,3]. The WHO and UNOS accept therapeutic kidney donations, including donations of kidneys with small renal tumors [4]. In Japan, clinical transplantation by utilizing therapeutic kidneys was empirically practiced without any ethical conflict up to 2007 [4]. An organ trafficking problem connected with the use of therapeutic kidneys occurred in 2006, and the Japanese government (Ministry of Health, Labor, and Welfare) issued an official notice in 2007 banning kidney transplantation using donors with therapeutic kidneys. After learning that a large number of usable therapeutic kidneys (e.g., with small renal tumors) were discarded every year [5], our medical group launched a restored kidney transplantation team to address the problem of saving “unrescuable” dialysis patients who are eager to undergo transplantation but do not have a suitable donor because of the limited donor supply for traditional kidney transplantation. Another official notification that was subsequently issued from the Ministry of Health, Labor and Welfare announced that for cases in clinical trials only, there is no limitation on using any kind of therapeutic kidney for restored kidney transplantation. This notification resulted in the motivation to initiate a clinical trial of restored kidney transplantation (2008). After the relevant literature and empirical reports were reviewed [3,6], a protocol for living renal transplantation with restored kidneys between
In this article, we focused on the clinical trial, namely, living kidney transplantation between family members using restored therapeutic kidneys, to evaluate the utility and safety of restored kidney transplantation between relatives using therapeutic donated kidneys as well as, if possible, to determine what disease conditions were suitable for therapeutic kidney donation and to estimate the incidence of potential donors as such.
Case A
The gap left open by difficulties or risks of open technique and endoscopic surgeries has been filled by laparoscopy, a technique that aims to replace classic pelvic lithotomy through lombotomy in cases where open surgery is indicated. In Europe and the United States, open surgeries for urinary calculi are only indicated for cases of large and hard stones, following the failure of extracorporeal lithotripsy, percutaneous nephrolithotripsy or ureterolithotripsy, or even in cases of anatomical abnormalities [9,10].
Subjects and Methods
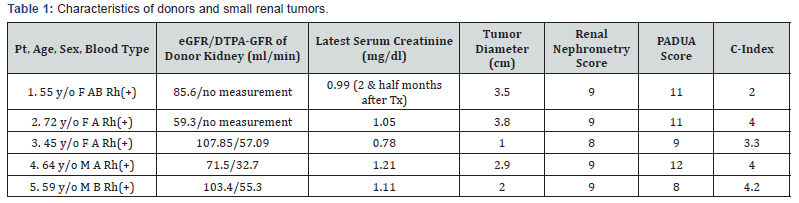
This clinical trial of restored kidney transplantation using therapeutic donated kidneys between family members with a planned enrollment of five cases was approved by the Tokushukai Joint Ethics Committee in July 2009. Initially, the potential therapeutic donated kidneys could contain a small (4 cm or less) renal tumor, renal stone, renal cyst, ureteral tumor, or ureteral stricture. The trial was financially supported by the Tokushukai Medical Group. The trial procedure was nearly identical to that of restored kidney transplantation between third parties reported in detail elsewhere [2] and lacked complicating factors that would have necessitated recipient registration (no recipient selection committee was required) or strict ethical consideration (the Restored Kidney Transplant Committee discussed whether the kidney donors and recipients met the study inclusion criteria). In Japan, therapeutic kidney donation is banned except for clinical trials; therefore, potential donors for therapeutic kidney donation sought entry into our trial. All donors had renal tumors, and no donor candidate had stones, ureter strictures, or other conditions except the fourth donor who had RCC and a renal stone (1 cm in diameter). Small renal tumors were incidentally identified during a donor preoperative examination for living related kidney transplantation in two men and three women (aged 45 to 72 years). They had visited various transplant centers, where the donations of therapeutic kidneys to their recipients were clearly and adamantly refused because of the legal restriction. Determined to donate their kidneys and fully understanding their medical status, the implications of donation on their future health, their psychosocial circumstances and their rights to proceed or decline the donor surgery without coercion, the donor candidates were referred to our transplant center for potential inclusion in our clinical trial. The five donor candidates arrived at our hospital from various distant areas of Japan and subsequently agreed to enter the trial. They opted to undergo nephrectomy for small RCC after extensive discussion of other possible treatment modalities. Their related recipients (two men and three women who were aged 43 to 62 years) agreed to enter our clinical trial; the recipients were on dialysis because of polycystic kidney disease, glomerulonephritis, or DM nephropathy. The eGFR of the donors ranged from 59.3 to 107.85 ml/min, and the DTPA-GFR of the donor kidneys ranged from 32.7 to 57.09 ml/min. The tumors ranged from 1.0 to 3.8 cm in size and were located as shown in Figure 1 and Table 1. All recipients were informed about the potential renal tumor pathology, tumor recurrence, renal function of the contralateral kidney, possible morbidity, and treatment options by reviewing current information on these topics [7-11]. Donor candidates were examined by imaging and classified according to the RENAL nephrometry score, PADUA score, and C-index as ranging from low to moderate complexity [12-14] (Figure 2 & Table 1). The RENAL nephrometry scoring system was introduced to objectively describe renal masses with respect to size, degree to which they are exo/endophytic, nearness to the collecting system, and location relative to polar lines as well as whether they are anterior or posterior [12]. PADUA scores involve similar components and methodology, enabling a comprehensive description of the tumor size, polarity, location and closeness to the collecting system [13]. The C-index method uses tumor size and proximity to the center of the kidney to calculate a number that represents tumor complexity [14]. The methodological analysis of the tumor location and standardization for the reporting of tumor data that are provided by the scoring system may be able to predict the success of partial nephrectomy (PN), risk of postoperative complications, and functional and oncological outcomes (e.g., functional renal loss attributed to PN) [12]. The kidney donors were two men aged 59-64 years and three women aged 45-72 years, each of whom harbored a small renal tumor (Table 1). Although we reiterated a recommendation for PN prior to the nephrectomy, they preferred to undergo nephrectomy because of their strong motivation to donate, the shorter hospital stay, the lower morbidity, and the lower risk of cancer recurrence despite the potential risk for chronic kidney disease (CKD) with nephrectomy compared to PN. Both the hospital ethics committee and the Restored Kidney Transplant Committee confirmed the patients’ intent to donate or receive, reviewed the documents, and discussed the cases for study inclusion. All recipients, aged 43-62 years, were on dialysis: two patients were blood type A, one was type B, one was type O, and one was type AB. No recipient had a history of kidney transplantation (Table 2). In all, three cases were ABOcompatible transplants, and two cases were ABO-incompatible transplants.

Pt, patient; Tx, transplantation
Nephrometry score of 4 to 6 = low complexity. Nephrometry score of 7 to 9 = moderate complexity. Nephrometry score of 10 to 12 = high complexity.
PADUA scores involve similar components and methodology. Low complexity score: 6-7; intermediate complexity score: 8-9; and high complexity score: ≥10.
A C-index of 0 indicates a tumor that is concentric with the center of the kidney, whereas a C-index of greater than 1 denotes a peripheral tumor that is increasingly distant from the kidney center.
Pt, patient; HD, hemodialysis; PD, peritoneal dialysis; Tx, transplantation; Hx, history
Results
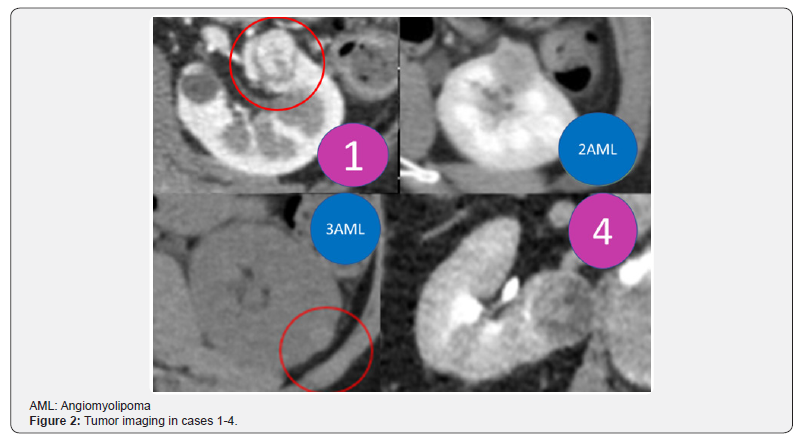
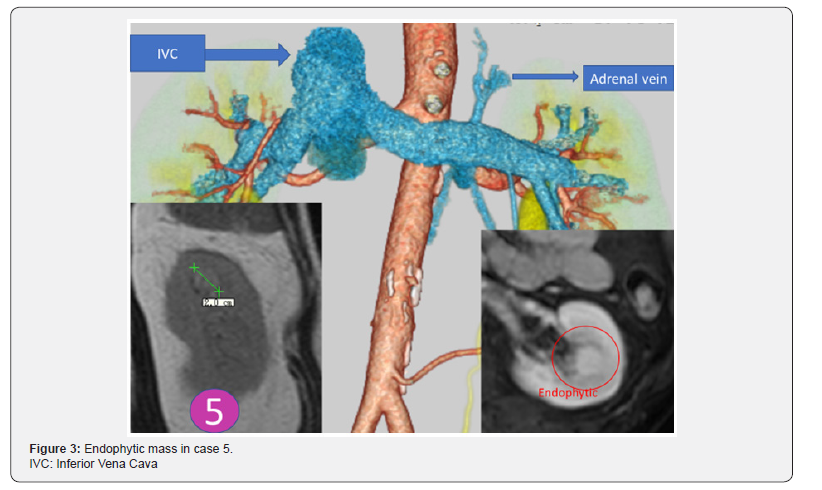
The first restored kidney transplantation between relatives was approved to be suitable by the Restored Kidney Transplant Committee and performed in March 2010, and the fifth restored kidney transplantation was performed in March 2017. All donor kidneys were procured, and all tumors were removed and restored ex vivo at Uwajima Tokushukai Hospital (Figure 2). Endoscopic simultaneous nephrolithotomy was required in one case (Figure 3). The tumor excised with an adequate border (1 cm) of healthy parenchyma was subjected to pathological examination, including the resected margin. As soon as the pathological report of the cut surface was confirmed to be cancer-free, the cut surface was approximated layer-to-layer to perform renorrhaphy, consisting of complete closure of the urinary collecting system and meticulous ligation of transected arteries and veins within the tumor bed using absorbable sutures. The tumor in case 5 was entirely endophytic without a substantial capsule and with a tumor texture similar to the normal parenchyma on imaging (Figure 3). It was not easy to identify and resect the tumor even with ex vivo surgery. The restored kidney was transplanted by the standard method, with end-to-side venous and arterial anastomoses between the renal and iliac vessels and ureteroneocystostomy according to the Lich-Gregoir technique with double-J ureteral stent inserted. A Foley catheter and double-J ureteral stent were inserted, with the catheter being removed 2 weeks after surgery and the stent being removed approximately 1 month after surgery. All recipients underwent restored kidney transplantation to the right iliac fossa. The surgical data included an ischemic time that ranged from 57 min to 4 hr 21 min, a donor nephrectomy time that ranged from 1 hr 21 min to 3 hr 53 min, a restoration time that ranged from 5 to 97 min (including stone removal in one case), and a transplant time that ranged from 3 hr 20 min to 6 hr 21 min (including simultaneous bilateral nephrectomy for polycystic kidneys in two cases). The tumor histology showed clear cell RCC in three cases and angiomyolipoma (AML) in two cases (Table 3). Living related kidney transplantation was performed in three ABO-compatible cases and two ABOincompatible cases. All patients initially received conventional triple immunosuppression (tacrolimus, mycophenolate mofetil, and methylprednisolone), which was reduced in dosage, and one recipient who received a kidney from an RCC donor is now taking everolimus. Basiliximab was administered on postoperative days 0 and 4. The two ABO-incompatible recipients were given rituximab at a dosage of 200 mg two weeks prior to surgery and underwent plasmapheresis and double filtration plasmapheresis to remove anti-AB antibodies prior to transplantation, similar to the desensitization protocol of Morath et al. [15]. A splenectomy was performed in the first patient because rituximab was not available at that time. After transplantation, the recipients were hospitalized for approximately one month and then discharged and followed up regularly. The first recipient with a functioning graft died suddenly of cardiac arrest 2.5 months after surgery. The four remaining recipients have experienced a satisfactory quality of life and an active lifestyle for 1 year & 3 months to 5 years & 10 months. Recipients 1 and 2 experienced rejection episodes, and recipient 1 experienced adverse events (urine leakage and sudden death). The other four patients now have functioning grafts. The serum creatinine levels ranged from 0.56 to 1.36 mg/dl, and no tumor recurrence has been observed in the donors or recipients to date (Table 4).
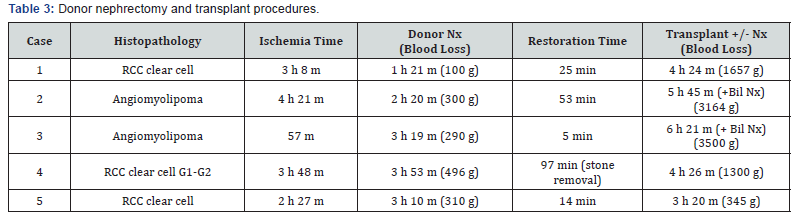
Bil Nx, bilateral nephrectomy; RCC, renal cell carcinoma
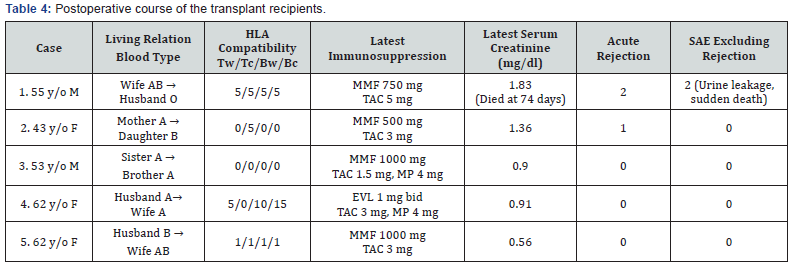
Tw: T cell warm; Tc: T cell cold; Bw: B cell warm; Bc: B cell cold; SAE: serious adverse event; MMF: mycophenolate mofetil; TAC: tacrolimus;
EVL: everolimus; MP: methylprednisolone
Discussion
Infrequently, renal tumors may be identified during donor screening for kidney transplantation between relatives. Such potential donors must make the difficult decision of whether to undergo a PN for themselves or to donate a therapeutic kidney to their relative. Except for clinical trials, no option exists to donate a therapeutic kidney in Japan. In cases of living related or altruistically donated kidney transplantation using therapeutic donatable kidneys, the surgical indication for a therapeutic donor nephrectomy may be elective, particularly in cases with a solitary small renal tumor. A donor’s motivation to provide a therapeutic organ depends on the relationship between the donor and the recipient. Some donor-recipient pairs, e.g., spouses, are willing to undertake the donation and transplantation of kidneys with incidentally identified renal masses. The ethical considerations regarding coercion and choice among family members regarding therapeutic living related kidney transplantation may not be as stringent as that for typical living related kidney transplantation because potential donors apparently understand that donor nephrectomy is a type of procedural treatment for their disease that may provide some benefit to the donor. Worldwide, 27 cases of restored kidney transplantation between relatives using kidneys containing small RCCs were published [1,16], along with 74 published cases using therapeutic kidneys in Japan [4]. The transplantation of a living donor kidney with a small renal tumor is controversial and considered high risk. Although the risk of disease transmission is reported to be low in wellscreened donors, all recipients of organs must be informed of the transmission risk of malignancy [17,18]. To balance the risk of dying on dialysis against that of cancer transmission [19], high-risk patients are suggested to be the most deserving of the procedure [20-22].
The current shortage of available deceased organs leads to a high mortality rate of patients on the waiting list for a kidney transplant. The donor shortage crisis in Japan prompts dialysis-intolerant patients to seek transplantation and donor kidneys in foreign countries, leading to an increase in transplant tourism [23-25]. In reality, dialysis patients seeking transplant abroad will incur a high cost (approximately $200,000), and the Japanese Health, Labor and Welfare ministry issued a notice to cover part of the expenses shouldered by patients traveling abroad to undergo organ transplants who are in need of urgent surgery (2017). Therefore, therapeutic kidney donation that is accepted widely might result in some changes/improvements to reduce transplant tourism and relieve the donor shortage.
Another scenario must remain a concern of ours, that is, whether widespread therapeutic kidney donation might be expected to help relieve the donor shortage. More than 80% of small renal tumors (< 4 cm) are treated by nephrectomy in Japan, so 2,000 kidneys with small RCCs have been estimated to be nephrectomized and discarded every year [5]. The Japanese Ministry of Health, Labor and Welfare conducted a questionnaire survey of treatment for a small RCC in 2012; 1082 patients with a small tumor underwent nephrectomy, while 1235 patients underwent PN in major teaching hospitals and cancer centers. The ratio of PN increased from 29.0%, 29.4%, 41.6%, and 53.3% in 2001, 2005, 2009, and 2011, respectively. More than 3,000 kidneys with tumors go untransplanted and are discarded in the United States each year [18,26]. Despite guidelines in the United States and Europe stating that a PN is a standard of care for a patient with a T1 renal mass, the application of PN for treating small renal masses varies widely. High-volume, young surgeons in academic settings often have a low radical nephrectomy (RN) threshold, and there might be low-volume surgeons over 50 years of age in private practices who have a very high RN threshold. Highly complex tumors as measured by RENAL nephrometry scores are more likely to be removed by RN than less complex tumors. The definition of the technical feasibility of a PN and the adherence to AUA and EAU guidelines are influenced by renal tumor case volume, PN% volume and practice setting [27]. These kidneys that are removed are otherwise discarded, and their use may provoke fewer ethical issues than the use of kidneys from healthy living donors. The great concept “Mottainai” by Prof. Wangari Maathai denoting the state of having sympathy and gratitude for world peace through environmental conservation so as not to waste resources and including the 3 Rs (“Reduce, Reuse, and Recycle”) should be invoked to solve the organ shortage crisis by utilizing kidneys with small renal tumors for transplantation. Utilizing these discarded kidneys may help to suppress the rise of organ trafficking and transplant tourism [23-25].
All donors in our trial had RCC, and we did not see any donor candidates with other diseases, e.g., stones or ureter conditions. Localized RCCs are best managed by PN rather than by RN as a first-line procedure whenever technically feasible, including in the presence of a normal contralateral kidney [7-10]. The recent AUA guideline recommends RN when all of the following criteria are met:
i. High tumor complexity and a potentially challenging PN, even for experienced surgeons;
ii. No preexisting CKD/proteinuria; and
iii. A normal contralateral kidney and new baseline eGFR likely to be >45 ml/min [11].
According to the ESMO clinical practice guidelines, PN is recommended as the preferred option in organ-confined tumors measuring up to 7 cm (elective indication) if negative margins are obtained and the risk of morbidity is acceptable [28]. Regarding surgery-related mortality, cancer-specific survival and time to recurrence, PN appears to result in a slight or no difference from RN [29]. The oncological equivalence of PN and RN was not definitively shown in the EORTC randomized study [8]; however, this equivalence is currently generally accepted. PN may be safely performed and results in a low percentage of patients with progression (4.5%) and renal cancer-related death (3%), supporting the existing arguments favoring PN in patients with T1 tumors. The preserved functional renal parenchymal volume after PN is the primary determinant of renal function [30]. Ischemia time is a factor only in the early phase of renal functional recovery and there would be near complete recovery within a few years [31]. Hypothetically, reduced blood flow and glomerular pressure in the partially resected kidney might cause renin release, inducing the Goldblatt model of hypertension; alternatively, surgical trauma might precipitate postoperative hypertension and renal injury, which might lead to postoperative hypertension manifesting as a Page kidney phenomenon after repair in cases of PN and nephrolithotomy [32]. However, there is weak evidence that PN is associated with greater urological complications, more bleeding, or a higher blood transfusion rate than RN, but there is evidence that RN causes more respiratory harm and acute kidney injury than PN [33]. The 5-year mean survival rate after open RN for localized RCC is 88.7%, according to pooled data of the three cohorts reported by Hemal 2007 (63/71 cases), Butler 1995 (34/42 cases), and Lee 2007 (50/56 cases) [34-36]. The 5-year recurrence rate after RN for pT1 RCC was 8.4% in a study by Stewart et al. [37]. Approximately 0.5% and 1.0% of kidney recipients are diagnosed as having RCC within 5 and 10 years after transplantation, respectively, and 90% of these cancers involve the native kidneys. The risk of RCC is approximately six-fold greater than in the general population, and the incidence is higher for papillary than for clear cell tumors [38]. The risk of ESRD-related kidney cancer increases with a longer time on dialysis and decreases following kidney transplantation. Perhaps the most important risk factor for ESRD-related kidney cancer is acquired cystic kidney disease (ACKD), a condition that develops in people with CKD and decreases in prevalence following kidney transplantation [39]. Approximately 60-80% of patients on dialysis for four years and 90% of those on dialysis for at least eight years develop ACKD. Of those with ACKD, approximately 10-20% develop RCCs [38]. The risk of transmitting a small RCC by restored kidney transplantation (0.015% to 1%) [40] appears negligible compared with the perioperative mortality rates for nephrectomy (0.03% for living donor nephrectomy and 0.3% to 1.7% for nephrectomy to remove RCC) and transplantation (1.7%) [2]. There is no clear-cut explanation for the discrepancy that the recurrence rate after nephrectomy for pT1 RCC is 8% but the transmission rate after restored transplantation is only 0.015-1%; however, surgical technique (tumor manipulation and spilling over into the operative field vs. ex vivo complete resection of the tumor from the restored transplant) and immunological milieu (cancer-bearing patient vs. immunesuppressed recipient) may play some role. To minimize tumor occurrence, we used mammalian target of rapamycin (mTOR) inhibitors (sirolimus and everolimus) which have antitumor and immunosuppressive effects. mTOR inhibitors have become established approved agents for treating RCC, and cumulative experience collectively points toward the potential to prevent the development of de novo malignancies during the posttransplant period [41]. In the new era of immunosuppression, despite lower occurrence, malignancy tends to appear earlier after the transplantation [42]. Sirolimus did not significantly impact the overall cancer-specific survival rates at 1 and 5 years of subjects who had de novo malignancies [43]. Therefore, trials of restored kidney transplant recipients using kidneys from which small RCCs are removed are warranted to assess the occurrence of malignancy.
By sharing these data with potential donor patients and becoming aware of their (well-informed) wishes, an experienced urologist may eventually conclude whether to perform PN or RN and whether a kidney should be donated, with renal salvage always maintained as the primary goal [44].
There are some limitations regarding our procedure and PN. We may rarely encounter an entirely endophytic tumor without a substantial capsule and with a tumor texture similar to the normal kidney parenchyma (Figure 3); therefore, tumor excision must be repeated several times because of positive surgical margins reported on frozen sections in our previous experience [2]. This case represents the difficulty in determining the surgical margin of the tumor within a short period of time, especially in cases of in situ PN without cooling or super-selective artery clamping. Positive surgical margins in PN are present in 0-7% of patients after open PN, in 0.7-4% after laparoscopic PN, and in 3.9-5.7% after robot-assisted PN. As indicated by intermediate followup data, the majority of patients with positive surgical margins after PN remain without disease recurrence, and a surveillance strategy seems preferable to surgical re-intervention [45].
A population-based study using the Ontario Cancer Registry (664 patients) suggests that, although positive surgical margins are fairly prevalent (10.7%), they are significantly associated with tumor stage and fat invasion and appear to have little to no impact on 5-year survival rates [46]. Several retrospective studies, including one by Besalah and colleagues, have failed to demonstrate a difference in survival for patients with positive surgical margins [47]. In the case of a positive surgical margin, the nephrectomy specimen will only have viable tumor in 6.9% to 15% of cases [48,49]. Tumor characteristics and surgical factors may contribute to positive surgical margins, which can be modified by the surgeon’s experience, intraoperative ultrasound or better clamping techniques [46]. A large, multicenter study investigated 943 patients undergoing robotic PN and found a low positive surgical margin rate (2.2%), but a higher rate of recurrence and metastases in patients with positive margins [50]. Considering the low risk of local recurrence, metastasis, and mortality, careful surveillance may be the best management option for most patients with positive surgical margins [51].
Solitary small renal tumors consist of heterogeneous lesions, including cysts, AML, and RCC. The enhancement pattern and presence of fat within renal tumors have been reported mainly in the context of the radiological differential diagnosis of AMLs. Although rare, AML and RCC are known to occur within the same kidney but separate from each other [52]. The Bosniak renal cyst classification has been used widely and a new category (category IIF) has been introduced. The frequency of malignancy in category IIF proved to be low (5% to 6%) after careful followup for at least 2 years [53,54]. Radiological features alone appear unable to easily detect RCC, and cyst size (>2 cm), male sex, and younger patient age (< 50 years) should also be taken into consideration [55]. However, currently there are some difficulties in making a definite diagnosis of complex renal cysts or complicated cysts. Percutaneous biopsy may play a key role but has not been widely accepted in part due to conflicting reports regarding its diagnostic accuracy and a rare complication of tumor seeding [56], so Volpe and colleagues recommended the use of a coaxial sheath to minimize the exposure of tumor cells to surrounding tissues [57]. Intraoperative tumor biopsy may be another solution.
Multifocal RCC remains an underappreciated and misunderstood entity with a prevalence of at least 6%. Bilateral multifocal disease is found in 11.7% of cases. The presence of multiple renal tumors in one kidney appears to increase the risk of having renal lesions in the contralateral kidney. Twenty percent of multifocal tumors are benign or indolent in nature. Resection of multiple renal lesions from the same renal unit in a single setting is feasible and may allow for excellent preservation of renal function [58].
We and other transplant surgeons tell our patients that living donor transplantations are usually safe procedures, extend the lives of recipients and provide better quality of life for recipients; however, in reality, living donor transplantation has a significant disadvantage. The perioperative mortality of live donor nephrectomy is 0.03%, and morbidity including minor complications is less than 10% [59]. The survival and health consequences of living donation have proven to be excellent and live donors have better outcomes than their population counterparts [60-62]. Thirty-day mortality after nephrectomy for RCC is 0.9% in the entire cohort of 24,535 patients (SEER database), and its foremost determinants are age and tumor stage. The thirty-day mortality of T1-2N0M0 is 0.2% and 0.4% in patients aged 60-69 and 70-79, respectively [63]. During and after operations in oncogeriatric populations, an impaired stress response, increased senescence, and decreased immunity impact the risk/benefit ratio associated with cancer surgery in the elderly. Increased awareness of such age-related differences is mandatory for those providing surgical treatment for the increasing elderly population of cancer patients [64].
Living unrelated transplant recipients have superior outcomes to deceased transplant recipients. The clinical outcome after transplantation between biological relatives differs from that between legal relatives. Spouses, particularly wives, represent an important potential source of kidney grafts [65]. ABO blood type incompatibility is another important barrier to kidney transplantation between living related pairs, though the higher immunosuppression that is required in ABO-incompatible kidney transplantation is increasing overall living donor kidney transplantation, particularly from a spousal donor (living unrelated), with clinical outcomes approaching ABO-compatible kidney transplantation [66, 67]. Therapeutic donated kidneys may be usable even in high-risk ABOincompatible recipients where the risks of dialysis outweigh the risks of surgery or tumor recurrence [68]. In such cases, no change in the immunosuppression protocol for standard ABO-incompatible transplantation is necessary [68]. To our knowledge, ABO-incompatible restored kidney transplantation using a therapeutic donated kidney is a novel intervention that may expand options for patient care. However, the performance of the procedure is limited to high-volume transplant centers with well-trained staff [68]. Notably, few centers worldwide are well qualified to capably perform ABO-incompatible restored kidney transplantation using therapeutic kidneys along with simultaneous bilateral nephrectomy for polycystic kidneys.
Our clinical trial of restored kidney transplantation using nephrectomized kidneys with small renal tumors is slow and steady in its progression. So far, we have performed 18 cases of restored kidney transplantation over 7 years in an effort to save “unrescuable” dialysis patients, and 10 recipients are still surviving with functional grafts without tumor recurrence. We are trying to expand the procedure into general practice; therefore, we are re-applying for advanced medical treatment (care) facilities regulated under the Medical Care Law to perform restored kidney transplantation at any certified hospital in Japan. This coming trial protocol, which requires the collection of 42 cases to obtain good evidence to verify the utility and safety of the procedure, has been approved conditionally, with a contingency that the problem from October 2017 will be solved; the problem will be worked out soon.
Conclusion
The donor shortage crisis in Japan is prompting dialysisintolerant patients to seek transplantation and donor kidneys in foreign countries, which may be increasing transplant tourism; therefore, highly motivated living donors with incidental renal tumors identified during donor screening may contribute to relieving some of the donor shortage. Potential living related donors in whom renal tumors are discovered during donor screening for kidney transplantation must make the difficult decision of whether to undergo a PN or to donate a therapeutic kidney to their relative. We report five cases of successful kidney transplantation by restoring the donor-provided therapeutic kidney. Our experiences suggest that well-motivated spousal kidney donors for ABO-incompatible or ABO-compatible recipients may provide a good chance for improved health and more active lifestyles for patients on dialysis, and these outcomes are anticipated to enhance their lives.
Acknowledgment
We wish to express our deep appreciation to M. Funama, J. Natsuhara, and N. Utada for their valuable and constructive assistance in coordinating this research. We would also like to thank all the hospital staff and committee members involved for their generous contributions to this project.
Authorship
YO, RM, MM, NM, MN, and HA: designed and performed the research, collected the data, and wrote the manuscript. SS, and KK: performed the research and collected the data.
References
- Yu N, Fu S, Fu Z, Meng J, Xu Z, et al. (2014) Allotransplanting donor kidneys after resection of a small renal cancer or contralateral healthy kidneys from cadaveric donors with unilateral renal cancer: a systematic review. Clin Transplant 28(1): 8-15.
- Ogawa Y, Kojima K, Mannami R, Mannami M, Kitajima K, et al. (2015) Transplantation of restored kidneys from unrelated donors after resection of renal cell carcinoma: Results from 10 patients. Transplant Proc 47: 1711-1719.
- Mannami M, Mannami R, Mitsuhata N, Nishi M, Tsutsumi Y, et al. (2008) Last resort for renal transplant recipients, ‘restored kidneys’ from living donors/patients. Am J Transplant 8(4): 811-818.
- Ogawa Y, Mannami R, Mannami M, Nishi M, Mitsuhata N (2016) What disease conditions could be considered for potential therapeutic kidney donations? J Clin Exp Transplant 1: 1.
- Tsutsumi Y (2007) Renal transplantation using elective benign and malignant kidneys: Objection against banning the transplantation as such. Microscopia 24(3): 200-205.
- Nicol DL, Preston JM, Wall DR, Griffin AD, Campbell SB, et al. (2008) Kidneys from patients with small renal tumours: a novel source of kidneys for transplantation. BJU Int 102(2): 188-192.
- van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, et al. (2007) A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 51(6): 1606- 1615.
- van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, et al. (2011) A prospective, randomized EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 59(4): 543- 552.
- MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, et al. (2012) Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol 61(5): 972-993.
- MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, et al. (2012) Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol 62(6): 1097-117.
- Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, et al. (2017) Renal mass and localized renal cancer: AUA guideline. J Urol 198(3): 520- 529.
- Kutikov A, Smaldone MC, Egleston BL, Manley BJ, Canter DJ, et al. (2011) Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL nephrometry score. Eur Urol 60(2): 241-248.
- Ficarra V, Novara G, Secco S, Macchi V, Porzionato A, et al. (2009) Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 56(5): 786-793.
- Simmons MN, Ching CB, Samplaski MK, Park CH, Gill IS (2010) Kidney tumor location measurement using the C index method. J Urol 183: 1708-1713.
- Morath C, Zeier M, Döhler B, Opelz G, Süsal C (2017) ABO-incompatible kidney transplantation. Front Immunol 8: 234.
- Lugo-Baruqui JA, Guerra G, Chen L, Burke GW, Gaite JA, et al. (2015) Living donor renal transplantation with incidental renal cell carcinoma from donor allograft. Transpl Int 28(9): 1126-1130.
- Sung RS, Abt PL, Desai DM, Garvey CA, Segev DL, et al. (2011) The right organ for the right recipient: the ninth Annual American Society of Transplant Surgeons’ State-of-the-Art winter symposium. Clin Transplant 25(6): E592-598.
- Flechner SM, Campbell SC (2012) The use of kidneys with small renal tumors for transplantation: who is taking the risk? Am J Transplant 12(1): 48-54.
- Watson CJ, Roberts R, Wright KA, Greenberg DC, Rous BA, et al. (2010) How safe is it to transplant organs from deceased donors with primary intracranial malignancy? An analysis of UK registry data. Am J Transplant 10(6): 1437-1444.
- Sener A, Uberoi V, Bartlett ST, Kramer AC, Phelan MW (2009) Livingdonor renal transplantation of grafts with incidental renal masses after ex-vivo partial nephrectomy. BJU Int 104(11): 1655-1660.
- Cohn JA, Englesbe MJ, Wolf JS (2008) Can urologic oncologists help expand the renal donor pool with “restored” kidneys? Urol Oncol 26(6): 573-574.
- Nicol D, Fujita S (2011) Kidneys from patients with small renal tumours used for transplantation: outcomes and results. Curr Opin Urol 21(5): 380-385.
- Shimazono Y (2007) The state of the international organ trade: a provisional picture based on integration of available information. Bull World Health Org 85: 955-962.
- Kokubo A (2009) The interaction of the international society concerning kidney transplants--a consideration of diseased kidney transplants in Japan and transplant tourism over the world. Leg Med (Tokyo) 11(suppl 1): S393-395.
- Fujita M, Slingsby BT, Akabayashi A (2010) Transplant tourism from Japan. Am J Bioeth 10: 24-26.
- Meng M, Whitson JM (2009) Planned renal allograft transplantation after tumor excision: increasing the availability of living-donor kidneys. Urol Oncol 27(4): 349-351.
- Weight CJ, Crispen PL, Breau RH, Kim SP, Lohse CM, et al. (2013) Practice-setting and surgeon characteristics heavily influence the decision to perform partial nephrectomy among American Urologic Association surgeons. BJU Int 111(5): 731-738.
- Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, et al. (2016) ESMO guidelines committee. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27(Suppl 5): v58–v68.
- Kunath F, Schmidt S, Krabbe LM, Miernik A, Dahm P,et al. (2017) Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev 5: CD012045.
- Donin NM, Suh LK, Barlow L, Hruby GW, Newhouse J, et al. (2012) Tumour diameter and decreased preoperative estimated glomerular filtration rate are independently correlated in patients with renal cell carcinoma. BJU Int 109(3): 379-383.
- Simmons MN, Hillyer SP, Lee BH, Fergany AF, Kaouk J, et al. (2012) Functional recovery after partial nephrectomy: effects of volume loss and ischemic injury. J Urol 187(5): 1667-1673.
- Ogawa Y, Kouno Y, Suzuki H (2017) Can renal denervation during nephron sparing surgery for renal cell carcinoma prevent de novo hypertension occurrence and potentially confer a survival benefit? SM J Urol 3(1): 1024.
- Pierorazio PM, Johnson MH, Patel HD, Sozio SM, Sharma R, et al. (2016) Management of renal masses and localized renal cancer: Systematic review and meta-analysis. J Urol 196(4): 989-999.
- Hemal AK, Kumar A, Kumar R, Seth A, Gupta NP (2007) Laparoscopic versus open radical nephrectomy for large renal tumors: a long-term prospective comparison. J Urol 177(3): 862-866.
- Butler BP, Novick AC, Miller DP, Campbell SA, Licht MR (1995) Management of small unilateral renal cell carcinomas: radical versus nephron-sparing surgery. Urology 45(1): 34-40.
- Lee JH, You CH, Min GE, Park JS, Lee SB, et al. (2007) Comparison of the surgical outcome and renal function between radical and nephronsparing surgery for renal cell carcinomas. Korean J Urol 48: 671-676.
- Stewart SB, Thompson RH, Psutka SP, Cheville JC1, Lohse CM, et al. (2014) Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol 32(36): 4059-4065.
- Karami S, Yanik EL, Moore LE, Pfeiffer RM1, Copeland G, et al. (2016) Risk of renal cell carcinoma among kidney transplant recipients in the United States. Am J Transplant 16(12): 3479-3489.
- Yanik EL, Clarke CA, Snyder JJ, Pfeiffer RM, Engels EA (2016) Variation in cancer incidence among patients with ESRD during kidney function and nonfunction intervals. J Am Soc Nephrol 27(5):1495-1504.
- Nalesnik MA, Woodle ES, DiMaio JM, Vasudev B, Teperman LW, et al. (2011) Donor-transmitted malignancies in organ transplantation: assessment of clinical risk. Am J Transplant 11(6): 1140-1147.
- Klintmalm GB, Saab S, Hong JC, Nashan B (2014) The role of mammalian target of rapamycin inhibitors in the management of post-transplant malignancy. Clin Transplant 28(6): 635-648.
- Watorek E, Boratynska M, Smolska D, Patrzalek D, Klinger M (2011) Malignancy after renal transplantation in the new era of immunosuppression. Ann Transplant 16(2): 14-18.
- Branco F, Cavadas V, Osório L, Carvalho F, Martins L, et al. (2011) The incidence of cancer and potential role of sirolimus immunosuppression conversion on mortality among a single-center renal transplantation cohort of 1,816 patients. Transplant Proc 43(1): 137-141.
- Breau RH, Crispen PL, Jenkins SM, Blute ML, Leibovich BC (2011) Treatment of patients with small renal masses: a survey of the American Urological Association. J Urol 185(2): 407-413.
- Marszalek M, Carini M, Chlosta P (2012) Positive surgical margins after nephron-sparing surgery. Eur Urol 61(4): 757-763.
- Ani I, Finelli A, Alibhai SM, Timilshina N, Fleshner N, et al. (2013) Prevalence and impact on survival of positive surgical margins in partial nephrectomy for renal cell carcinoma: a population-based study. BJU Int 111(8): E300-305.
- Bensalah K, Pantuck AJ, Rioux-Leclercq N, Thuret R, Montorsi F, et al. (2010) Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol 57(3): 466-471.
- Raz O, Mendlovic S, Shilo Y, Leibovici D, Sandbank J, et al. (2010) Positive surgical margins with renal cell carcinoma have a limited influence on long-term oncological outcomes of nephron sparing surgery. Urology 75(2): 277-280.
- Sundaram V, Figenshau RS, Roytman TM, Kibel AS, Grubb RL, et al. (2011) Positive margin during partial nephrectomy: does cancer remain in the renal remnant? Urology 77(6): 1400-1403.
- Khalifeh A, Kaouk JH, Bhayani S, Rogers C, Stifelman M, et al. (2013) Positive surgical margins in robot-assisted partial nephrectomy: a multi-institutional analysis of oncologic outcomes (leave no tumor behind). J Urol 190(5): 1674-1679.
- Borghesi M, Brunocilla E, Schiavina R, Martorana G (2013) Positive surgical margins after nephron-sparing surgery for renal cell carcinoma: incidence, clinical impact, and management. Clin Genitourin Cancer 11(1): 5-9.
- Aron M, Aydin H, Sercia L, Magi-Galluzzi C, Zhou M (2010) Renal cell carcinomas with intratumoral fat and concomitant angiomyolipoma: potential pitfalls in staging and diagnosis. Am J Clin Pathol 134(5): 807-812.
- Israel GM, Bosniak MA (2005) An update of the Bosniak renal cyst classification system. Urology 66(3): 484-488
- O’Malley RL, Godoy G, Hecht EM, Stifelman MD, Taneja SS (2009) Bosniak category IIF designation and surgery for complex renal cysts. J Urol 182(3): 1091-1095.
- Han HH, Choi KH, Oh YT, Yang SC, Han WK (2012) Differential diagnosis of complex renal cysts based on lesion size along with the Bosniak renal cyst classification. Yonsei Med J 53(4): 729-733
- Mullins JK, Rodriguez R (2013) Renal cell carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J 7(3-4): E176-179.
- Volpe A, Mattar K, Finelli A, Kachura JR, Evans AJ, et al. (2008) Contemporary results of percutaneous biopsy of 100 small renal masses: a single center experience. J Urol 180(6): 2333-2337.
- Sorbellini M, Bratslavsky G (2012) Decreasing the indications for radical nephrectomy: a study of multifocal renal cell carcinoma. Front Oncol 2: 84.
- Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, et al. (2010) Perioperative mortality and long-term survival following live kidney donation. JAMA 303(10): 959-966.
- Poggio ED, Braun WE, Davis C (2009) The science of stewardship: due diligence for kidney donors and kidney function in living kidney donation--evaluation, determinants, and implications for outcomes. Clin J Am Soc Nephrol 4(10): 1677-1684.
- Ibrahim HN, Foley R, Tan L, Tan L, Rogers T, et al. (2009) Long-term consequences of kidney donation. N Engl J Med 360(5): 459-469.
- Morgan BR, Ibrahim HN (2011) Long-term outcomes of kidney donors. Curr Opin Nephrol Hypertens 20(6): 605-609
- Cloutier V, Capitanio U, Zini L, Perrotte P, Jeldres C, et al. (2009) Thirtyday mortality after nephrectomy: clinical implications for informed consent. Eur Urol 56(6): 998-1003.
- Kowdley GC, Merchant N, Richardson JP, Somerville J, Gorospe M, et al. (2012) Cancer surgery in the elderly. Sci World J 2012: 303852.
- Kute VB, Shah PR, Vanikar AV, Gumber MR, Goplani KR, et al. (2012) Long-term outcomes of renal transplants from spousal and livingrelated and other living-unrelated donors: a single center experience. J Assoc Physicians India 60: 24-27.
- Yu JH, Chung BH, Yang CW (2017) Korean Organ Transplantation Registry Study Group. Impact of ABO incompatible kidney transplantation on living donor transplantation. PLoS One 12(3): e0173878.
- Park WY, Kang SS, Park SB, Park UJ, Kim HT, et al. (2016) Comparison of clinical outcomes between ABO-compatible and ABO-incompatible spousal donor kidney transplantation. Kidney Res Clin Pract 35(1): 50- 54.
- Ali AM, Rajagoppal P, Sayed A, Hakim N, David T, et al. (2012) Transplant of kidneys with small renal cell carcinoma in incompatible, heavily immunosuppressed recipients. Ann R Coll Surg Engl 94(6): e189-e190






























