Assessment of Sperm Functional Parameters and Sperm Genomic Integrity Status in Different Infertile Conditions in Male Subjects
Abdul Salam Ansari1, Asha Sharma1, Trilok Chand Sadasukhi2 and Nirmal Kumar Lohiya1
1Department of Zoology, Centre for Advanced Studies, University of Rajasthan, Jaipur-302004, India
2Department of Urology, Mahatma Gandhi Medical College and Hospital, Jaipur-302004, India
Submission: January 11, 2018; Published: January 24, 2018
*Corresponding author: AS Ansari, Department of Zoology, Centre for Advanced Studies, University of Rajasthan, Jaipur-302004, India, Tel: +91141 - 2701809; Fax: +91-141 - 2701809; Email: abdulsansari@yahoo.com
How to cite this article: Ansari A S, Sharma A, Sadasukhi T C, Lohiya N K. Assessment of Sperm Functional Parameters and Sperm Genomic Integrity Status in Different Infertile Conditions in Male Subjects. JOJ uro & nephron. 2018; 4(5): 555650. DOI: 10.19080/JOJUN.2018.04.555650
Abstract
Background: This paper aims to evaluate and correlate the sperm functional parameters, i.e., hypo-osmotic swelling score (HOS), acrosome intactness test (AIT) with sperm DNA integrity of infertile men with different infertile conditions.
Materials and methods: A total of 147 infertile subjects were recruited for the study from SMS Medical College & Hospital, Jaipur, Rajasthan, India. The study group was categorized into five different conditions according to the WHO protocol. Parameters of seminal characteristics and sperm functional tests and DNA integrity of spermatozoa were analyzed.
Results: The HOS and AIT shows decrement in mean value for all infertile subjects compared with the normozoospermic fertile men. However, in teratozoospermic and asthenoteratozoospermic conditions HOS and AIT values decreased significantly (p<0.05, 0.01) as compared to normozoospermic condition. DNA damage in spermatozoa was also increased in teratozoospermic condition as compared to other infertile conditions. A significant and inverse correlation (r=-0.04, P<0.05) was observed between percentage of DNA damage and HOS score. Whereas, AIT score showed an insignificant inverse (r=-0.4, p>0.05) correlation.
Conclusion: The sperm functional tests are objective markers of sperm DNA damage. Thus, DNA integrity assessment have a pivotal diagnostic and prognostic role in infertile men opting for assisted reproduction techniques and sperm functional tests can provide valuable clinical insights into defects causing male infertility.
Keywords: Infertility; Sperm functional test; Hypo-osmotic swelling test; Acrosome intactness test; DNA integrity
Abbreviations: HOS: Hypo-Osmotic Swelling; AIT: Acrosome Intactness Test; ICSI: Intracytoplasmic Sperm Injection
Introduction
Routine semen analysis provides useful information pertaining to sperm production by the testis, sperm motility, viability, the patency of the male genital tract, the secretions of the accessory organs, as well as ejaculation and emission. For initial assessment of infertile male these informations are obvious. However, these tests do not provide insights into the fertilizing potential of the spermatozoon [1-3]. Thus, the semen analysis results alone cannot distinguish the fertile from the infertile subject except the man is azoospermic. To achieve pregnancy spermatozoa must be competent of penetrating and passing through the cervical mucus, and through the uterus to the ampullae of the oviducts, for capacitation, acrosomal reaction, binding and penetration of the zona pellucida, and ultimately the ovum. Once the spermatozoon penetrates the ovum, it must then undergo nuclear decondensation to deliver the appropriate haploid chromosome complement. Defects in any of these complex events can result in male infertility [4]. Now a days there is a significant decrement in these tests with the onset of intracytoplasmic sperm injection (ICSI), despite the fact that evaluation prior to treatment could prevent over treatment with the most advanced and costly technology, as well as unexpected IVF (in vitro fertilization) failure for men with normal semen parameters (as measured by the semen analysis), but with unrecognized functional sperm deficiencies. With ICSI, many physicians treating the infertile couple no longer cared to determine the source of the infertility. If a spermatozoon could be found, there was the likely to fertilize an ovum, regardless of the functional deficiency [5]. Therefore, besides motility and viability parameters, sperm functional tests, i.e., hypo-osmotic swelling test, acrosomal integrity and sperm DNA integrity tests would also be useful in IVF outcome. The hypo-osmotic swelling test assesses the functional integrity of the sperm plasma membrane. The plasma membrane of a spermatozoon is also essential for capacitation, acrosome reaction, binding of the sperm to the egg surface and fertilization. It has also been suggested that the level of acrosin activity of the sperm may be a useful determinant of sperm fertility potential [6]. Sperm chromatin structure protects genetic integrity during transport of the paternal genome through the male and female reproductive tracts [7,8]. Various in vivo and in vitro studies have suggested that disturbances in the organization of the genomic material in sperm nuclei are negatively correlated with the fertility potential of spermatozoa [9-11]. Here, an attempt has been made to assess the quality of sperms through the sperm functional tests and DNA integrity of spermatozoa in different types of diagnosed infertility conditions.
Materials and Methods
The study population comprised from couples who presented themselves for various infertility problems at the Division of Infertility, Department of Urology, SMS Medical College & Hospital, Jaipur, India during March, 2006 to February, 2015. Five hundred infertile couples were selected for initial screening. After exclusion of infertility through female factors, aspermic and subjects with erectile dysfunction and obstructive azoospermic conditions, 147 infertile subjects were recruited for the present investigation. The details of occupation, status of infertility, viz., primary/secondary, details of previous ailments/treatment, lifestyle, occupation, information on age, health problems, etc., were recorded. The age of the recruited subjects ranged 18 to 45 years. From the recruited subjects 34 were businessmen, 41 professionals, 37 casual laborers, 26 farmers and 9 others (students). Based on the subjects profile 28 subjects were the case of excessive alcohol consumption, 40 subjects had the habit of cigarette smoking, 60 subjects were the habit of tobacco chewing and 19 subjects had a high stressful job profile (long driving and long sitting). A complete urogenital examination of each subject was recorded in a predesigned proforma. The study was approved by the Institutional Ethical Committee (IEC) and a written consent to participate in the study was obtained from each subject.
Semen analysis
The semen samples were collected by masturbation into a clean sterile sample collection vial, under aseptic conditions. Subjects were instructed to abstain for at least 48 hours prior to collection of the semen sample. The samples were liquefied for at least 20 minutes in a water bath at 37 °C, but no longer than 1 hour prior to performing a routine semen analysis. Semen analysis was performed within one hour after collection of semen. Semen color, pH, liquefaction, consistency, agglutination, semen volume, sperm count, motility, vitality and sperm morphology were analyzed [12].
Sperm functional test
The hypo-osmotic swelling (HOS) test measures, sperm membrane integrity by examining its ability to swell when exposed to hypo-osmotic media, undamaged sperm tail membrane permits passage of fluid into the cytoplasmic space causing swelling and the pressure generated leads to curling of tail fibers. Damaged cells fail to this property which can be observed under a phase contrast microscope. Spermatozoa with >60% coiled tail considered healthy spermatozoa or normal value of HOS for fertile male, spermatozoa with 50-59% HOS considered subfertile and 0-49% spermatozoa with coiled tail considered infertile male [13]. For sperm functional tests, i.e., HOS and AIT were recorded using the validated test kits obtained from the National Institute of Health & Family Welfare (NIHFW), New Delhi. For HOS test, 50μL semen sample and 500μL HOS solution was added and mixed gently at room temperature for 5min. After the addition of 50μL stop solution, observation was made under light microscope after placing a drop of mixture on a clean glass slide.
The acrosome of spermatozoa contains a number of proteases which play a crucial role in penetration of spermatozoa through outer investments of oocyte. For understanding the status of acrosome membrane integrity test was performed. AIT in fertile males >50% with >30μm halo diameter, subfertile 40-49% with 20-29μm halo diameter and in infertile male 0-39% AIT was considered. For AI 50μL semen sample, 500μL AI solution was added and mixed gently at room temperature for 5min. A smear was made smoothly on a gelatin coated slide. The slide was placed at 50 °C in a moist chamber and incubated for a period of 30min. The slide was air dried and observed under a light microscope [13].
Genomic integrity evaluation
After liquefaction sperms were separated by swim up technique [14] and the sperm concentration at 20*106/mL was adjusted. A medium thick smear was prepared on cleaned glass slide and being air dried, fixed in Carnoy's fixative, stained in 0. 1% acridine orange in citrate phosphate buffer (pH 7.4) and then washed in double distilled water. Slides were observed for normal sperm heads (green in color) and disrupted sperms heads (red or orange in colour) under a fluorescent microscope using 490nm excitation filter and 530nm barrier filter and per cent DNA damage was calculated [15].
Statistical analysis
Values are represented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was employed for statistical comparison. The difference between means was analyzed by Holm-Sidak multiple comparison test to detect the inter-group difference by using the statistical software SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). The P value less than 0.05 was considered as significant. Relationship between two variables was determined by Karl Pearson's coefficient of correlation.
Results
Semen colour, pH, liquefaction, consistency, agglutination and semen volume was insignificantly different in all infertile subjects. Asthenozoospermic condition shows decreased mean sperm vitality than the other conditions. Table 1 shows the sperm functional parameters and per cent DNA damage in different infertile conditions. The HOS and AIT shows decreased mean value for all infertile conditions, compared with normozoospermic condition. Though, in teratozoospermic (p<0.05) and asthenoteratozoospermic (p<0.01) conditions HOS and AI decreased significantly as compared to normozoospermic condition Asthenoteratozoospermic condition showed the highest per cent DNA damage as comparison to other infertile conditions. Figures 1 & 2 show HOS and acrosomal intactness status in different infertile conditions.
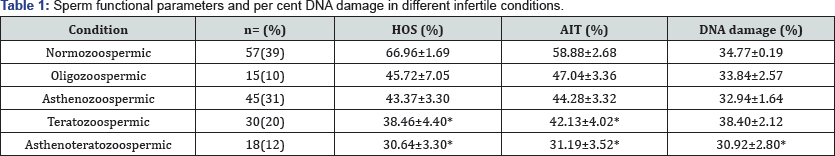
All values are expressed as mean ± SD; HOS: Hypo-osmotc swelling test, AIT: Acrosome Intactness Test, Significant *P<0.05
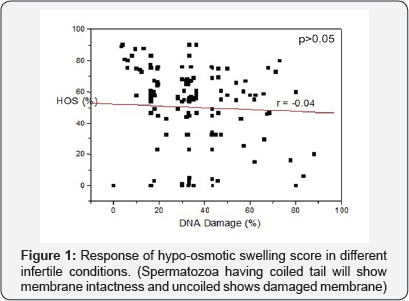

A significant and inverse correlation (r=-0.04, p<0.05) was observed between per cent DNA damage and HOS score. However, acrosomal intactness test insignificantly inversely (r=-0.4, p>0.05) correlated with DNA damage of spermatozoa (Figures 3 & 4). The sperm motility shows a non significant inverse (r =-0.02, p>0.05) correlation with per cent DNA damage of spermatozoa (Figure 5). Sperm motility showed a positive and non significant correlation (r= 0.17, p= 0.41) with HOS scores.

Discussion
The HOS test can be considered an easy, inexpensive, and reliable test for predicting male fertility potential and for identifying among subfertile men those who have a greater possibility of conceiving. In complete asthenozoospermic condition, ahead of ICSI sperm functional parameters are more handy as less time consuming and low cost process to discriminating viable and nonviable spermatozoa [16]. When the condition of severe asthenozoospermia and complete absence of motility, HOS is the only clinical test and also way to find metabolically live spermatozoa for further assisted reproductive treatments [17]. In the present study, a positive and insignificant correlation (r=0.17, p=0.41) was observed between HOS and sperm motility in accordance with the study of Fuse et al. [17]. These findings are supported by the fact that sperm motility depends partly on the transport of compounds across the membrane and consequently would be related to the structural and functional integrity of the membrane. In mammalian spermatozoa high amount of hydrolytic enzyme acrosin is present in acrosome which is very essential for gamete fusion, penetration of zona pellucida and for cervical mucus penetration [18]. In our investigation we found a significantly less AI in all infertile conditions as compared to normozoospermic condition, our results are in agreement with the study carried out by Emokpae and Uadia [19]. Though, in our investigation we found a significant decrement in HOS and AI activity in teratozoospermic and asthenoteratozoospermic conditions as compared to normozoospermic condition. Sreenivasa et al. [20] also reported a reduced AI in infertile males as compared to fertile males. Most of the abnormalities in the acrosomal region and mitochondrial sheath are associated with reduced sperm motility and fertility [21].
With the advancement in infertility management techniques,i. e., assisted reproductive techniques (ARTs) and ICSI, sperm DNA integrity assessment in addition to routine sperm parameters (sperm count, motility, vitality and morphology) has become essential because these techniques bypass several natural selection barriers that are present throughout the male and female reproductive tracts and until the sperm enters the oocyte [22]. Investigations have also shown that if any disorders occur results into failure of ICSI and intrauterine insemination (IUI). These disorders are also one of the main causes of defective offspring [23]. In the present study, a significant inverse correlation was observed between per cent DNA damage and HOS score. However, AIT score shows a nonsignificant inverse correlation with DNA damage of spermatozoa. Ramos and Wetzels [24] had also provided evidences for the importance of sperm motility as a marker for identification of intact DNA in spermatozoa. Sperm motility depends largely on the mitochondrial volume within the mid piece and factor affecting mitochondrial energy production could be responsible for reduction in sperm motility [25]. The DNA damage can be measured directly (fragmentation and oxidation) or indirectly (sperm chromatin compaction). Direct assessment of DNA damage can be obtained by means of single stranded gel electrophoresis assay or "COMET" assay and terminal deoxynucleiotidyl transferase-mediated dUTP-nick end labeling or "TUNEL" assay. DNA damage can be assessed indirectly by means of sperm chromatin integrity assay by acridine orange [14,26-28] and nuclear protein level include slide based sperm nuclear protein stain, viz., aniline blue or toluidine blue (detects histones), CMA3 (detects under protamination) [26,27] and DNA. The sperm chromatin structure assay uses flow cytometry to estimate the percentage of spermatozoa with DNA denaturation [29].
The idea to test sperm chromatin with acridine orange after DNA denaturation occurred in the 1960s [30-32]. Acridine orange staining of mammalian spermatozoa has been used to probe the chromatin structure of the sperm nucleus in relation to the sperm fertility. Acridine orange intercalates into doublestranded DNA as a monomer, whereas it binds to single stranded DNA as an aggregate. Upon excitation at 470nm to 490nm, the monomeric acridine orange bound to double-stranded DNA and fluorescence green, with an emission maximum at 530nm. The aggregated acridine orange on single-stranded DNA, fluorescence red, with an emission at about 640nm [33,34]. The deletion with acridine orange staining differentiates the spermatozoa of fertile males to that of subfertile males [29]. The nuclei of most spermatozoa from fertile males exhibited green fluorescence before and after heat denaturation, whereas those from subfertile males showed green fluorescence before heating, but red fluorescence after heat denaturation. This was interpreted that DNA of infertile spermatozoa is more sensitive to heat-induced denaturation than the DNA of fertile spermatozoa [29]. Acridine orange staining of spermatozoa after acid alcohol treatment has also revealed differences between fertile and infertile men [14]. In the present study, DNA damage was insignificantly and negatively correlated to sperm motility which is in agreement with the studies conducted by Sheikh et al. [35]. The study of sperm DNA characteristics had a great significance because it gives us a clear view of the variation among sperm DNA of different categories of human semen.
Conclusion
The sperm functional tests are objective markers of sperm DNA damage. Thus, DNA integrity assessment with HOS and AIT test have a pivotal diagnostic and prognostic role in infertile men. Incorporating sperm functional tests mainly HOS and AIT into the routine andrology workup will have an important step for diagnosis of male infertility.
Acknowledgment
The investigation was supported by the Indian Council of Medical Research, New Delhi. The Head, Department of Zoology, University of Rajasthan, Jaipur for infrastructural facilities and the Director, NIHFW, New Delhi for sperm functional test kits are gratefully acknowledged.
References
- Khanna J (1994) Biennial Report 1992-1993 Challenges in Reproductive Health Research. World Health Organisation, Geneva, Switzerland.
- Seshagiri PB (2001) Molecular insights into the causes of male infertility. J Biosci 26(4 Suppl): 429-435.
- Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, et al. (2002) Best practice policies for male infertility. Fertil Steril 77(5): 873-882.
- Lamb DJ (2010) Semen analysis in 21st century medicine: The need for sperm function testing. Asian J Androl 12(1): 64-70.
- Jequier AM (2010) Semen analysis: A new manual and its application to the understanding of semen and its pathology. Asian J Androl 2: 1113.
- Kennedy WF, Kaminski JM, Vander Ven HH, Jeyendran RS, Reid, et al. (1989) A simple clinical assay to evaluate the acrosin activity of human spermatozoa. J Androl 10(3): 221-231.
- Zini A, Bielcki R, Phang D, Zenzes MT (2001) Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation in fertile and infertile men. Fertil Steril 75(4): 674-677.
- Evenson DP, Larson KL, Jost LK (2002) Sperm chromatin structure assay: Its clinical use for detecting sperm DNA fragmentation in male fertility and comparisons with other techniques. J Androl 23: 25-43.
- Spano M, Bonde JP, Hjollund HI (2000) Sperm chromatin damage impairs human fertility. Fertil Steril 73(1): 43-50.
- Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A (2010) Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertil Steril 94(4): 1465-1472.
- Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A (2009) Role of male factor in early recurrent embryo loss: Do antioxidants have any effect? Fertil Steril 92(2): 565-571.
- World Health Organization (1999) WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. (4th edn), Cambridge University Press, Cambridge, UK.
- Misro MM, Chaki SP (2008) Development of a rapid, sensitive and reproducible laboratory test kit for the assessment of plasma membrane integrity of human sperm. Fertil Steril 89(1): 223-227.
- Tejada RI, Mitchell JC, Normal A, Marik JJ, Friedman S (1984) A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril 42(1): 87-91.
- Verheyen G, Joris H, Crits K, Nagy Z, Tournaye H, et al. (1997) Comparison of different hypo-osmotic swelling solutions to select viable immotile spermatozoa for potential use in intracytoplasmic sperm injection. Hum Reprod 3(3): 195-203.
- Jothipriya R, Sasikumar S, Madhankumar EK, Pranetha A, Kalaiselvi S (2014) A study of hypoosmotic swelling test in human spermatozoa. Int J Curr Res Rev 2: 47-63.
- Fuse H, Ohta S, Sakamoto M, Kazama T, Katayama T (1993) Hypoos- motic swelling test with a medium of distilled water. Arch Androl 30(2): 111-116.
- Schill WB, Töpfer-Petersen E, Heisslerm E (1988) The sperm acro- some: Functional and clinical aspects. Hum Reprod 3(2): 139-145.
- Emokpae MA, Uadia PO (2006) Acrosin activity in spermatozoa of infertile Nigerian males. J Clin Biochem 21(1): 199-201.
- Sreenivasa G, Vineeth VS, Kavitha P, Malini SS (2012) Evaluation of ac- rosome intactness status in male infertility in Mysore, South India. Int J Appl Bas Med Res 2(1): 31-33.
- Gopalkrishnan K, Anand Kumar TC (1990) Scanning electron microscopy in the assessment of sperm morphology. Indian J Med Res 92: 169-174.
- Agarwal A, Allameneni SSR (2004) The effect of sperm DNA damage in assisted reproduction outcomes. Min Ginecol 56: 235-245.
- Dada R, Gupta NP, Kucheria K (2004) Yq microdeletions azoospermia factor candidate genes and spermatogenic arrest. J Biomole Tech 15(3): 176-183.
- Ramos L, Wetzels AM (2001) Low rates of DNA fragmentation in selected motile human spermatozoa assessed by the TUNEL assay. Hum Reprod 16(8): 1703-1707.
- Ruiz-Pesini E, Diez C, Lapena AC, Perez-Martos A, Montoya J, et.al. (1998) Correlation of sperm motility with mitochondrial enzymatic activities. Clin Chem 44: 1616-1620.
- Bianchi PG, Manicardi GC, Bizzaro D, Bianchi U, Sakkas D (1993) Effect of deoxyribonucleic acid protamination on fluorochrome staining and In situ nick-translation of murine and human mature spermatozoa. Biol Reprod 49(5): 1083-1088.
- Erenpreiss J, Bars J, Lipatnikova V, Erenpreisa J, Zalkalns J (2001) Comparative study of cytochemical tests for sperm chromatin integrity. J Androl 22(1): 45-53.
- Guzick DS, Overstreet JW, Factor-Litvak P (2001) Sperm morphology, motility and concentration in infertile and fertile men. The New Engl J Med 345(19): 1388-1393.
- Evenson DP, Darzynkiewicz Z, Melamed MR (1980) Relation of mammalian sperm chromatin heterogeneity to fertility. Science 210(4474): 1131-1133.
- Gledhill BL, Gledhill MP, Rigler R, Ringertz NR (1966) Changes in de- oxyribonucleoprotein during spermiogenesis in the bull. Exp Cell Res 41: 652-665.
- Ringertz NR, Gledhill BL, Darzynkiewicz Z (1970) Changes in deoxyri- bonucleoprotein during spermiogenesis in the bull. Sensitivity of DNA to heat denaturation. Exp Cell Res 62(1): 204-218.
- Darzynkiewicz Z (1994) Acid-induced denaturation of DNA in situ as a probe of chromatin structure. Meth Cell Biol 41: 527-541.
- Ichimura S, Zama M, Fujita H (197l) Quantitative determination of single stranded sections in DNA using the fluorescent probe acridine orange. Biochim Biophys Acta 240(4): 485-495.
- Peacocke AR (1973) The Interaction of Acridines with Nucleic Acids. In: Acheson RM (Eds.), Chemistry of Heterocyclic Compounds: Acrid- ines, Volume 9 (2nd edn), John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 723-754.
- Sheikh N, Amiri I, Farimani M, Najafi R, Hadeie J (2008) Correlation between sperm parameters and sperm DNA fragmentation in fertile and infertile men. Iranian J Reprod Med 6(1): 13-18.






























