Urinary Bladder Schistosomiasis
Noha Said Helal*
Department of Pathology, Theodor BUharz Research Institute, Giza, Egypt
Submission: November 10, 2017; Published: January 22, 2018
*Corresponding author: Noha Said Helal, Department of Pathology, Theodor Bilharz Research Institute, El-Nile Street, Warrak El-Hadar, Imbaba, Tel: +20225401019; Fax: +20235408125; Giza 12411, Egypt. Email: nohasaidhelal@yahoo.com
How to cite this article: Noha 004 Said Helal. VUrinary Bladder Schistosomiasis. JOJ uro & nephron. 2018; 4(5): 555648. DOI: 10.19080/JOJUN.2018.04.555648
Abstract
Schistosomiasis is a parasitic disease caused by flukes (Trematodes) of the genus Schistosoma. Urinary tract disease is caused by Schistosomahematobium species. Urinary schistosomiasis (Bilharziasis) is often chronic and can cause pain, secondary infection and even bladder cancer. In this review, the histopathological manifestations of urinary bladder schistosomiasis are displayed with emphasis on impact of Schistosomal cystitis on development of urinary bladder cancer.
Keywords: Urinary bladder, Schistosoma haematobium, Bladder Cancer
Introduction
Schistosomiasis is the third most devastating tropical disease in the world after malaria and intestinal helminthiasis; being a major source of morbidity and mortality for developing countries. It is estimated that at least 92% of those requiring treatment for Schistosomiasis live in Africa. Schistosomiasis mostly affects poor communities without access to safe drinking water and adequate sanitation and in rural communities, particularly agricultural and fishing populations. Women doing domestic chores in infested water, such as washing clothes, are also at risk. Inadequate hygiene and contact with infected water make children especially vulnerable to infection [1].
In Egypt, Schistosomes are well-preserved parasites that have been documented to cause urinary disease in humans since ancient times, being mostly documented in Egyptian papyri, notably the Eber's and Edwin Smith's [2].
There are six Schistosomal species affecting human: S. haematobium, S. guineensis, S. intercalatum, S. japonicum, S. mansoni and S. mekongi.
Urinary tract disease is caused by Schistosoma haematobium (S. hematobium) species. Adult worm pairs live in the venous plexus surrounding the bladder and ureters. Schistosomal bladder lesions usually start in the trigone and base [3].
Pathological Features of the Schistosomal Bladder
The histopathological lesions in the bladder due to Schistosomiasis are classified into four stages according to Von Lichtenberg et al. [4].
o Active Schistosomal granulomatous stage, characterized by granulation tissue with numerous plasma cells and eosinophils around viable bilharzia eggs.
o Chronic active stage; the Schistosomal granulomata are still present,the lymphoid cells and eosinophils are the main cellular elements and 50% of the eggs are calcified.
o Late residual stage; 80% of the eggs are calcified and there is an evidence of healing granulomata with few eosinophils.
o Inactive stage; characterized by dense fibrous scar, sparse lymphocytic infiltrate, and absent eosinophils. All the eggs are particularly calcified.
Schistosomal bladder lesions are most common conveniently described on a topographical basis. Thus mucosal, submucosal and mural lesions are recognized.
Mucosal Lesions
Congestion of the mucosa
This is due to the inflammatory reaction associated with extrusion of the ova. The resulting trauma from rupture of vessels and penetration of ova through the mucosa cause transient edema, inflammatory changes, hyperemia of the mucosa, submucosal hemorrhage and minute epithelial erosions [5].
Schistosomal ulcers
Ulceration occurs mainly during the active stage of ova deposition. With proper treatment they heal, unless they develop into chronic stage due to supervention of secondary infection or because of their location in a relatively a vascular posterior wall [6]. Ulcers in chronic active, late residual or chronic inactive stages are superficial, irregular in outline, with sloping edges and granular yellow floor [7].
The surface of the ulcer is usually devoid of urothelium. The ulcer base shows either sloughing tissue; calcified eggs or viable eggs; inflammatory tissue; or most often a narrow band of fibrous tissue with numerous minute blood vessels. The lamina propria under the ulcer is always thickened by dense fibrous tissue, by granulomatous reaction around viable eggs or by calcified eggs in dense fibrous tissue [8].
Von Brunn's nests (Islands) (Figure 1A)
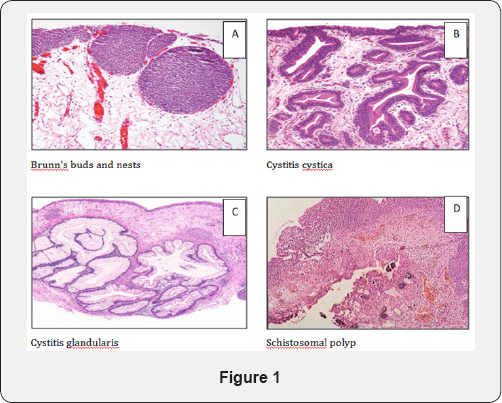
They are pathognomonic of Schistosomiasis. These are late lesions in which the mucosa is roughened, raised and grayish- golden-brown in color. They are proliferative invaginations of urothelium either retaining continuity with the surface (von Brunn's buds) or separate from the mucosa forming well-defined solid nests of urothelial cells within the superficial lamina propria (von Brunn's nests) [9]. The overlying urothelium may be irregularly thickened or atrophic and areas of squamous metaplasia have been described [8].
They are most commonly found in the trigone and bladder neck. It is considered as an early stage of manifestation of a basic metaplastic condition. When very florid, these changes may mimic urothelial carcinoma [10].
Cystitis cystic (Figure 1B)
Cystitis cystic is a common chronic reactive inflammatory disorder, which occur in the setting of chronic irritation. The trigone is the area most commonly affected. Grossly, they usually present as irregular mamillated lesions that may be confused cystoscopically with carcinoma. Microscopically, cystitis cystica is similar to Von Brunn’s nests except that the centers of the nests have undergone eosinophilic liquefaction, when the central lumen of a nest exceeds several millimeters, the remaining urothelial cell in the periphery will be flattened and attenuated liquefaction [11].
Cystitis glandularis (Glandular Metaplasia) (Figure 1C)
It is similar to cystitis cystica except that the urothelial cells which line the cystic lesion have undergone glandular metaplasia with goblet cells identical to those of the large bowel. Gross cystoscopic picture may show it as a papillary lesion. It appears histologically as submucosal nests of columnar epithelial cells surrounding a central liquefied region of cellular degeneration. Patients in whom the intestinal metaplasia is very extensive are at high risk for the development of adenocarcinoma of the urinary bladder [12]. If that epithelium acquires intestinal-type goblet cells, then the term cystitis glandularis with intestinal metaplasia is used [13].
Submucosal Lesions
Schistosomal tubercles
Tubercles are the earliest specific lesions of Schistosomiasis. Grossly, they are seen as multiple shiny yellowish elevated nodules and by time they become brownish and surrounded by a zone of hyperemia. They are most often found on the trigone or posterior wall of the bladder. Histologically, these are small lesions formed of living ova surrounded by neutrophils and eosinophils. They may leave a pit on the surface of the mucosa [14] .
Schistosomal polyps (Figure 1D)
Schistosomal polyps develop in 13% of Schistosomal cystitis [15] . Polypoid lesions result from irritation of the mucosa by Schistosomal products, so that the epithelium is pushed in the direction towards the bladder cavity [6]. They may be single or multiple but are few in number and mostly located in the trigone and near by the ureteric orifices [8]. They increase the frequency of developing carcinoma [16].
Schistosomal granulomata
The basic tissue reaction to Schistosoma eggs is the formation of granulomata around them [17]. The granulomatous reaction can be divided into three stages according to histological criteria:
i. Early active granuloma characterized by a marked eosinophilic response followed by diffuse infiltration with numerous macrophages and multinucleated giant cells surrounding the ova, with a peripheral mantle of eosinophils, plasma cells and lymphocytes;
ii. Intermediate lesions showing macrophages and giant cells in the center surrounded by spindle cells and thin layers of fibrous tissue;
iii. Late lesions showing few or no macrophages and giant cells, increased number of spindle cells and prominent layers of fibrous tissue of variable thickness [18].
Mural Lesions
Weakening of the muscle layer occurs due to trapping of Schistosoma ova associated with fibrosis and endarteritis obliterans leading to ischemia of the muscle layer [19].
The Impact of Schistosomiasis on the Pathology of Bladder Carcinoma
The mechanism by which Schistosomiasis produces bladder cancer remains unknown, but may be related to:
i. The prolonged chronic mechanical irritation of the bladder epithelium caused by Schistosomal ova deposition provoke an intense inflammatory reaction, associated with the production of oxygen-derived free radicals, which may induce genetic mutations or promote the production of carcinogenic compounds such as N-nitrosamines and polycyclic aromatic hydrocarbons) leading to malignant transformation [20].
ii. Schistosomiasis is often accompanied by chronic bacterial super-infectionoftenly gram-negative bacteria that can:
o change urinary nitrites and nitrates into nitrosamines which have carcinogenic effects;
o secrete beta-glucouronidase enzyme that may cleave conjugated carcinogens yielding free carcinogenic products; and
o produce hyperplasia, metaplasia, and dysplastic changes in the urothelium that play an important role in initiating bladder cancer [21-22].
iii. The tryptophan metabolites released from the worms in the blood and excreted in urine may be carcinogenic, as high levels have been reported to correlate with tumor recurrence rates [23].
Schistosoma-associated urinary bladder cancer has some distinctive features regarding
Age: Schistosoma-associated bladder cancer (SA-BC) with positive Schistosomal eggs tends to occur at a relatively young age with a high tendency towards bladder muscle invasion, compared to non-Schistosomal associated cancers in western countries [24-25].
In Egypt, SA-BC was reported in younger age group than non-Schistosomal carcinoma (median age is 46 years) [26]. However, more recent studies showed changing trends of the urinary bladder tumor incidence as a result of the control of Schistosomal infection with increase of median age of diagnosis [27-28].
Sex: In Egypt, El-Bolkainy [26] reported a male predominance in SA-BC. Male to female ratio was 5.6: I. However a recent study by Salem and Mahfouz [28] found the male/female ratio changed to be 4.2:1.
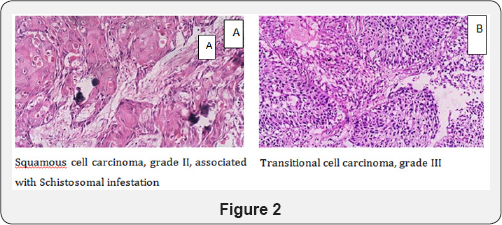
Histological type: A 54-81% incidence of squamous cell carcinoma was found in all cases of bladder cancers in endemic areas, opposed to 3-10% in Western countries [21]. In Egypt, cancers of the urinary bladder accounts for 30.3% of all cancers, of which the majority are squamous cell carcinoma related to Schistosomal infection (Figure 2A). This is similar to other African countries, such as Sudan, Kenya, Uganda, Nigeria and Senegal [29-30]; however other studies showed a changing of the urinary bladder tumor incidence as a result the control of Schistosomal infection with decrease in incidence of squamous cell carcinoma. Transitional cell carcinoma was the commonest form of cancer with low eggs positivity (Figure 2B) [27,31]. Furthermore, Salem and Mahfouz [28] reported a decrease in incidence of SA-BC from 80% to 50%, a significant increase in transitional cell carcinoma from 20% to 66%, with a significant decrease in squamous cell carcinoma from 73% to 25%.
Genetic changes in Schistosomal associated bladder cancer (SA-BC)
Among the most common genetic changes in bladder cancer is the loss of heterozygosity (LOH) on chromosomes 9p and 9q, which is found regardless of tumor grade and stage [32].
The over expression of the Bcl-2 gene in SA-BC patients was found to be up-regulated in squamous but not transitional cell cancers. Mutations of TP53 were detected in 73% of tumors, Bcl- 2 expression in 32% and abnormalities of both TP53 and Bcl-2 in 13% [33].
The cyclooxygenase-2 role in the complex multi-stage process of SA-BC carcinogenesis was proposed: pro-inflammatory cytokines such as interleukin-1, tumor growth factor-B and tumor necrosis factor-alpha. H-RAS, deletion of p16 and p15, increased epidermal growth factor receptor, c-erb-2 and tumor necrosis factor-alpha are additional mutation reported. These changes increase tumorigenicity by decreasing cell apoptosis and/or creating immunosuppression [34].
References
- WHO (2010) Schistosomiasis, Fact Sheet No 115. World Health Organization, Geneva, Switzerland.
- Paul G (1965) Magic and medical science in ancient Egypt. Barnes and Noble, Inc., New York, UK.
- Fried B, Reddy A, Mayer D (2011) Helminths in human carcinogenesis. Cancer Lett 305 (2): 239-249.
- Von Lichtenberg F, Edington GM, NwaBuebo L, Tavlor JR, Smith JH (1971) Pathologic effects of schistosomiasis in Ibadan, Western state of Nigeria. II Pathogenesis of lesions of the bladder and ureters. Am J Trop Med Hyg 20(2): 244-254.?
- Abdulla A, Mousa AH (1984) Schistosomiasis and other trematode infections. In: Woodruff AW, Wright SG (Eds), Medicine in the tropics. (2nd edn), Churchian Livingstone. Edinburg, UK, pp: 163-201.
- Abdel SE and Ehsan A (1978) Cystoscopic picture of Sachistosomahematobium in Egypt children correlated to intensity of infection and morbidity. Am J Trop Med And Hyg 27(4): 774-778.
- Smith JH, Kelada AS, Khalil A (1977) Schistosomal ulceration of the urinary bladder. Am J Trop Med Hyg 26(1): 89-95.
- Elwi AM (1976) Pathology of Schistosomiasis. In complied view on schistosomiasis. National information and Documental center, Dokki, Giza, Egypt, pp: 223-224.
- Weiner DP, Koss LG, Sablay B, Freed SZ (1979) The prevalence and significance of Brunn's nests, cystitis cystica and squamous metaplasia in normal bladders. J Urol 122(3): 317-321.
- Volmar KE, Chan TY, De Marzo AM, Epstein JI (2003) Florid Von Brunn nests mimicking urothelial carcinoma. A morphologic and immunohistochemical comparison to the nested variant of urothelial carcinoma. Am J Surg Pathol 27: 1243-1252.
- Wong-You-Cheong JJ, Woodward PJ, Manning MA, Davis CJ (2006) Inflammatory and Nonneoplastic Bladder Masses: Radiologic- Pathologic Correlation. RadioGraphics 26:1847-1868.
- Kiernan M, Gaffney EF (1990) The endocrine-paracrine cells of Von Brunn's nests and glandular metaplasia in the supramontanal prostatic urethra. Histopathology 16(4): 365-369.
- Hameed O, Humphrey PA (2010) Pseudoneoplastic Mimics of Prostate and Bladder Carcinomas. Arch Pathol Lab Med 134: 427-443.
- Singh M (1976) Tropical parasitic infestations of the urinary tract. In: Blandy J and Blach W (Eds.), Urology. (1st edn), Scintific puplications, Oxford, UK, pp: 261-290.
- Abdel Wahab MF (1982) Clinical and pathological aspects of Schistosomiasis in Egypt. CRC press, Inc Boca Roton Florida, pp: 101110.
- Young RH (1988) Pseudoneoplastic lesions of urinary bladder. In: Rosen PP, Fechner RE (Eds.), Pathology Annual. (1st edn), Appleton and Lange. Norwalk, connectical, San Mateo, California, USA, pp: 67-104
- Garfield E (1986) Schistosomiasis. The scoring of the third world. Current Comments 9: 3-7.
- Al Adnani MS (1985) Concomitant immunohistochemical localization of fibronectin and collagen in schistosomal granulomata. J Pathol 147(2): 77-85.
- Gelfand M (1982) Incomplete emptying of the fibrosed bladder in schistsomiasis. Tropical Doctor 12: 117-119.
- Rosin MP, Zaki SS, Ward AJ, Anwar WA (1994) Involvement of inflammatory reactions and elevated cell proliferation in thedevelopment of bladder cancer in Schistosomiasis patients. Mutat Res 305(2): 83-92.
- Shokeir AA (2004) Squamous cell carcinoma of the bladder: pathology, diagnosis and treatment. BJU Int 93(2): 216-220.
- El-Aasar AA, and El-Merzabani MM (1981) Biochemical detection of etiologic factors. In: El Bolkainy MN, Chu EW (Eds.), Detection of bladder cancer associated with schistosomiasis. Cancer Institute, Al Ahram Press, Cairo, pp: 157.
- Teulings FA, Peters HA, Hop WJ, Fokkens W, Haije WG, et al. (1978) A new aspect of the urinary excretion of tryptophan metabolites in patients with cancer bladder. Int J Cancer 21(2): 140-145.
- Cooppan RM, Bhoola KD, Mayet FG (1984) Schistosomiasis and bladder carcinoma in Natal. S Afr Med J 66(22): 841-843.
- Zarzour AH, Selim M, Abd-Elsayed AA, Hameed DA, Abdelaziz MA (2008) Muscle invasive bladder cancer in Upper Egypt: the shift in risk factors and tumor characteristics. BMC Cancer 8: 250.
- EL Bolkainy MN (2000) Topographic pathology of cancer. (2nd edn), AL- Ahram Press, Cairo, pp: 57-63.
- Gouda I, Mokhtar N, Bilal D, El-Bolkainy T, El-Bolkainy NM (2007) Bilharziasis and bladder cancer: a time trend analysis of 9843 patients. J Egypt Natl Canc Inst 19(2): 158-162.
- Salem HK, Mahfouz S (2012) Changing Patterns (Age, Incidence, and Pathologic Types) of Schistosoma-associated Bladder Cancer in Egypt in the Past Decade. Urol 79(2): 379-383.
- El-Mawla NG, El-Bolkainy MN, Khaled HM (2001) Bladder cancer in Africa: update. Semin Oncol 28(2):174-178.
- Fedewa SA, Soliman AS, Ismail K, Hablas A, Seifeldin IA, et al (2009) Incidence analyses of bladder cancer in the Nile delta region of Egypt. Cancer Epidemiol 33(3-4): 176-181.
- Zaghloul MS, Nouh A, Moneer M, El-Baradie M, Nazmy M, et al (2008) Time-trend in epidemiological and pathological features of schistosoma-associated bladder cancer. J Egypt Natl Canc Inst 20(2): 168-174.
- Jacobs BL, Lee CT, Montie JE (2010) Bladder cancer in 2010: how far have we come? CA: Cancer J Clin 60(4): 244-272.
- Chaudhary KS, Lu QL, Abel PD, Khandan-Nia N, Shoma AM, et al. (1997) Expression of bcl-2 and p53 oncoproteins in schistosomiasis- associated transitional and squamous cell carcinoma of urinary bladder. Br J Urol 79(1):78-84.
- El-Sheikh SS, Madaan S, Alhasso A, Abel P, Stamp G, et al. (2001) Cyclooxygenase-2: a possible target in schistosoma-associated bladder cancer. BJU Int 88(9): 921-927.






























