Mirabegron Combined with Solifenacin Versus solifenacin Monotherapy for Overactive Bladder: A Systematic Review and Meta-Analysis
Wei Sheng1*, Hongwei Zhang2, Kirschner-Hermanns Ruth1
1Department of Neuro-Urology, Rheinisch Friedrich-Wilhelms University, Germany
2Department of Urology, The first people’s hospital of Changde, Changde China
Submission: August 04, 2017; Published: September 11, 2017
*Corresponding author: Wei Sheng, University Clinic, Rheinisch Friedrich-Wilhelms University, Clinic of Urology/Neuro-Urology Bonn, Germany, Tel: 04915776513089; Email: sheng_wei_2007@126.com
How to cite this article: Wei S, Hongwei Z, Kirschner H R. Mirabegron Combined with Solifenacin Versus solifenacin Monotherapy for Overactive 002 Bladder: A Systematic Review and Meta-Analysis. JOJ uro & nephron. 2017; 4(1): 555628. DOI: 10.19080/JOJUN.2017.04.555628
Abstract
Objective: To evaluate the efficacy and safety of mirabegron and solifenacin combined therapy compared with solifenacin monotherapy for overactive bladder.
Methods: We searched the Cochrane Central Register of Controlled Trials, PubMed and EMBASE from inception until March 2017 to identify all eligible studies that compared mirabegron and solifenacin combined therapy with solifenacin monotherapy for overactive bladder.
Results: Five articles were identified as eligible for this meta-analysis, with a total of 2614 participants. Synthetic data showed combined therapy had significant increase in mean volume voided per micturition (mean difference [MD]=9.02; 95% confidence interval [CI] 3.87 to 14.16, P =0.0006), statistically significant improvement in HRQoL (heath related quality of life) total score (mean difference [MD]=3.87; 95% CI 1.30 to 6.45, P=0.003) and decrease of OAB-q symptom bother score (mean difference [MD]=-3.33; 95% CI -4.90 to -1.76, P<0.0001), without significant differences on drug-related adverse effects (AEs),except that it showed combined therapy had a significant low rate of dry mouth than monotherapy therapy in our study (RR=0.57; 95% CI 0.43 to 0.77, P=0.0002).
Conclusion: Mirabegron and solifenacin combined therapy showed a superiority over solifenacin monotherapy, no matter in effectiveness or safety and maybe an alternative for those who experience resistance to solifenacin monotherapy or discontinuation because of severe adverse effects.
Keywords: Solifenacin; Mirabegron; Overactive bladder; Anti-muscarinic drugs; Adverse events
Abbreviations: MD: Mean Difference; AEs: Adverse Effects; OAB: Overactive Bladder; ICS: International Continence Society; QoL: Quality of Life; AR: Adrenoceptor; WMD: Weighted Mean Difference; RR: Risk Ratio: SD: Standard Deviation; SE: Standard Error; MVV: Mean volume voided; HRQoL: Heath Related Quality of Life; UTI: Urinary Tract Infection; TEAEs: Treatment-Emergent Adverse Events
Introduction
Overactive bladder (OAB) is a very common syndrome in elderly people, especially for women, which is defined as urgency with or without urinary in continence, usually associated with frequency and nocturia by the International Continence Society (ICS) [1]. OAB has a great impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation, particularly for those who have experienced the incontinence [2]. That’s the reason why we often call it a “social disease”, not too lethal but really troublesome. Currently, we have several methods for treatment of OAB anti-muscarinic agents are the pharmacological mainstay at present [3,4]. Solifenacin as a first-line drug for OAB has been wildly used in recent years, and it functions well at the beginning [5]. However, the symptom control is often insufficient over time, leading to increase of dosage. That often aggravates anti- cholinergic adverse events (AEs), which can cause treatment discontinuation [6,7].
Mirabegron, a selective β3-adrenoceptor (AR) agonist, is a first in class drug for the treatment of OAB [8]. It has a totally different mechanism for the treatment of OAB from anti-muscarinic drugs. Comparing to the main blockage of M3 receptor from anti-muscarinic drugs, mirabegron mediate human detrusor relaxation through activation of ARs. Whereas, β3-ARs account for more than 95% of all β-AR mRNA in the human bladder [8,9], it suggests mirabegron as an effective and well-tolerated drug in treating OAB. The goal of our study is to evaluate the efficacy and safety of mirabegron and solifenacin combined therapy in comparison with solifenac in mono therapy.
Materials and Methods
Search strategy
To identify relevant studies until March 2017 according to PRISMA, we searched PubMed, EMBASE and Cochrane Library database. All RCTs were retrieved in which patients were randomly selected to receive either solifenacin or solifenacin combined with mirabegron for the treatment OAB. Searches included combinations of the following terms: solifenacin, mirabegron, α-blocker oradrenergic α1- receptor, muscarinic antagonist or anti-muscarinic or anti-cholinergic, overactive bladder or OAB, randomised trial. We also reviewed the references of the included studies to identify potential relevant studies and tried to contact all corresponding authors when data were found missing. There was no restriction regarding to the language of the publications.
Study selection
Publications had to meet the following inclusion criteria [1] the investigation was performed in patients associated with OAB [2] the patients were treated with mirabegron and solifenacin combined therapy versus solifenacicn monotherapy [3] the study reported at least one of the following end points: mean volume voided per micturition, episodes of incontinence per day, episodes of micturition per day, episodes of urgency per day, OAB related questionnaire, adverse effects. The exclusion criteria were as follow [1] the studies reported on OAB associated with other pathological neurological diseases [2] patients had a history of surgery that affected the function of bladder [3] patients had an active urinary tract infection.
Data extraction and quality assessment
For each study, information was carefully collected from all eligible publications, including first author, year of publication, countries, main outcomes, inclusion details, intervention of the trial groups, For studies including different doses of combined therapy and monotherapy, we chose the mirabegron (50mg) and solifenacin (5mg) as the combined therapy and solifenacin (10mg) as the monotherapy. If more than one paper with the same study was found, we included all the studies with available data. If the data for meta-analysis were missing or only expressed graphically, we tried to contact authors to inquire for further information or calculated by ourselves if available. The methodological quality of RCTs was assessed independently using the Cochrane Handbook for Systematic Reviews of Interventions. Two investigators independently evaluated the methodological quality of the included articles. Disagreements were resolved through consensus or discussed with another author.
Statistical analysis
We used the weighted mean difference (WMD) for continuous data (e.g. changes in MVV) and risk ratio (RR) for dichotomous data (e.g. AEs) as the estimated effect measures, and both were reported with 95% confidence intervals. Mean and standard deviation (SD) were calculated for the continuous pooled results. Four articles expressed the data as mean and standard error (SE) [10,11], so we transformed the standard error of the mean into SD by the following formula:
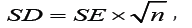
n=Population number.
For the assessment of change of urgency episodes, we used the values which were calculated from adjusted difference versus solifenacin 5mg. In an article [12] they didn’t calculate the change value of solifenacin (10mg) adjusted from solifenacin 5mg. The mean value was the subtraction from solifenacin (10mg) by solifenacin (5mg). The standard error (SE) was calculated from the following formula.
For all statistical comparisons, differences with a P<0.05 were considered significant. Heterogeneity between studies was assessed using the I2 test (I2 value: <50% was considered low heterogeneity; 50% to 75% means moderate heterogeneity; 75% indicates high heterogeneity [13]. When heterogeneity was present (I2>50%), the data was analyzed using the randomeffects model, otherwise, the fixed-effects model was applied when heterogeneity was low. All statistical analyses were performed with Review Manager 5.3 software (The Cochrane Collaboration, Oxford, UK).
Database searches revealed 537 publications up to March 2017. Of these, 245 were immediately because of duplicated series. A further 283 papers were excluded owing to unrelated abstracts, reviews, comments. 11 papers were selected for intensive reading. 2 papers were excluded because of lack of useful data, and another 4 papers were ruled out because the comparison were only between monotherapy or combined therapy (Figure 1). Finally, 3 RCT studies (5 papers) involving a total of 2676 patients were included in the meta-analysis [10,11,13-15] (Table 1).

AEs: Adverse Effects; MVV: Mean Volume Voided per Micturition; EI: Episodes of Incontinence; EM: Episodes of Micturition; EU: Episodes of Urgency; MCC: Maximum Cystometric Capacity; HRQoL total score: Heath Related Quality of Life Total Score
Risk of bias in included studies
We utilized the criteria recommended by Cochrane Handbook for Systematic Reviews to assess the risk of bias in the 3 RCTs included. All the studied had a randomization and described the method of random sequences generation. An adequate concealment of allocation procedure was also used in each study. Non-selective reporting was found in the three trials. Therefore, all of the included trials were considered to have a low risk of bias. The methodological quality of each study was summarized in Table 2.

Primary outcome
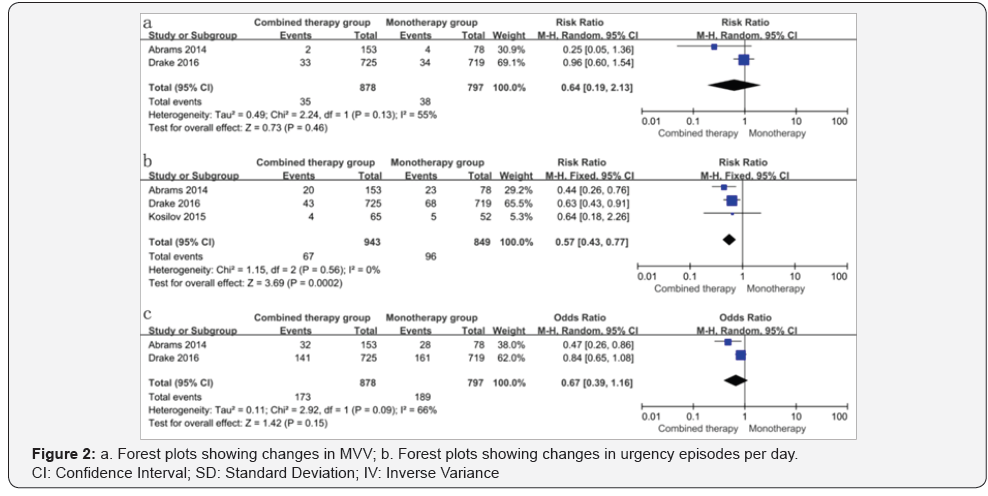
Mean volume voided per micturition (MVV) and urgency episodes per day: MVV was reported in only two studies and were pooled in our meta-analysis. The result revealed that mirabegron and solifenacin combined therapy was associated with dramatically greater increase in MVV compared with solifenacin monotherapy (mean difference WMD=9.02; 95% confidence interval [CI] 3.87 to 14.16, P=0.0006). There was no significant difference between combined therapy and monotherapy over the change of urgency episodes per day. (WMD=-0.37; 95% confidence interval [CI] -0.75 to 0.01, P =0.05) (Figure 2).
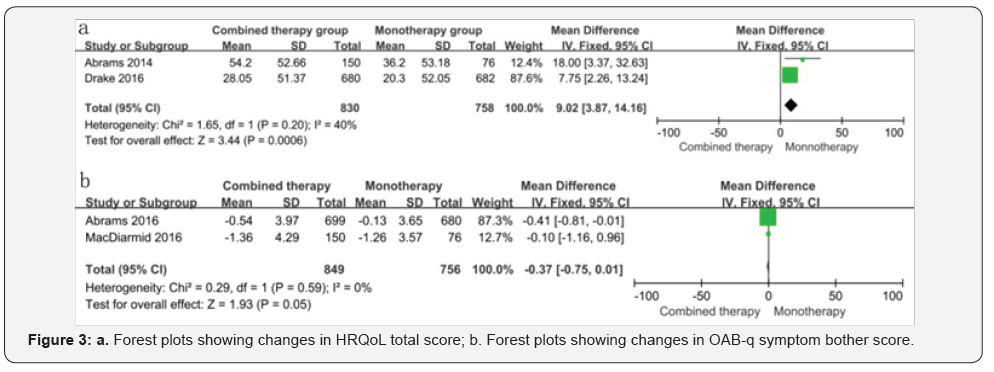
HRQoL (heath related quality of life) and OAB-q symptom bother score: HRQoL (heath related quality of life) total score showed statistically significant improvement in combined therapy over monotherapy (mean difference WMD=3.87; 95% CI 1.30 to 6.45, P =0.003) and decreased OAB-q symptom bother score in combined therapy group (mean difference WMD=-3.33; 95% CI -4.90 to -1.76, P<0.0001) (Figure 3).
Safety
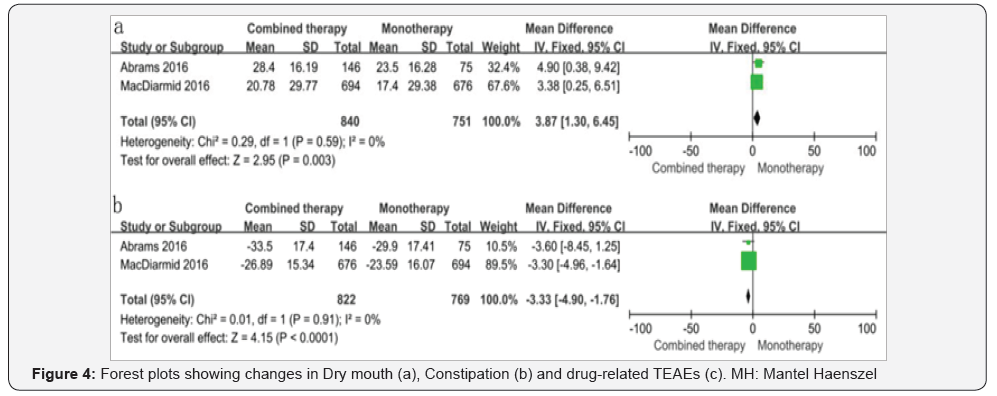
Dry mouth, constipation and drug-related treatmentemergent adverse events (TEAEs): Dry mouth and constipation were discussed in all papers and TEAEs were discussed in two papers. Our results showed that there were no significant differences between the combined therapy group and the monotherapy group in terms of constipation (RR=0.64; 95% CI 0.19 to 2.13, P=0.46) and drug-related TEAEs (RR=0.67; 95% CI 0.39 to 1.16, P=0.15)., However, a statistically significant difference could be observed in the evaluation of Dry mouth, and monotherapy group had a higher rate.(RR=0.57; 95% CI 0.43 to 0.77, P=0.0002) ( Figure 4).
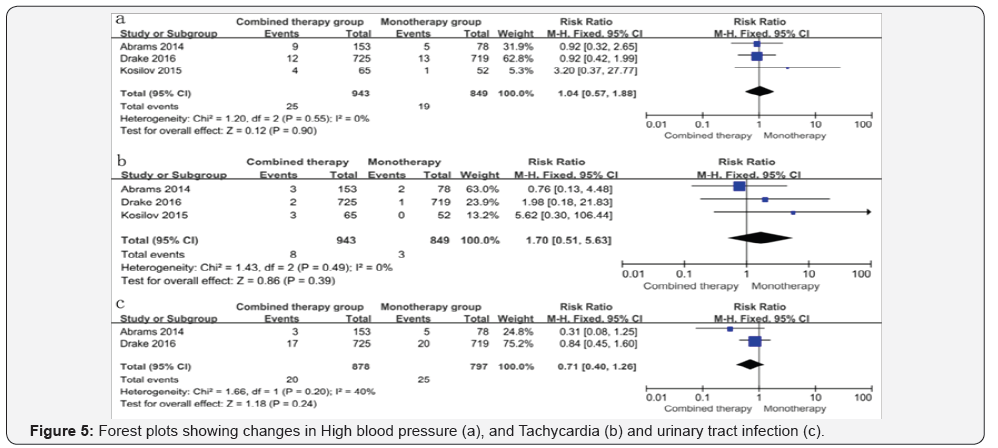
High blood pressure, Tachycardia and Urinary tract infection (UTI): In the consideration of cardiovascular effects and UTI, our analysis suggested that there were no significant differences between the combined therapy group and the monotherapy group regarding to high blood pressure (RR=1.05; 95% CI 0.64 to 1.71, P=0.84), tachycardia (RR=1.01; 95% CI 0.41 to 2.53, P=0.85), UTI (RR=1.18; 95% CI 0.40 to 1.26, P=0.24) (Figure 5).
Discussion
To date, there are a variety of methods available in treating OAB, including bladder and behavioral training, biofeedback procedures, pharmacotherapy, botulinum toxin, electrical or magnetic stimulation, surgery. The pharmacotherapeutic treatment is always the first priority. Because of its effectiveness in alleviating OAB symptoms, while the other treatments such as biofeedback procedures, electrical stimulation or botulinum toxin are either less effective or invasive. There are several muscarinic receptor antagonists that used in clinical practice, for instance solifenacin, tolterodine, fesoterodine, which are the current first-line drugs. Especially for solifenacin, which is a high selective M3 receptor blocker. However, OAB patients may have a suboptimal response or find that anti-muscarinic therapy is limited by associated AEs [16,17].
Mirabegron, a high selective β3-AR agonist, is the first in a new class of agents developed for the treatment OAB, which mediates relaxation of the detrusor during the storage phase of the micturition cycle, improving bladder storage capacity without hindering bladder voiding, and no muscarinic side effects such as dry mouth and constipation [18]. It provides us another effective alternative drug for OAB. Nevertheless, in a phase 3B noninferiority trial in OAB patients refractory to previous anti-muscarinic therapy, mirabegron 50mg did not demonstrate superiority versus solifenacin 5mg [19] That’s why we think mirabegron cannot replace solifenacin completely, the combined therapy maybe a proper option for those who suffer from severe OAB symptoms and muscarinic side effects.
As far as we know, we are the first meta-analysis to evaluate the efficacy and safety of mirabegron and solifenacin combined therapy compared with solifenacin monotherapy for OAB, which is based on RCTs. The most eminent finding in our study is that we observed that combined therapy (mirabegron 50mg+ solifinacin 5mg) was associated with more significant increase in MVV, HRQoL total score and decrease of OAB-q symptom bother score than monotherapy (solifenacin 10mg). It also showed no inferiority for combined therapy over the change of urgency episodes per day (WMD=-0.37; 95% confidence interval [CI] -0.75 to 0.01, P=0.05). No significant difference was found between combined therapy and solifenacin monotherapy concerning drug-related AEs, except the dry mouth rates, which showed monotherapy group had a higher rate of getting dry mouth.
Concerning AEs, the primary side effects of solifenacin are dry mouth and constipation, especially the dry mouth, which is the most common reason for majority of discontinuation [20]. From our results, it suggests the combined therapy is a good option if patients suffer from dry mouth severely. Another worrying adverse effect is the cardiovascular effect for mirabegron. However, no significant differences were found between the two groups concerning the high blood pressure, tachycardia. In a study of systematic review and meta-analysis of phase III trials in efficacy and safety of mirabegron in treating OAB, the rates of getting hypertension, cardiac arrhythmia TEAEs, urinary retention didn’t exceed placebo [14]. That suggests mirabegron is a well-tolerated drug for clinical use. Probably, the combined therapy should not be worried too much about the cardiovascular effect in treating OAB. However, because lack of data of patients with poorly controlled hypertension, arrhythmia, or cardiac heart failure, periodic blood pressure and heart rate measurements are recommended for patients with significant cardiovascular risk factors, such as coronary heart disease and cardiomyopathy, and those aged >80 yr [21-23].
Some limitations of this meta-analysis should be acknowledged. First of all, the number of included studies is not too much, but we believe they are all high quality studies and included enough patients in each research, particularly the two cross-boundary studies, it is robust to draw a conclusion. Secondly, in one study from Kosilov etc., They used the mirabegron 50mg and solifenacin 10mg as combined group, which was different from the other two studies. However, no primary outcomes were included from that study when performing the meta-analysis. What’s more, theoretically, the larger dosage of solifenacin, the more adverse events it will get. In reality, the AEs would be less in the combined group in our study. Because of the relative small number of included patients. It would have a little impact on the results. Thirdly, the longer term safety, efficacy cannot be extrapolated from this article. More high-quality long term trials should be proposed to learn more about the comparison between the combined therapy and solifenacin monotherapy.
Conclusion
Mirabegron and solifenacin combined therapy showed a superiority over solifenacin monotherapy, no matter in effectiveness or safety and may be an alternative for those who experience resistance to solifenacin monotherapy or discontinuation because of severe adverse effects.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, et al. (2002) The standardisation of terminology of lower urinary tract function: Report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn 21(2): 167-178.
- Bartoli S, Aquzzi G, Tarricone R (2010) Impact on quality of life of urinary incontinence and overactive bladder: a systematic literature review. Urology 75(3): 491-500.
- Chapple CR (2000) Muscarinic receptor antagonists in the treatment of overactive bladder. Urology 55(5A suppl): 33-46.
- Garely AD, Borrows LJ (2002) Current pharmacotherapeutic strategies for over active bladder. Expert Opin Pharmacother 3(7): 827-833.
- Cardozo L, Lisec M, Millard R, et al. (2004) Randomized, doubleblind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol 172(5 Pt 1): 1919-1924.
- Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, et al. (2010) Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 105(9): 1276-1282.
- Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, et al. (2011) Persistence and adherence in the treatment of overactive bladder syndrome with anti-cholinergic therapy: a systematic review of the literature. Int J Clin Pract 65(5): 567-585.
- Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, et al. (1999) Evidence for beta3-adrenoceptor subtypes in relaxation of the humanurinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther 288(3): 1367-1373.
- Igawa Y, Aizawa N, Homma Y (2010) β3-adrenoceptor agonists: possible role in the treatment of overactive bladder. Korean J Urol 51(12): 811-818.
- Kosilova K, Loparevb S, Ivanovskayac M, Kosilova L (2015) A randomized, controlled trial of effectiveness and safety of management of OAB symptoms in elderly men and women with standard-dosed combination of solifenacin and mirabegron. Arch Gerontol Geriatr 61(2): 212-216.
- Abrams P, Kelleher C, Staskin D, Rechberger T, Kay R, et al. (2015) Combination Treatment with Mirabegron and Solifenacin in Patients with Overactive Bladder: Efficacy and Safety Results from a Randomised, Double-blind, Dose-ranging, Phase 2 Study (Symphony). Eur Urol 67(3): 577-588.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta analysis. BMJ 327(7414): 557-560.
- Drake MJ, Chapple C, Ahmet A, Athanasiou S, Cambronero, et al. (2016) Efficacy and Safety of Mirabegron Add-on Therapy to Solifenacinin Incontinent Overactive Bladder Patients with an Inadequate Response to Initial 4-Week Solifenacin Monotherapy:A Randomised Doubleblind Multicentre Phase 3B Study (BESIDE). Eur Urol 70(1): 136-145.
- MacDiarmid S, Al-Shukri S, Barkin J Fianu-Jonasson A, Grise P, et al. (2016) Mirabegron as Add-On Treatment to Solifenacin in Patients with Incontinent Overactive Bladder and an Inadequate Response to Solifenacin Monotherapy. J Urol 196(3): 809-818.
- Abrams P, Kelleher C, Staskin D, Kay R, Martan A, et al. (2017) Combination treatment with mirabegron and solifenacin in patients with overactive bladder: exploratory responder analyses of efficacy and evaluation of patient-reported outcomes from a randomized, double-blind, factorial, dose-ranging, Phase II study (SYMPHONY). World J Urol 35(5): 827-838.
- D’Souza AO, Smith MJ, Miller LA, D oyle J, Ariely R (2008) Persistence, adherence, and switch rates among extended-release andimmediate release overactive bladder medications in a regional managed care plan. J Manag Care Pharm 14(3): 291-301.
- Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, et al. (2010) Patient reported reasons for discontinuing over active bladder medication. BJU Int 105(9): 1276-1282.
- Aizawa N, Homma Y, Igawa Y (2012) Effects of mirabegron, a novel β3- adrenoceptor agonist, on primary bladder afferent activity and bladder micro contractions in rats compared with the effects of oxybutinin. Eur Urol 62(6): 1165-1173.
- Batista JE, Kolbl H, Herschorn S, Rechberger T, Cambronero J, et al. (2015) The efficacy and safety of mirabegron compared with solifenacin in over active bladder patients dissatisfied with previous anti-muscarinic treatment due to lack of efficacy: results of a non inferiority, randomized, phase IIIb trial. Ther Adv Urol 7(4):167-179.
- Fu R, Vandermeer BW, Shamliyan TA, O’Neil ME, Yazdi F, et al. (2008) Handling Continuous Outcomes in Quantitative Synthesis. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality, USA.
- Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, et al. (2010) Patient reported reasons for discontinuing overactive bladder medication. BJU Int 105(9): 1276-82.
- Cui Y, Zong H, Yang C, Yan H, Zhang Y (2014) The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials. Int Urol Nephrol 46(1): 275-284.
- Rosa GM, Ferrero S, Nitti VW, Wagg A, Saleem T, et al. (2016) Cardiovascular safety of β3-adrenoceptor agonist for the treatment of patients with over active bladder syndrome Eur Urol 69(2): 311-323.






























