Careful Interpretation of HLA Typing and Cross-Match Tests in Kidney Transplant
Mohanka R1,2, El Kosi M2,3, Jin JK2,4, Sharma A2,5 and Halawa A2,6*
1Depatment of Hepato-Biliary Surgery and Transplantation, Global Hospital, Mumbai, India
2Department of Health and Science, University of Liverpool, UK
3Doncaster Royal Infirmary, UK
4Nottingham Children Hospital, UK
5Royal Liverpool University Hospitals, UK
6Sheffield Teaching Hospitals, UK
Submission: July 03, 2017; Published: August 04, 2017
*Corresponding author: Ahmed Halawa, Consultant Transplant Surgeon, Sheffield Teaching Hospital,United Kingdom, Tel: +447787542128; Fax: +441142714604; Email: Ahmed.Halawa@sth.nhs.uk
How to cite this article: Mohanka R, E Kosi M, Jin J, Sharma A, Halawa A. Careful Interpretation of HLA Typing and Cross-Match Tests in Kidney Transplant.JOJ uro & nephron. 2017; 3(5): 555625. DOI: 10.19080/JOJUN.2017.03.555625
Abstract
HLA typing and cross-matching plays a significant role in organ allocation and optimizing graft utilization in kidney transplantation. The types of tests, information received and interpretation have evolved, expanded and increased in complexity with time. Various techniques used i.e., Complement Dependent Cytotoxicity (CDC), Solid Phase assays using ELISA, flow cytometry and Luminex have been described, compared and limitations discussed. The case discussed is an immunologically low-risk patient with no history of sensitization, negative DSA by Luminex and single antigen mismatch with a positive T and B cell CDC cross-match. There is a high possibility of the CDC being false positive as the given patient has auto-immune disease i.e., lupus nephritis, thereby demonstrating the importance of carefully interpreting available reports understanding its limitations. Further testing to negate IgM and auto-antibodies has been suggested for the given case.
Keywords: Transplantation; Crossmatch; Complement dependent cytotoxicity; Flow cytometry; Virtual crossmatch
Abbreviations: CDC: Complement Dependent Cytotoxicity; CKD5: Chronic Kidney Disease 5; HD: Haemodialysis; FCXM: Flow Cytometry Cross Match; DSA: Donor Specific Antibodies; SAB: Single Antigen Beads; cPRA: Calculated PRA; MFI: Median Fluorescence Intensity; PRA: Panel Reactive Antibodies; cRF: calculated Reaction Frequency; HSPs: Highly Sensitized Patients
Case Details for Discussion
History
A 30 year old male patient with Chronic Kidney Disease 5 (CKD5) had been on haemodialysis (HD) for the last 5 years, secondary to lupus nephritis. There was no history of blood transfusion or previous transplantation.
Transplant status
The patient received the offer of a kidney from a deceased donor.
Laboratory results at admission for transplantation
His laboratory results when he was admitted to the unit for preparation for transplantation were as follows:
• 1-0-0 mismatch.
• Complement dependent cytotoxicity (CDC) cross match reported positive for B and T cell, but flow cytometry cross match (FCXM) was reported negative for both B and T
• Luminex-SAB did not identify any Donor Specific Antibodies (DSA).
Introduction
Kidney transplant offers survival and quality of life benefit over dialysis in patients with chronic kidney Disease (CKD). However, its potential benefit has not been fully realized because of organ shortage. Most patients typically wait for a few years for the transplant and some are never able to receive one. To maximize the benefit of available organs, they are distributed equitably using the newest HLA and cross-matching techniques to minimize immunological risks of graft loss, as demonstrated in the given case.
Discussion
The given patient is a young male with Chronic Kidney Disease (CKD) due to lupus nephritis on Hemodialysis (HD) with no history of blood transfusion or transplantation, which are sensitizing events for developing anti-HLA antibodies. Women may get sensitized to their partner’s HLA antigens during pregnancy. In absence of any history of sensitizing events, the given patient is low-risk for pre-formed anti-HLA antibodies [1]. Pre-formed antibodies are known to cause hyper-acute rejection in the transplanted kidney and are avoided by allotting the kidney to another recipient who does not have pre-formed antibodies against the donor’s antigens [2,3]. At pre-transplant evaluation, the recipient’s serum is collected, preserved and tested for HLA antibodies with the following goals
i. Determine level of HLA sensitization.
ii. Cross-match against donor’s antigens.
The clinical significance of the HLA antibodies should be interpreted with the following context
i. IgG antibodies evoke sensitization response whereas IgM antibodies may not elicit true anti-HLA response.
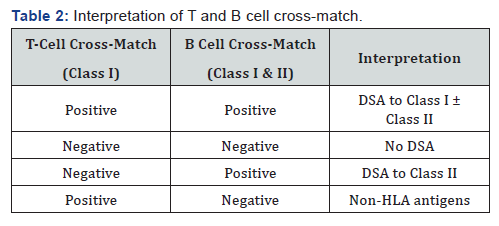
ii. T cells only express HLA Class I antigens whereas B cells express both HLA Class I and II antigens. Class I antibodies manifesting as both T and B cell positivity represents clinically significant sensitization. Class II antibodies manifest only as B cell positivity and is considered clinically less significant.
iii. Complement fixing tests activate the complement cascade leading to cyto toxicity and are considered clinically important. The clinical significance of non-complement fixing antibodies is unclear in the short term but may be associated with increased risk of rejection and graft loss in the long term [4].
Determine level of HLA sensitization
At pre-transplant evaluation, the recipient’s HLA antibody levels are estimated to determine the level of sensitization using one of the following methods
Panel Reactive Antibodies (PRA): The recipient’s serum is tested against a pool (20-100) of local donor antigens using Complement-Dependent Cytotoxicity (CDC) in which donor’s lymphocytes (from the buffy layer of centrifuged blood) are mixed with patient’s serum and rabbit complement and a vital dye. Cell lysis is examined under an inverted phase microscopy and the percentage of reactions reported as PRA [2]. A certain recipient’s PRA levels may change either due to change in antibody levels (due to sensitizing events) or change in the composition of HLA in the assay utilized [1]. PRA is not comparable between laboratories because of differences in composition of cell panel. Falsely high PRAs could be obtained due to IgM or allo-antibodies.
Calculated PRA (cPRA): Antibodies in the recipient’s serum may also be detected using one of the following techniques
ELISA: Allows many HLA antibodies to be characterized, specificities accurately determined and measured semiquantitatively. It is more sensitive than CDC, but differences between testing kits and HLA composition of antigen pool used limits it’s utility and has mostly been replaced by flow cytometry based methods.
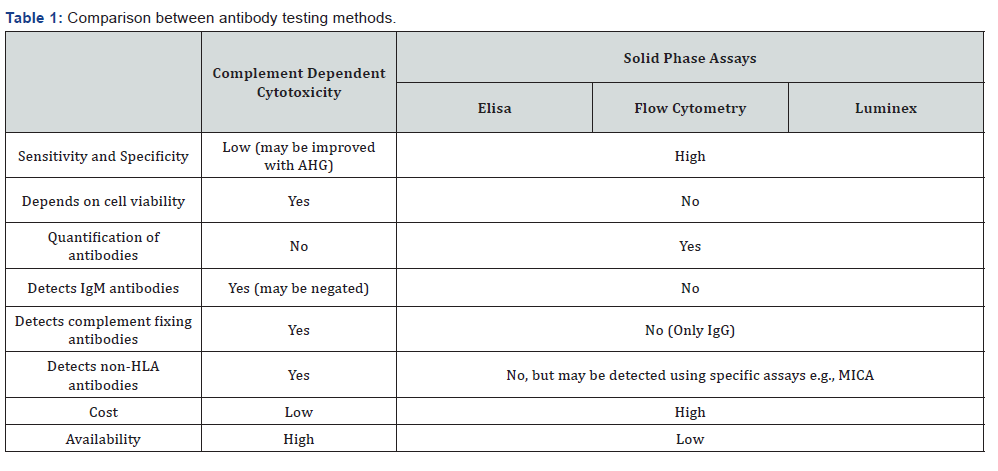
Flow cytometry techniques using single antigen beads (SAB): There are two common methods using this technique and the florescence is most commonly reported as Median Fluorescence Intensity (MFI). One of the aspects to keep in mind when interpreting the results of SAB assays is the prozone phenomenon i.e. when diluted serum gives a higher MFI than the undiluted serum and false negative results at high HLA antibody concentration, which can be negated by pre-heating the test serum, adding ethylene-diamine-tetra-acetic acid (EDTA) or dithiothreitol (DTT) [5,6]. The first method involves flow cytometry using micro-particles, each coated with soluble single HLA antigen to detect and measure the channel shift associated with antibody binding to the beads. It is highly sensitive in detecting various HLA antibodies, more than CDC. The second, which is more popular, is the use of the Luminex® fluorocytometry utilizing two lasers, one of which excites the fluorochrome in the bead and the other laser exciting the detection antibody. The first readout therefore identifies the unique signal of the bead and hence the specificity of the bound HLA molecule, while the second readout indicates if antibody is bound to the specific HLA molecule (Table 1) (Figure 1).
Virtual Cross-match: The ELISA, flow cytometry or Luminex assay is run against a fixed panel of HLA antigens from past organ donors in a large computerized database and the percentage of specific unsuitable antigens reported. This “virtual cross-match” is called as calculated PRA (cPRA) in the US and calculated Reaction Frequency (cRF) in UK [7,8]. PRA/cPRA measure the level of HLA sensitization, the recipients’ likelihood of a positive cross-match but cannot determine the presence of antibodies against a certain donor kidney. The recipient antibody panel is re-tested after every 3 months, additionally when a sensitizing event is recorded and both the highest historical and current PRA are reported. The virtual cross-match is quick, tests against a large number of fixed and consistent HLA antigens. Highly Sensitized Patients (HSPs) have PRA≥80% to 85% and constitute about 15% of listed patients. Once a kidney is available for allocation, the donor antigens are entered in the computer, patients with a positive virtual cross-match are excluded, and the kidney is preferentially allotted to HSPs with a negative virtual cross-match followed by others. The algorithm enables quick and efficient organs allocation with highest likelihood of a negative CDC cross-match. Using the virtual cross-match, HSPs also have higher probability of preferential allotment of suitable organs compared to the past. In the given case, the Luminex SAB did not identify any DSA, which indicates a high chance of the cross-match being negative and the kidney being acceptable.
Donor’s lymphocytes are also tested for the HLA type and matched against the recipient’s known HLA type to obtain the number of mismatches (more often in living donor transplants). The panel includes HLA antigens A, B and DR and excludes HLA Cw, DP and DQ, as they do not generally invoke a strong immune allo-repose and therefore not considered clinically significant [9]. An attempt is made to allot the kidney to recipients with least mismatches as the number of mismatches inversely influence graft outcomes [9-11]. The given case has a single antigen mismatch (1-0-0) and therefore low immunological risk. Crossmatch against donor’s antigens at the time of a deceased donor kidney offer, lymphocytes from the donor blood sample are cross-matched against the recipients’ serum. Various methods of doing the cross-match are used depending on its availability at the centre and time available.
Cell based CDC cross-match: The technique is similar to the one described above, except that for cross-match, actual donor’s cells, instead of pooled donor cells are used. The crossmatch is performed at 4 °C, 22 °C and 37 °C to detect warm and cold reactive antibodies. It is reported qualitatively (positive or negative) or semi-quantitatively either as percentage of dead cells to live cells (0-no dead cells, 2-20% lyses, 4, 6, 8-80% lyses) or repeated dilutions (titres) [2,12]. CDC is performed separately with T and B cells with T cells only expressing Class I antigens and B cells expressing both Class I and II anti gens, to determine the class of reactive antibodies. However, B cell crossmatch has high false positive rates (up to 50%) and therefore not considered relevant nor used by all centers [13] (Table 2).
CDC cross-match may be false negative when
If the donor lymphocytes are not viable or has low antigen expression on its surface.
Low titres, in which case, the cross-match reaction can be enhanced by adding antihuman globulin (AHG) to amplify complement activation and cell lysis [14] by inducing crosslinking of antibodies.
Depletion of antibody with time (when the cross-match was positive in the past). These patients may have a preserved immunological memory and may cause rejection [15].
If the recipient antibodies are non-complement activating.
On the other hand, CDC may be false positive if
Antibodies of auto-immune diseases may cause cell lysis, which can be tested by an auto-cross-match using recipient’s lymphocytes and serum [15,16].
HLA IgM antibodies, in which case, CDC may be repeated after adding Dithiothreitol (DTT) which reduces disulfide bonds in IgM to obtain a negative cross-match. [17-19] IgM antibodies which are reactive at 4 °C may also be inactivated by incubating at 55 °C [20]. Extended incubation by increasing the incubation time of recipient serum, donor cells and complement improves its sensitivity [21].
Clinically irrelevant non-HLA antibodies, may be washed using Amos (3 wash) or modified Amos (1 wash), to remove nonspecific antibodies and increase specificity. The washing is done after primary incubation, before adding complement to remove unbound serum from the lymphocyte suspension to reduce false negative results [21].
Use of Thymoglobulin or Rituximab [22].
Solid Phase Flow Cytometry Cross-Match (FCXM)
In which recipient’s serum is mixed with donor lymphocytes and incubated with fluorescein-labelled antibodies against human IgG. Fluorescein-labelled antibodies bind to donor specific antibodies (DSA) which in turn bind to antigens on donor lymphocytes and read on a flow cytometer. FCXM is more sensitive than CDC and detects complement-activating and complement-independent IgG DSA. Although a MFI of 1000 is commonly used, cut-offs for a positive result is variable between labs [18-22].Generally, a MFI of 10,000-20,000 would result in a positive CDC cross-match in most cases [23-27]. In about 15% cases, the CDC is negative with a positive FXCM, which most commonly indicates low antibody titres. The strength of antibody detected and prior antigen exposure history should also be considered, using such grafts may cause rejection and early graft loss and therefore should be closely monitored [18]. Sometimes, the CDC may be negative for the current sample, but positive from a historical sample. In such a situation, there is a risk of an early rejection due to preserved immunological memory [25].
The patient in the given case is low risk because of lack of history of sensitization, single antigen mismatch on donorrecipient HLA, and negative virtual cross-match. However, a positive CDC cross-match to both T and B cells suggests that the patient may have HLA Class I ± Class II Antibodies against the donor organ. Routinely a positive CDC cross-match would be a contraindication to allot the kidney to this patient because of high risk of rejection however, the FCXM and Luminex SAB DSA are both negative, which is a surprise. The reasons for this discrepancy need to be investigated. Since the patient has lupus nephritis related ESRD, which is an auto-immune disease, there is a possibility of a false positive CDC due to auto antibodies. In this situation, the non-HLA auto-antibodies may be negated using DTT or another technique and a CDC may be repeated. An auto-cross-match may also be performed using the recipient lymphocytes and serum. If either or both reveal it to be a false positive CDC, with a negative FCXM and absence of DSA, it would be safe to offer the kidney to this recipient and proceed with transplant.
Since deceased donor organs are very scare societal resources, cross-match guided allocation is used to minimize immunological graft loss after transplant and maximize utilization of the available kidneys. Therefore, an attempt is made to preferentially allot kidneys to patients with
• Zero mismatch, as they are likely to have the best immunological outcomes [9,10].
• HSPs have low likelihood of finding a suitable match and therefore less access to transplantation and are preferentially allotted kidneys with negative DSA [28- 29].
While on one hand, the above techniques and protocols help us reduce antibody mediated rejections, the time required for testing may increase the graft’s cold ischemia time, which is also detrimental for its function. To balance the two, there is a trend to use virtual cross-match, to select recipients for CDC and allot cross-matched kidneys regionally before being offered nationally in many countries [30].
HLA null alleles
Do not express HLA antigens on cell surface and are denoted with a suffix ‘N’. Molecular methods of HLA typing and crossmatch may detect these DNA sequences, although because of lack of antigen expression, they have little relevance. Mutations in these alleles may lead to expression of antigens similar to one of the regularly expressed HLA antigens. Such antigens are not part of the virtual cross-match panel and therefore will be missed. Depending on the degree of expression of the antigen, the misidentified antigen may form denovo DSA after transplant or lead to a humeral rejection, although typically the risk is low.
Conclusion and Plan
Cross-match techniques currently used in minimizing immunological risk allocation of kidneys are complementary to each other and should be used with an understanding of their limitations. Both a CDC and a solid phase cross-match and additional testing should be used in case of discrepancy in results to optimize allocation and prevent undue denial of a transplant opportunity to any patient. The patient should be further investigated with an auto-cross-match and a repeat CDC with DTT. If one or both of them are positive, it will explain the discrepancy in CDC and FCXM and allow safe transplantation of the offered kidney.
References
- Picascia A, Grimaldi V, Sabia C, Napoli C (2016) Comprehensive assessment of sensitizing events and anti-HLA antibody development in women awaiting kidney transplantation. Transpl Immunol 36: 14- 19.
- Patel R, Terasaki PI (1969) Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280(14): 735-739.
- Cai J, Terasaki PI (2005) Humoral theory of transplantation: Mechanism, prevention, and treatment Hum. Immunol 66(4): 334-342.
- Bartel G, Wahrmann M, Exner M, Regele H, Schillinger M, et al. (2007) Determinants of the complement-fixing ability of recipient presensitization against HLA antigens. Transplantation 83(6): 727- 733.
- Kosmoliaptsis V, Bradley JA, Peacock S, Chaudhry AN, Taylor CJ (2009) Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigenbeads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies. Transplantation 87(6): 813- 820.
- Carey BS, Boswijk K, Mabrok M, Rowe PA, Connor A, et al. (2016) A reliable method for avoiding false negative results with luminex single antigen beads; evidence of the prozone effect. Transpl Immunol 37: 23-27.
- Cecka JM (2010) Calculated PRA (CPRA): The New Measure of Sensitization for Transplant Candidates. Am J Transplant 10(1): 26-29.
- Tait BD, Hudson F, Cantwell L (2009) Luminex technology for HLA antibody detection in organ transplantation. Nephrology (Carlton) 14(2): 247-254.
- Opelz G (1985) Correlation of HLA matching with kidney graft survival in patients with or without cyclosporine treatment. Transplantation 40(3): 240-243.
- Williams RC, Opelz G, McGarvey CJ, Weil EJ, Chakkera HA (2016) The Risk of Transplant Failure with HLA Mismatch in First Adult Kidney Allografts From Deceased Donors. Transplantation 100(5): 1094-102.
- Doxiadis II, Smits JM, Schreuder GM, Persijn GG, van Houwelingen HC, et al. (1996) Association between specific HLA combinations and probability of kidney allograft loss: the taboo concept. Lancet 348(9031): 850-853.
- Terasaki PI, McClelland JD (1964) Microdroplet assay of human serum cytotoxins. Nature 204: 998-1000.
- Mahoney RJ, Taranto S, Edwards E (2002) B-Cell crossmatching and kidney allograft outcome in 9031 United States transplant recipients. Hum. Immunol 63(4): 324-325.
- Karpinski M, Rush D, Jeffery J, Exner M, Regele H, et al. (2001) Flow cytometric cross-matching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol 12(12): 2807-2814.
- Ten Hoor GM, Coopmans M, Allebes WA (1993) Specificity and Ig class of preformed alloantibodies causing a positive crossmatch in renal transplantation. Transplantation 56(2): 298-304.
- Ting A, Morris PJ (1983) Successful transplantation with a positive T and B cell crossmatch due to autoreactive antibodies. Tissue Antigens 21(3): 219-226.
- Tardif GN, McCalmon RT (1995) Successful renal transplantation in the presence of donor specific HLA IgM antibodies. Transplant Proc 27 (1): 664-665.
- Tellis VA, Matas AJ, Senitzer D, Louis P, Glicklich D, et al. (1989) Successful transplantation after conversion of a positive crossmatch to negative by dissociation of IgM antibody. Transplantation 47(1): 127- 129.
- Vaidya S, Ruth J (1989) Contributions and clinical significance of IgM and autoantibodies in highly sensitized renal allograft recipients. Transplantation 47 (6): 956-958.
- Al-Muzairai IA, Mansour M, Almajed L, Alkanderi N, Alshatti N, et al. (2008) Heat inactivation can differentiate between IgG and IgM antibodies in the pretransplant cross match. Transplant Proc 40(7): 2198-2199.
- Howard MG, Robert AB, Peter N (2003) Pre-Transplant Assessment of Donor-Reactive, HLA-Specific Antibodies in Renal Transplantation: Contraindication vs. Risk. Am J Transplant 3(12): 1488-1500.
- Philippe G, Isabelle J, Gilles P, Magdelaine C, Bridoux F, et al. (2013) Very low residual concentrations of rituximab long after infusion still induce positive B-cell complement-dependent cytotoxicitycrossmatch. Hum Immunol 74(12): 1616-1618.
- Böhmig GA, Fidler S, Christiansen FT, Fischer G, Ferrari P (2013) Transnational validation of the Australian algorithm for virtual cross match allocation in kidney paired donation. Hum Immunol 74(5):500- 505.
- Karpinski M, Rush D, Jeffery J, Exner M, Regele H, (2001) Flow cytometric crossmatching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol 12(12): 2807-2814.
- Bryan CF, Baier KA, Nelson PW, Luger AM, Martinez J, et al. (1998) Longterm graft survival is improved in cadaveric renal retransplantation by flow cytometric crossmatching. Transplantation 66(12): 1827-1832.
- Garovoy MRRM, Bigos M, Perkins H, Colombe BFN, Salvatierra O (1983) Flow cytometry analysis: A high technology crossmatch technique facilitating transplantation. Transplant Proc XV: 1939.
- Vaidya S, Partlow D, Susskind B, Noor M, Barnes T, et al. (2006) Prediction of cross match outcome of highly sensitized patients by single and/or multiple antigen bead luminex assay. Transplantation 82: 1524.
- Limaye S, O’Kelly P, Harmon G, O’Neill D, Dorman AM, et al. (2009) Improved graft survival in highly sensitized patients undergoing renal transplantation after the introduction of a clinically validated flow cytometry crossmatch. Transplantation 87(7): 1052-1056.
- De Meester J, Doxiadis IIN, Persijn GG, Claas FHJ (2002) Renal Transplantation of Highly Sensitised Patients via Prioritised Renal Allocation Programs. Nephron 92(1): 111-119.
- Bhavna C, Kalathil KS (2015) Changing organ allocation policy for kidney transplantation in the United States. World J Transplant 5(2): 38-43.
- William RM, John K (2011) Understanding cross-match testing in organ transplantation: A case-based guide for the general nephrologist. Nephrology 16 (2): 125-133.






























