Localized Amyloidosis of Ureter Secondary to Genitourinary Tuberculosis: A Case Report
Yuting Kao1, Kwai-Fong Ng2 and Cheng-Keng Chuang3*
1Chang Gung University, Taiwan
2Department of Pathology, Chang Gung Memorial Hospital, Taiwan
3Division of Urology, Chang Gung University, Taiwan
Submission: July 21, 2017; Published: July 27, 2017
*Corresponding author: Cheng-Keng Chuang, Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, Chang Gung University, Taoyuan, Taiwan, Tel: +886-3-32-81200; Fax: +886-3-3285818; Email: ckchuang@gmail.com
How to cite this article: Yuting K, Kwai-Fong N, Cheng-Keng C. Localized Amyloidosis of Ureter Secondary to Genitourinary Tuberculosis: A Case Report. JOJ uro & nephron. 2017; 3(5): 555623. DOI: 10.19080/JOJUN.2017.03.555623
Abstract
Amyloidosis is a heterogeneous group of diseases that characterized by the extracellular proteins with an insoluble fibril structure called amyloid. The amyloid deposits in tissues with sufficient amount to deteriorate the normal function of the affected tissue. It can be classified into primary or secondary based on immunocyte dyscrasia or complication of chronic inflammatory disorder. It can be systemic or localized when affecting specific organs. Localized amyloid deposition in the urinary tract is rare, especially of the ureter. With the clinical features of painless hematuria, flank pain, ureteral stricture and hydroureteronephrosis, ureteral amyloidosis can be similar to neoplasm or genitourinary tuberculosis (GUTB). It is difficult to diagnose this condition preoperatively. We report the clinical presentation and management of a 39-year-old woman with left ureter amyloidosis secondary to GUTB and review the literature.
Keywords: Congo red; Hydronephrosis; Secondary amyloidosis; Tuberculosis; Ureter
Abbreviations: GUTB: Genitourinary Tuberculosis; AL: Immunoglobulin Light Chain; AA: Amyloidosis (Secondary Amyloidosis)
Introduction
The most common sites of localized secondary amyloidosis is kidney, stomach, rectum and fat, its occurrence in ureter is rare [1]. Localized amyloidosis has a benign clinical course and usually is cured by complete resection. The case is difficult to diagnose this condition preoperatively and histopathologic examination is required for definitive diagnosis and proper management. Early detection and proper treatment for associated inflammatory disorders could avoid grave outcome for these patients.
Case Report
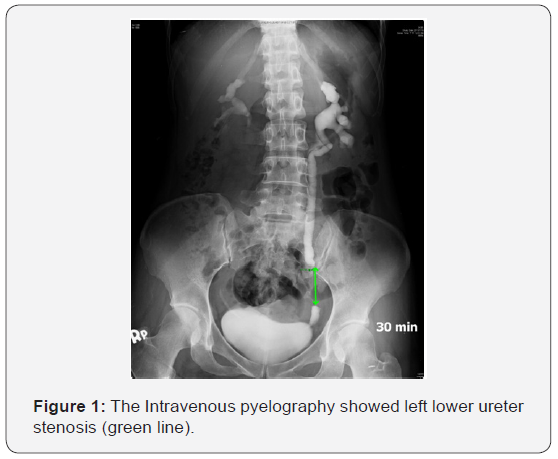
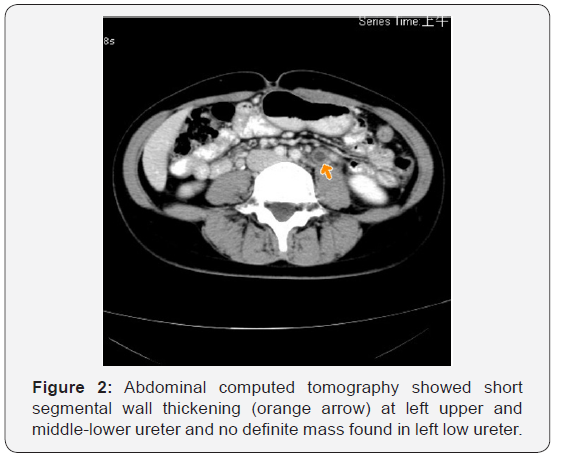
A 39-year-old woman went to a local regional hospital in July 2013 due to painless hematuria followed by left flank pain for one month. She had no history of urologic or chronic medical disorders. The blood analysis, chest X-ray and electrocardiography (ECG) were normal. Urinalysis showed hematuria and proteinuria. The intravenous pyelography (IVP) and computed tomography (CT) were done which disclosed left hydronephrosis and hydroureter with left low ureter stenosis (Figure 1) and wall thickening of left middle and upper ureter without mass found in low ureter (Figure 2). Left ureteroscopy and ureter biopsy was done which did not find any neoplasm, urine from left ureter was positive for acid-fast bacillus test (AFB), left ureter genitourinary tuberculosis was suspected. A double J stent was placed in left ureter. The patient came to our hospital in August 2013, though the voided urine AFB test, mycobacterium smear and culture, polymerase chain reaction (PCR) for tuberculosis (TB) were negative. Because of positive urine AFB from left ureter, anti-TB management with Rifater (combined Rifampicin, Isoniazid, Pyrazinamide) was prescribed. Renal sonography two months later disclosed both kidneys are normal in size with mildly irregular contour and increased cortical echogenicity. There is no evidence of renal stone, mass or cyst found in kidney.
In November, after three-month of anti-TB therapy, left ureteroscopy revealed several polypoid lesions in left ureter with swelling of ureter mucosa, biopsy showed ureter tissue with deposition of amorphous pink materials and focal lymphocytic infiltrates. The amorphous material exhibited apple-green birefringence under polarized light on Congo-red staining, which was indicative of amyloidosis (Figure 3). Blood and urine protein electrophoresis (PEP) and immunoglobulin electrophoresis (IEP) showed no monoclonal (M) protein. There was no evidence of systemic involvement by abdominal CT, the secondary localized amyloidosis secondary to GUTB was impressed.

After six months of anti-TB medication treatment. The patient underwent segmental resection of left low ureter with ureteroneocystostomy in March 2014. The histopathology revealed amyloid deposits. The urine from left ureter was negative for AFB test. Postoperative medication with non steroidal anti-inflammatory drugs (NSAID) Ibuprofen was prescribed for two months. Until this writing, follow-up IVP revealed no evidence of recurrence.
Discussion
Amyloidosis is a generic term that refers to the extracellular deposition of fibrils composed of low molecular weight subunits serum proteins. The presence of amyloid fibrils can be confirmed by their ability to bind Congo red (leading to green birefringence under polarized light) [2]. It can be primary, secondary or hereditary (biochemical classification) and the deposits can be systemic or localized (clinical classification).
Immunoglobulin light chain (AL) amyloidosis (as primary amyloidosis) in which the fibrils are composed of fragments of monoclonal light chains. The diagnosis of AL amyloidosis requires evidence of a monoclonal plasma cell proliferative disorder as displayed by the presence of a serum or urine monoclonal (M) protein. Diagnostic criteria for AL amyloidosis have been developed by the Mayo Clinic and the International Myeloma Working Group and require the presence of all of the following four criteria [3-5].
a. Presence of an amyloid-related systemic syndrome (e.g., renal, liver, heart, gastrointestinal tract or peripheral nerve involvement).
b. Positive amyloid staining by Congo red in any tissue.
c. Evidence that the amyloid is light chain-related established by direct examination of the amyloid using spectrometry-based proteomic analysis or immunoelectron microscopy.
d. Evidence of a monoclonal plasma cell proliferative disorder (e.g., presence of a serum or urine M protein, abnormal serum free light chain ratio, or clonal plasma cells in the bone marrow). Testing of serum and urine for monoclonal immunoglobulins and of serum for free light chains to exclude AL amyloidosis is recommended. In this report, we did not find the presence of blood or urine M protein.
AA amyloidosis (previously referred to as secondary amyloidosis) occurs as a complication of a variety of chronic inflammatory conditions, such as rheumatoid arthritis and its variants, tuberculosis, bronchiectasis, Crohn’s disease and other inflammatory bowel diseases, osteomyelitis, and familial Mediterranean fever. Especially tuberculosis is the main cause [6]. Inflammation leads to increased hepatic production of the acute phase reactant serum amyloid A, which is then degraded in circulating macrophages into smaller amyloid A fragments that are then deposited as fibrils in the tissues. The diagnosis of AA amyloidosis may be suggested by clinical features and by the presence of a predisposing rheumatic or a chronic inflammatory disease (e.g., TB). This patient received anti-TB treatment due to positive urine AFB test, for GUTB, AFB test has low sensitivity and high specificity (38.1% vs 74.5%) [7]. The use of PCR can improve the detection of Mycobacterium tuberculosis in urine or renal tissue, with sensitivity and specificity of 87 to100% vs 92.2 to 98% [6]. Despite the rarity of secondary localized amyloidosis, we should be aware of this entity to avoid misinterpretation and over treatment.
References
- Gertz MA, Kyle RA (1991) Secondary systemic amyloidosis: response and survival in 64 patients. Medicine (Baltimore) 70(4): 246-256.
- Ding X, Yan X, Ma X, Wang C, Du Y, et al. (2013) Localized amyloidosis of the ureter: A case report and literature review. Can Urol Assoc J 7(11- 12): E764-767.
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, et al. (2014) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15(12): e538-548.
- Rajkumar SV (2011) Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol 86 (1): 57-65.
- Kyle RA, Rajkumar SV (2009) Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23 (1): 3-9.
- Agab Eldour AA, Mohmed Salih EN, Ahmed HG (2014) Incidence of Tuberculosis and Amyloidosis among Sudanese Patients Presented with Enlarged Nodes. J Trop Med 2014: 832029.
- Swai HF, Mugusi FM, Mbwambo JK (2011) Sputum smear negative pulmonary tuberculosis: sensitivity and specificity of diagnostic algorithm. BMC Res Notes 4: 475.






























