Safety of Breastfeeding by Mothers on Immunosuppressive Medication for Renal Transplantation: Obsession, Myth and Truth
Sameh M1,2, Mohsen El kossi2,3, Jon Jim Kim2,4, Ahmed H2,5 and Ajay Sharma2,6*
1Department of Nephrology Unit, Ibri regional hospital, Sultanate Oman
2Faculty of Health and Science, Institute of Learning and Teaching, University of Liverpool, UK
3Doncaster Royal Infirmary
4Nottingham Children Hospital
5Sheffield Teaching Hospitals
6Royal Liverpool University Hospitals, Liverpool, UK
Submission: June 06, 2017; Published: June 19, 2017
*Correspondence Address: Ajay Sharma, Renal Transplant Unit, Royal Liverpool University Hospital, University of Liverpool-Prescot Street-L7 8XP, United Kingdom, Tel: +447772362144, Fax: +44 111517065819; Email: ajay.sharma@rlbuht.nhs.uk
How to cite this article: Sameh M, Mohsen E k, Jon J K, Ahmed H, Ajay S. Safety of Breastfeeding by Mothers on Immunosuppressive Medication for Renal Transplantation: Obsession, Myth and Truth. JOJ uro & nephron. 2017; 3(3): 555612. DOI: 10.19080/JOJUN.2017.3.555612
Abstract
It is very natural to be concerned that immunosuppressive medications might be deemed hazardous to infant's breastfed by mothers who are transplanted. This is due to lack of good quality guidelines supported by robust evidence. The drugs that are not safe during pregnancy are not necessarily unsafe during breastfeeding. On reviewing non-transplant patients, we noted that there was no adverse effect reported in infants who were breastfed by mothers receiving steroids even in a high dose of prednisolone 50mg/day. Azathioprine levels were undetectable in breast milk or in infant blood and were not associated with increased risk of infection on a long-term follow-up. Cyclosporine levels are undetectable or at insignificant levels in infants breastfed by mothers on this drug. Tacrolimus levels were detected in infants' serum but at insignificantly low levels. In regards to mycophenolate mofetil and belatacept, the data based on animal studies is rather limited. There is still no conclusive evidence regarding the safety of motor inhibitors during breastfeeding. In conclusion, it is safe for mothers to breastfeed while on immune suppression that includes steroids, cyclosporine, tacrolimus or azathioprine. Nonetheless, a close follow-up of these infants is of paramount importance
Keywords: Breast feeding; Lactation; Immune suppression; Renal transplantation
Abbreviations: CsA: Cyclosporine; TAC: Tacrolimus; MMF: Mycophenolate Mofetil; CNI: Calcineurin Inhibitors; IBD: Inflammatory Bowel Diseases; NTPR: National Transplantation Pregnancy Registry; TGN: Thioguanine; MMPN: Methyl Mercapto Purine Nucleotide
Introduction
Women of childbearing age, who have had a renal transplant and wish to conceive, are faced with a dilemma in regards to the safety of breastfeeding. This question is pertinent because the number of renal transplantation patients has increased over the last two decades. The benefits of normal lactation, physical and psychological elements, for the infant as well as the mother, are well established and well known. Our aim, as clinicians, is to facilitate and encourage them live life as normal as possible. A plethora of new immunosuppressants has become available over the last 2 decades. There are only a few studies performed to address the safety of immune suppression, especially the novel immunosuppressive drugs, from the point of view of breastfeeding by a transplanted mother. This review is aimed to critically appraise current evidence to answer with a focus on immune suppression for renal transplantation.
Methodology
An electronic search was performed for scientific papers using the following keywords: safety of breastfeeding and immunosuppression, renal transplantation, cyclosporine (CsA), tacrolimus (TAC), azathioprine, mycophenolate mofetil (MMF), everolimus, sirolimus, belatacept, and steroid.
Results
We observed the following findings:
- There are no specific trials on MMF, sirolimus, everolimus or belatacept that addressed their safety during breastfeeding.
- Most of the trials explored breastfeeding in patients treated with calcineurin inhibitors (CNI), azathioprine and steroid.
- Studies that looked at usage of steroids for autoimmune diseases.
Analytical Review
Steroids
Grekas et al. [1] reported that oral maintenance doses of prednisolone and azathioprine in 2 lactating mothers did not affect growth, the risk of infections or hematological complications in the infants. They found that immunoglobulin levels in the breast milk did not decrease. Greenberger et al. [2] studied the effect of 50mg of prednisolone, a dose much higher dose than that used in renal transplant recipients, in breast milk of three asthmatic nursing mothers and demonstrated that the levels of prednisolone are at very low levels in breast milk and have no any significant medical effect on infants [3]. It is also noted that this study reported relatively. No side effect or any harm has been observed in infants who were breastfed by mothers receiving steroid. Nyberg et al. [4], Morreti et al. [5], and Bramham et al. [6], demonstrated the safety of CNI in combination with steroids in breastfeeding mothers. Arakali et al. [7] reported no side effects in infants of breastfeeding mothers.
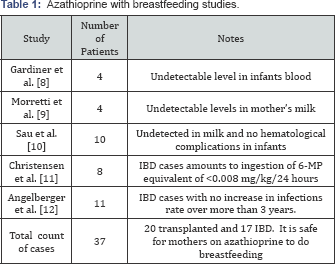
Azathioprine
Gardiner et al. [8] studied four cases of women receiving from 1.2-2.lmg/kg/day of azathioprine daily. They looked for metabolites of azathioprine, 6-thioguanine (6-TGN) and 6-methylmercaptopurine nucleotides (6-MP), in the infant's blood. Both the 6-TGN and 6-MMPN were undetectable. Morretti et al. [9], analyzed breast milk to detect 6-MP, a metabolite of azathioprine. It was undetectable in collected mothers' milk samples and there was no side effect noted in their infants. Sau et al. [10], collected31 breast milk samples from 10 women and reported that 6-MP was undetectable 29 samples .Only 2 samples had a very low level (1.2-7.6ng/ml) compared to mother's blood level (50ng/ml). 6-TGN was not detected in the 31 milk samples. No hematological abnormalities were found in neonates.
Christensen et al. [11], looked for 6-MP in breast milk of lactating mothers (n=8) with inflammatory bowel diseases who received azathioprine (7 of 8 taking 100mg or more, range 75mg to 200mg/day). Samples collected hourly following administration of the treatment. The maximum level of 6-MP in the breast milk was at 4 hours post administration of the azathioprine that amounts to ingestion of 6-MP equivalent of <0.008mg/kg/24 hours. The authors concluded that azathioprine is safe in mothers who are breastfeeding.
Angelberger et al. [12], studied the risk of infection in babies (age 3.3-4.7years) who were breast-fed by mothers with inflammatory bowel diseases (IBD) on azathioprine. The infants of mothers receiving azathioprine (n=11) did not show any significantly increased rate of infection when compared to the control group (n=12) (Table 1)
Cyclosporine (CsA)
Lewis et al. [13] found that CsA levels in the infant blood were 2% of mothers’ serum level. Ziegenhagen et al. [14], reported a case study that maternal milk contains one-sixth of cyclosporine blood level of the lactating mothers. Although Behrens et al. [15], showed that milk cyclosporine levels ranged from 15 to 90% higher than simultaneous serum levels. However, the authors estimated that a breastfed infant would receive less than 5% of an immunosuppressive dose.
Onstensen et al. [16], demonstrated that serum CsA levels in 6 infants were less than 2% of the mother CsA serum level. In an observational study, Thiru et al. [17], demonstrated that the CsA dose was less than 0.1mg/kg in the infant while the mother was receiving 3mg/kg. The infant was followed up for 2 years without any evidence of growth retardation or any reported side effects of CsA affecting the child.
Nyberg et al. [4], showed that breastfeeding can be allowed in the renal transplanted mothers. Their study included 7 infants breastfed by renal transplanted mothers receiving prednisolone, azathioprine and CsA. Blood CsA levels were below the detection limit of 30ng/ml in all infants. Breast milk concentration ranged from 87 to 440ng/ml in 16 samples obtained at different time points from one mother. Infants' serum creatinine ranged from 25 to 54|imol/L at 1 week after birth and 23-52|imol/L after breastfeeding. All babies are thrived. This study concluded that no nephrotoxic effect or other side effect was occurred or noticed in these infants. Similar observations were confirmed by drug safety report by Armenti et al. [18]. Easterling et al. [19], reported that CsA level was undetectable in an infant blood who was breastfed for 10.5 months by a mother treated by CsA.
Excretion of CsA in breast milk in five mothers was studied by Moretti et al. [5]. He concluded that CsA level in human milk varies from 16 micrograms/L to 596 micrograms/L. There was variability in breast milk drug levels, ranging from lower concentration in the fore-milk in comparison to higher concentration in the hind milk. That is explained by lesser fat in foremilk than in hind-milk. Three of them were below 0.5% of mother's weight-adjusted dose and one had 2.1% of mother weight adjusted the dose [20]. Osadchy et al. [21], observed that CsA was undetectable in the blood of a breastfed infant (<10ng/L). Morton et al. [22], in failed to detect CsA in the blood of 2 infants who were breastfed 14 months and 4 months. No side effects were noted in both infants in regards to growth and renal function (Table 2).

Tacrolimus (TAC)
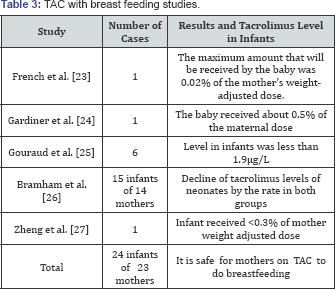
French et al. [23], studied liver transplanted mother receiving 0.1mg/kg/day of tacrolimus. The mean concentration of tacrolimus in milk was 0.429ng/ml. TAC intake infant was estimated at 0.06 microgram/kg/day. They reported also that maximum amount of TAC that will be received by the baby was 0.02% of the mother's weight-adjusted dose. In this study, the researcher followed the development of the infant for 2.5 months only. Physical and neurological development was normal.
Another case reported by Gardiner et al. [24], was a mother, a recipient of any solid organ, who was on TAC. Milk to blood TAC levels ratio was 0.23. The baby received about 0.5% of the maternal dose. Gouraud et al. [25], reported the first long-term follow-up study of 6 infants received breastfeeding for up to 180 days with follow-up of 30 months. TAC level in infants was less than 1.9|ig/L. They advised that lactation in TAC-treated patients was safe with close monitoring of the infant.
Bramham et al. [6] studied 14 mothers who were receiving TAC while breastfeeding their infants. These infants absorbed less than 0.23 % of the maternal weight-adjusted dose. The breastfed infants have been compared to other bottle-fed infants whose mothers were receiving tacrolimus during pregnancy. There was no difference in TAC levels in these 2 groups that were declining after birth. Zheng et al. [26] found tacrolimus level in an infant who received breastfeeding tacrolimus-treated mother was less than 0.3% of mother weight adjusted the dose. They concluded that breastfeeding is safe to another receiving tacrolimus (Table 3).
Sirolimus and everolimus
There are no specific trials for sirolimus and everolimus. However, one of the fourteen infants was breastfed by a mother receiving sirolimus in addition to TAC in a trial reported by Bramham et al. [6]. No clinical harm occurred, but the investigators did not check sirolimus level in infant’s blood. Therefore, it remains uncertain if breastfeeding by mums on sirolimus or everolimus is safe or not.
Mycophenolic acid (MPA)
It is well known that MPA is contraindicated during pregnancy. It could cause spontaneous abortion and other foetal anomalies. A higher incidence of structural malformations was seen with MMF exposures during 28 pregnancies in 18 kidney transplant recipients compared to the overall kidney transplant recipient offspring. MPA in breastfeeding was not studied in humans. Animal studies have demonstrated that MPA can be transferred to breast milk in rats [27].
Belatacept
The author did not find any clinical studies that looked at safety profile in infants being breastfed by mothers on belat accept. Product information revealed that belatacept secreted in breast milk of rats. They recommended that belatacept should not be used while breast-feeding [28].
Discussion
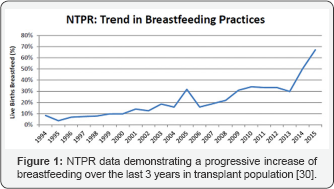
There is an expansion of the numbers of young female patients who are coming through successful solid organ transplantation. Planning conception when the allograft function is stabilised is one of their high priority as suggested by Yusif et al. [29]. The annual report of National Transplantation Pregnancy Registry (NTPR) reported in June 2016 [30] that transplanted mothers who breastfeed while they are on immunosuppression medications are increasing progressively over the last 20 years (Figure 1). As shown in Figure 1 there has been a progressive increase of breastfeeding over the last 3 years by mothers who have received transplantation.
Transplant clinicians should encourage breastfeeding whenever it is possible for a multitude of reasons. Firstly, the increasing number of women in childbearing age group deserves a normal life after transplantation. Secondly, most of the cases that have been published show that breastfeeding while receiving CNI, azathioprine and steroids are relatively safe. Finally, in spite of a lack of clear guidelines, a large percentage of mothers, as shown in the annual report of NTPR30, have preferred to breastfeed their infants.
Conclusion
Based on our review of the literature, it is fine to state that breastfeeding by mothers (who are allograft recipient and are on steroid, azathioprine and CNI) is safe. Many small trials or case studies demonstrated no risk or harmful affect the infants of these mothers. Large size studies with long-term follow-up are needed, however. Drugs serum levels of these mothers should be reviewed more frequently. In addition, the drug level in breast milk and in the blood samples of the infant may need to be checked. The authors suggest a careful and regular evaluation of mothers as well as her infant in order to ensure normal physical and mental growth. Nephrotoxicity in these infants of CNI- treated mothers and hematological complications in infants of azathioprine-treated mothers should be looked for. It is still not clear if mTOR inhibitors are safe during breastfeeding. MMF and belatacept, however, have been proved to be unsafe in animal studies.
References
- Grekas DM, Vasiliou SS, Lazarides AN (1984) Immunosuppressive therapy and breast-feeding after renal transplantation. Nephron 37(1]: 68.
- Greenberger PA (1993] Pharmacokinetics of prednisolone transfer to breast milk. Clin Pharmacol Ther 53(3]: 324-328.
- Ito S1, Blajchman A, Stephenson M, Eliopoulos C, Koren G (1993] Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication. Am J Obstet Gynecol 168(5]: 13931399.
- Nyberg G, Haljamäe U, Frisenette-Fich C, Wennergren M, Kjellmer I (1998) Breast-feeding during treatment with cyclosporine. Transplantation 65(2]:253-255.
- Moretti ME, Sgro M, Johnson DW, Sauve RS, Woolgar MJ, et al. (2003] Cyclosporine excretion into breast milk. Transplantation 75(12): 2144-2146.
- Bramham K, Chusney G, Lee J, Lightstone L, Piercy NC (2013] Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol 8(4]: 563-567.
- Arakali SR, Mealey KJ, Cardonick EH, Gaughan WJ, Davison JM, et al. (2013] Safety considerations: breastfeeding after transplant. Prog Transplant 23(2]: 137-146.
- Gardiner SJ, Gearry RB, Roberts RL, Zhang M, Barclay ML, et al. (2006) Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs. Br J Clin Pharmacol 62(4]: 453-456.
- Moretti ME, Verjee Z, Ito S, Koren G (2006) Breast-feeding during maternal use of azathioprine. Ann Pharmacother 40(12): 2269-2272.
- Sau A, Clarke S, Bass J, Kaiser A, Marinaki A, et al. (2007) Azathioprine and breastfeeding-is it safe? BJOG 114(4): 498-501.
- Christensen LA, Dahlerup JF, Nielsen MJ, Fallingborg JF, Schmiegelow K (2008) Azathioprine treatment during lactation. Alimentary pharmacology & therapeutics. 28(10]: 1209-1213.
- Angelberger S, Reinisch W, Messerschmidt A, Miehsler W, Novacek G, et al. (2011) Long-term follow-up of babies exposed to azathioprine in utero and via breastfeeding. J Crohns Colitis 5(2): 95-100.
- Lewis GJ, Lamont CA, Lee HA, Slapak M (1983] Successful pregnancy in a renal transplant recipient taking cyclosporin A. Br Med J (Clin Res Ed] 286(6365]: 603.
- Ziegenhagen DJ, Crombach G, Dieckmann M, Zehnter E, Wienand P, et al. (1988) Pregnancy during cyclosporin medication following a kidney transplant. Deutsche Medizinische Wochenschrift 113(7]: 260-263.
- Behrens O, Kohlhaw K, Günter H, Wonigeit K, Niesert S (1989) Detection of cyclosporin A in breast milk--is breast feeding contraindicated? Geburtshilfe Frauenheilkd 49(2): 207-209.
- Ostensen M (1992) Treatment with immunosuppressive and disease modifying drugs during pregnancy and lactation. American Journal of Reproductive Immunology 28(3-4): 148-152.
- Thiru Y, Bateman DN, Coulthard MG (1997) Successful breast feeding while mother was taking cyclosporin. BMJ 315(7106): 463.
- Armenti VT, Moritz MJ, Davison JM (1998) Drug safety issues in pregnancy following transplantation and immunosuppression. Drug Saf
- Easterling T, Davis C, Bond EF (2001) Breast-feeding by a cyclosporine- treated mother. Obstet Gynecol 97(5): 816-818.
- Munoz FTKD, Easterling T, Davis C, et al. Breast-feeding by a cyclosporine-treated mother. Obstet Gynecol 97(5): 816-818.
- Osadchy A, Koren G (2011) Cyclosporine and lactation: when the mother is willing to breastfeed. Ther Drug Monit 33(2): 147-148.
- Morton A (2011) Cyclosporine and lactation. Nephrology (Carlton) 16(2): 249.
- French AE, Soldin SJ, Soldin OP, Koren G (2003) Milk transfer and neonatal safety of tacrolimus. Ann Pharmacother 37(6) :815-818.
- Gardiner SJ, Begg EJ (2006) Breastfeeding during tacrolimus therapy. Obstet Gynecol 107(2): 453-455.
- Gouraud A, Bernard N, Millaret A, Bruel M, Paret N, et al. (2012) Follow- up of tacrolimus breastfed babies. Transplantation 94(6): e38-e40.
- Zheng S, Easterling TR, Hays K, Umans JG, Miodovnik M, et al. (2013) Tacrolimus placental transfer at delivery and neonatal exposure through breast milk. Br J Clin Pharmacol 76(6): 988-996.
- Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 82(12): 1698-1702.
- Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, et al. (2014) Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol 28(8): 1163-1173.
- Yousif ME, Bridson JM, Halawa A (2016) Contraception After Kidney Transplantation, From Myth to Reality: A Comprehensive Review of the Current Evidence. Exp Clin Transplant 14(3): 252-258.
- National Transplantation Pregnancy Registry (NTPR) 2015 Annual Report. 2016 June. Gift of Life Institute, Philadelphia.






























