Naimi’s Structure and Migration: A Tissue-Specific Pattern of Organization Discovered in Normal Bladder Urothelia
Bouhout Sara and Bolduc Stephane*
Research Center in Experimental Organogenesis at Laval University/LOEX, Hopital Enfant-Jesus, Quebec
Submission: February 22, 2017; Published: March 06, 2017
*Corresponding author: Bolduc Stephane, Research Center in Experimental Organogenesis at Laval University/LOEX, Hopital Enfant-Jesus, Quebec, Email: stephane.bolduc@ihied.ulaval.ca
How to cite this article: S Bouhout, S Bolduc. Naimi's Structure and Migration: A Tissue-Specific Pattern of Organization Discovered in Normal Bladder Urothelia. JOJ uro & nephron. 2017; 2(2): 555583. DOI: 10.19080/JOJUN.2017.2.555583.
Abstract
Introduction: Sequential migration and organization are critical events in tissue development. The urothelium, the epithelium lining urologic tissues, is highly specialized regarding the watertight function and adaptation to large and frequent changes in urine volume. To establish the functional state of this tissue, horizontal cells growth is required to allow the vertical development into a multilayered uroepithelium. If this first step is already documented, the mechanism implicated in the switch toward the vertical growth is not yet described.
Methods: We had elaborated a three-dimensional bladder model, made of bladder mesenchymal cells (BMC) seeded in a collagen matrix which can promote bladder urothelial cells (BUC) development.
Results: This in vitro model allowed us to discover a behavior specific to BUC, resulting in a specific urothelial stratification and differentiation pattern. Cellular alignment into a circular arrangement, named Naimi’s structure, followed by coordinated gyratory cellular movement, named Naimi’s migration, were sequentially observed in our in vitro model and were also confirmed in native bladder
Conclusion: We describe here the steps that led to Naimi’s structure and migration. Ultrastructural observations are also provided to document the remarkable organization of urothelial cells and the underlying mesenchymal rearrangement.
Keywords: Urinary bladder; Urothelium; Cell migration; Extracellular matrix
Abbreviations: BM: Tissue-Engineered Three-Dimensional Bladder Model; BMC: Bladder Mesenchymal Cell; BUC: Bladder Urothelial Cell; DME: Dulbecco-Vogt modification of Eagle’s Medium; EGF: Epidermal Growth Factor; FCS: Fetal Calf Serum.
Introduction
Cell migration is a function that plays a crucial role in normal physiology as well as in various pathologic conditions. Spatiotemporal coordination of cell migration leads several steps of organ development, apoptosis, wound repair, and tissue regeneration [1]. The literature describes matrix- dependent adhesion molecules, such as integrins, that allow cell anchoring and displacement in-vitro and in-vivo [2]. Cell migration is polarized by various signals, such as chemo attractants, electrical influx [3], magnetic fields [4], cell-cell and cell-matrix interactions, dynamic and static tensions or stress [5,6], or spontaneous movement through intermediate filament gene modulation. Cell differentiation and specialization induce morphological and functional changes that influence migration and tissue organization. All these factors must be considered prior to perform tissue reconstruction in-vitro, notably through the tissue-engineering approach. Isolating, growing and characterizing various cell types in culture, demands frequent observations performed under different conditions. Bladder urothelial cells (BUC) cultured on plastic and on a collagen matrix repeatedly showed a new and unique pattern of organization and migration that we named Naimi’s structure and migration.
The uroepithelium is a distensible epithelial barrier which is able to accommodate the variable volume of urine content and prevent the infiltration of urine into the vascular system [7]. It is composed of multi-layers of urothelial cells that differentiate into highly specialized umbrella cells that exhibit strong watertight function-related features [8]. Among them, the unique morphology of their apical plasma membrane displays scalloped features [9,10]. This particular pattern results from membrane extension, containing integral membrane proteins called uroplakins, which initiate and maintain urothelial membrane remodelling, essential to urine barrier function [11,12]. In the developing phase, it is known that urothelial cells adopt high proliferation activity to entirely recover the mesenchymal layer [13], but the mechanisms that trigger the switch of horizontal toward vertical development into mature and multilayered urothelium are not well documented.
We have established a tissue-engineered three-dimensional bladder model (BM), based on bladder mesenchymal cells (BMC) seeded in a collagen matrix covered with BUC. In order to provide conditions close to the physiological tissue state, we stimulated mesenchymal-epithelial interactions through urine exposure, in our in-vitro three-dimensional bladder model. These conditions allowed BUC to reach a confluent state that led to a spectacular mode of organization and migration, that was never reported in any cell culture system nor in native tissues up until now, including bladder. After the completion of the horizontal development, progressive circular alignment of these cells induced a massive gyratory movement in counterclockwise or clockwise direction, combined to a lateral translation of the whole structure. Following the formation of these remarkable structures, Naimi’s migration was observed simultaneously at several sites on the same tissue-engineered uroepithelium. The ultrastructural organization of BUC and the steps leading to the Naimi’s migration pattern are described.
Materials and Methods
Porcine urothelial and mesenchymal cell isolation
Porcine bladder tissue samples were collected from at least 3 different adult pigs, with the approval ofthe local ethics committee. The tissue processing was performed within 2-3 hours after removing the porcine bladders postmortem. The urothelium was surgically resected from the mesenchymal components of each bladder biopsies. The epithelium and the mesenchyme were set apart. Samples of mesenchyme were cut into pieces of 5mm2 in order to be subjected to enzymatic treatment. Collagen being the major constituent of bladder matrix, collagenase was chosen to digest the native mesenchymes. The tissues were digested in 0.1% (0.2U/ml) collagenase H (Boehringer Mannheim, Montreal, Canada) prepared in DME culture medium containing 10mM CaCl2 without any supplement, overnight at 4°C. Homogenates were centrifuged for 10 min at 300 g and the BMC pellets were resuspended in DME supplemented with 10% fetal calf serum (FCS). BUC were isolated from pig’s urothelium digested with thermolysin, as previously described [14].
Human bladder cancer cell lines
Four bladder cancer cell lines were used: MGHU-3 had been generously provided by Dr Y. Fradet’s lab from a transitional cell carcinoma of a 76 years old Caucasian male (grade 1, passage 97), RT4 (ATCC HTB-2, grade 1, passage 237) from a transitional cell papilloma of a 63 years old Caucasian male, SW780 (ATCC CRL-2169, grade 1, passage 88) from transitional cell carcinoma of an 80 years old Caucasian female, and T24 (ATCC HTB-4, grade 3, passage 56) from a transitional cell carcinoma of a 81 years old Caucasian female.
Bladder cell culture
Porcine BUC and human bladder carcinoma cells lines were cultured in a combination of Dulbecco-Vogt modification of Eagle’s medium (DME) with Ham’s F12 in a 3:1 proportion (Flow Lab., Mississauga, Ontario, Canada), supplemented with 10µg/ml human epidermal growth factor (EGF, Chiron Corp., Emeryville, CA, USA), 5µg/ml crystallized bovine insulin, 5µg/ ml human transferrin, 2 X 10-9M 3,3’,5’, tri iodo-L-thyronin (Sigma Chemicals, St-Louis, MO, USA), 0.4µg/ml hydrocortisone (Calbiochem, La Jolla, CA, USA), 10% FCS (Gibco BRL, Life technologies Inc, Grand Island, NY, USA), 100IU/ml penicillin G and 25µg/ml gentamycin (Sigma). This culture medium was defined as the proliferation medium. It was changed three times a week. After 7 days in culture, urothelial cells had reached 85% confluence and were ready to be stored and sub cultured. BMC were cultured in DME supplemented with 10% FCS (Gibco), 100 IU/ml penicillin G and 25µg/ml gentamycin (Sigma). Culture medium was changed three times a week. Cultures of BUC and BMC were both kept in an 8% CO2 atmosphere at 39°C, and human bladder carcinoma cells lines at 37 °C.
Urothelial cell monolayers culture
BUC and human bladder carcinoma cells lines were grown to confluence (n=6/each urothelial cells type) on plastic culture- treated dishes and on a cellular and BMC seeded collagen gels. All these cells were cultured in the proliferation medium, as described above.
Production of porcine BM
Step 1: Preparation of the mesenchymal layer of the BM: According to the protocol of Paquette, et al. [15], a mixture of native bovine Type I collagen (2.0mg/ml) was prepared by dissolving the powder overnight at 4 °C in sterile 0.017M acetic acid. A solution of 0.84 ml of DME 2.7X containing 200 IU/ml penicillin G and 50 µg/ml of gentamycin, pH 8.0, was mixed with a second solution containing 0.56 ml of FCS, 1.43 ml of the stock collagen solution, 30 µl of NaOH 0.7N and 0.15 ml of a BMC suspension (4 x 105cells/ml of DME supplemented with 10% FCS, 100 IU/ml penicillin G and 25 µg/ml gentamycin).
Step 2: Coating of the mesenchymal layers with complete porcine urine: Porcine urine samples were collected directly from bladders of adult porks immediately postmortem. The urine was frozen without any filtration and stored at -30 °C until use. When the cell-seeded collagen matrices had polymerized (after 20-25 min), a volume of 1 ml was poured onto each mesenchymal layer for 20 min at room temperature. This step aimed at coating the surface of the collagen gel with the natural constituents of porcine urine. Then, the urine was removed by gentle aspiration, and the mesenchyme was ready for epithelialization (n=6). Groups of uncoated mesenchymes (n=6) were used as negative controls. Porcine urine exposure of the BM was repeated at every change of culture medium.
Step3: Epithelialization of the BM: The epithelialization was performed by seeding porcine BUC (4 x 105 cells/BM) on the mesenchymal constructs, pre coated or not with porcine urine. During the first 5-6 days after epithelialization, all BM were maintained in the proliferation medium, supplemented with 50 Hg/ml ascorbic acid, until confluent state was obtained.
Step4: Culture of the BM: The BM were cultured in the differentiation medium as soon as a confluent layer of BUC had covered the mesenchymal constructs (5-6 days after epithelialization, depending on cell growth rates). The differentiation medium was defined as the proliferation medium, supplemented with 5x10-8 M retinoic acid (RA, Sigma) but without EGF, to reduce the secretion of gelatinases by the cells, which degrade the collagen matrix in culture [16]. The culture medium was changed every other day, during 10 days. Before each change of culture medium, a volume of 1 ml of pure porcine urine was poured on the urothelium, and kept for 20 min at room temperature (n=6). Then, the urine was removed by gentle aspiration in order to be replaced by the differentiation medium. Groups of uncoated mesenchymes were cultured without any urine supply (n=6).
Electron microscopy analyses
The electron microscopy analyses were performed by an independent service. BM and native bladder samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4 at 4 °C. Then, they were rinsed with cacodylate buffer and a post fixation in 1% osmium tetroxide. The biopsies for transmission electronic microscopy were stained with uranyl acetate and dehydrated through a graded series of ethanol then embedded in Epon (Polysciences, Warrington, PA). They were cut in ultra-fine sections and counterstained with lead citrate and uranyl acetate. The sections were examined with a JEM 1230 (Tokyo, Japan). The BM and native bladder biopsies for scanning electronic microscopy were dehydrated then critical point dried. Samples were spattered with gold and viewed with a Jeol JSM-63060LV (Tokyo, Japan).
Time-lapse observations of Naimi’s structures and migration
BUC cultured on plastic (n=6), and on reconstructed mesenchymes (n=6) were observed for 48 hrs under a timelapse fluorescence microscopy using a Zeiss Axio Observer Z1, to monitor the evolution of Naimi’s structure and migration from the beginning. The microscope allowed taking pictures in 6 wells in rotation, so that 6 fields could be monitored every 30 min, over a period of 48 hrs.
Results
BUC and BMC morphology in culture
Morphologically stable cell populations (initially at passage 0), were sub cultured over more than 3 passages. Forty eight hours after epithelialization of tissue-engineered mesenchymes pre coated with urine, BUC showed high plating efficiency (Figure 1A). Colonies of BUC grew well (Figure 1B), to reach a confluent state after 5 days of culture in medium of proliferation (Figure 1C). In the 3D collagen matrix of the mesenchymes, BMC progressively spread among the collagen fiber network (Figure 1D). BMC progressively aligned themselves in the horizontal plane (Figure 1E & 1F), in response to the radial tension applied on the collagen gel by the peripheral anchorage.
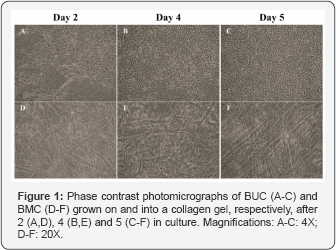
BUC behavior during the formation of Naimis structures
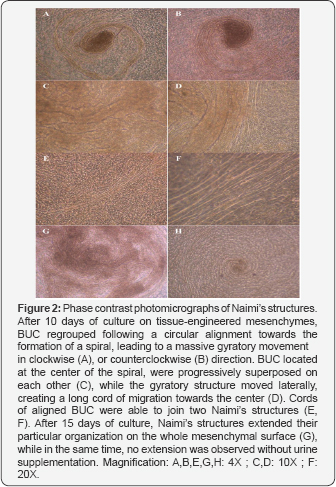
At various sites, at random and in a spontaneous fashion, confluent BUC progressively regrouped to adopt a semi-circular alignment at various sites of the reconstructed urothelium. This first step was followed by the orientation of the cells in a spiral, that led to a massive gyratory movement oriented in a clockwise (Figure 2A) or counterclockwise (Figure 2B) direction. During this migratory process, all surrounding BUC remained morphologically similar, without any sign of degeneration. The center of Naimi’s structure was always dense, filled with BUC aligned in superposed layers, which could be described as cords or ropes, organized in a comparative fashion to the eye of a tropical cyclone (Figure 2C). BUC surrounding the center of Naimi’s structure adopted an elongated morphology and formed parallel lanes of cells organized in concentric circles, like a spiral (Figure 2D-2F). After 15 days of culture in presence of urine, the Naimi’s structures exhibited an important extension through the mesenchymal layer (Figure 2G). In the same time, BUC grown on mesenchymes that were not exposed to urine were capable to create Naimi’s structures, but of smaller diameters and the whole process occurred at slower rate (Figure 2H). Similarly, Naimi’s structures were observed in BUC monolayers, grown to confluence on culture-treated dishes.
Ultrastructural analyses of Naimi’s structures: BUC organization
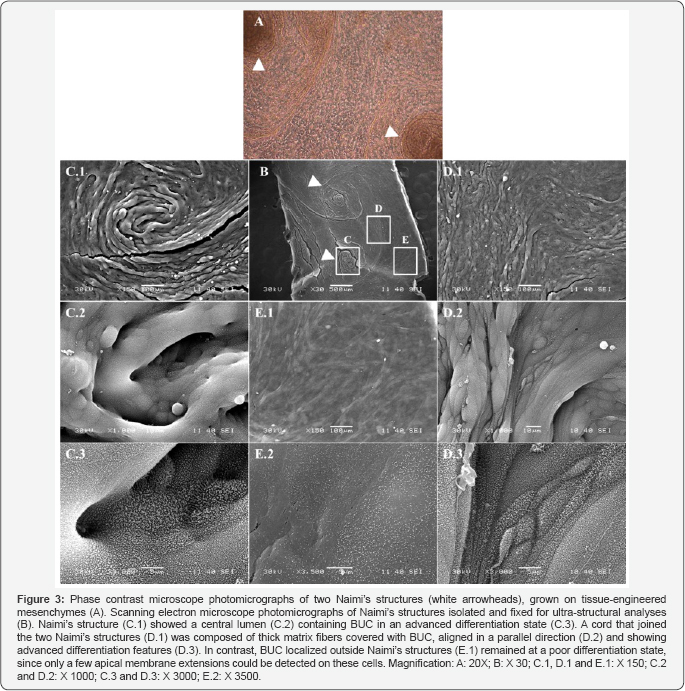
Naimi’s structures observed in our BMs (Figure 3A), were analyzed under electron microscope (Figure 3B). Scanning electron analyses of Naimi’s structures (Figure 3C.1), showed that the center of these spirals-like configurations included a lumen (Figure 3C.2), were well-circumscribed and differentiated, as confirmed by the advanced organization of BUC’s apical membranes (Figure 3C.3). Indeed, the apical surface of BUC was covered with abundant membrane extensions, which interactions between each other allow scalloped features to be established and present on the apical surface of mature urothelium. These membrane extensions are also exposed on BUC that form a cord that links the two Naimi’s structures (Figure 3D.1-3D.3). In contrast, BUC located outside Naimi’s structure show a weak remodeling of the apical (Figure 3E.1, & 3E.2).These observations strongly suggest that Naimiís structures are triggering urothelium maturation. Observations made on porcine native bladder are in agreement with this hypothysis.
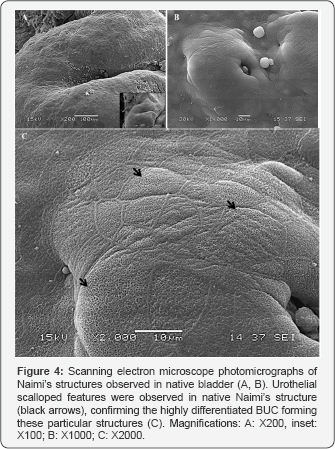
Interestingly, scanning the surface of native urothelium, observations confirmed the presence of more complex Naimi’s structures associated with high level of urothelial maturation in native bladder ex-vivo (Figure 4A & 4B). Native Naimi’s structures of diametrical sizes, ranging from about 20 up to several hundreds of mm, were all characterized by a lumen at their center and scalloped features specific to fully differentiated urothelium (Figure 4C). At the center of the Naimi’s structures analyzed in native bladder samples, concentric cords of BUC were organized in the same pattern observed in our BM (Figure 5A & 5B). The BUC surrounding the center of Naimi’s structures were aligned to form several parallel folds, each row being composed of a dense cell-matrix network (Figure 5C). In contrast with BUC located between Naimi’s structures, and in agreement to observations made in-vitro, cells adopted an elongated morphology in these rows (Figure 5D), showing well defined or in progress scalloped features.
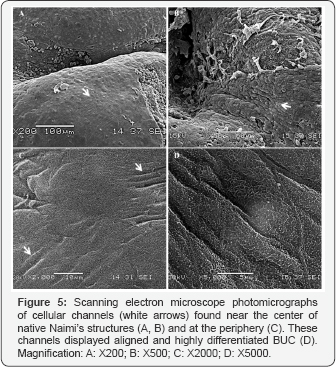
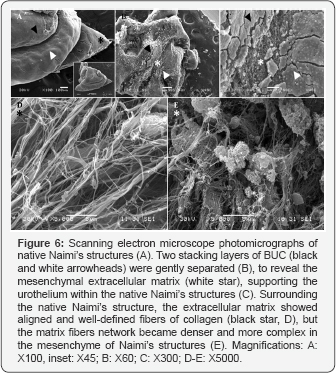
We observed different levels of maturity of Naimi’s structures in the native bladder tissues, like in our BM in vitro. To investigate cell-matrix interactions and matrix fibers orientation at the interface between two dense concentric folds within Naimi’s structure (Figure 6C), we gently separated them (Figure 6A & 6B). Longitudinal collagen fibers alignment was obvious (Figure 6D), at the superficial matrix layer located outside of the two superposed cell cords. However, the density of the extracellular matrix fibers network and complex cell-matrix arrangement could be observed inside each cord that composed Naimi’s structures (Figure 6E).
Ultra-structural analysis of Naimi’s structures: Lateral view
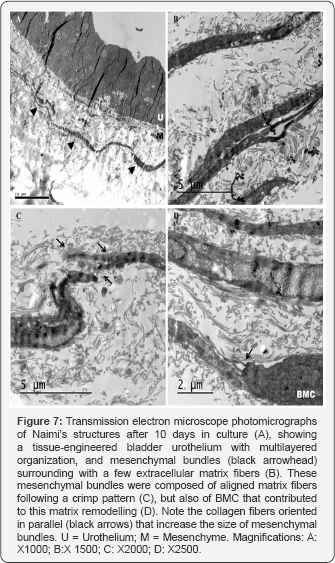
Transmission electron microscopy analyses of our BM’s urothelium confirmed that Naimi’s structures were covered with a multilayered-like urothelium, under the culture conditions established (Figure 7A), highly comparable to the urothelium of Naimi’s structures observed in native bladder samples. Interestingly, wide matrix bundles were present under the urothelium of Naimi’s structures (Figure 7B), and the space between these bundles was filled with collagen fibers of variable calibers (Figure 7B). At higher magnification, the bundles were too dense to detect the typical periodicity of collagen (67 nm) among their fibers, but collagen fibrils were observed all along the periphery of the bundles (Figure 7C), with BMC sparsely distributed nearby them (Figure 7D).
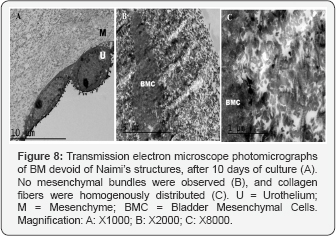
The bundles ran densely packed, parallel, and straight changing their direction only in periodic crimps where fibrils showed a local deformation. In areas of the urothelium divided of Naimi’s structures, matrix bundles were not observed (Figure 8A), but BMC were surrounded of collagen fibrils distributed randomly around the cells (Figure 8B), which did not show any specific alignment (Figure 8C).
Genesis of Naimi’s migration
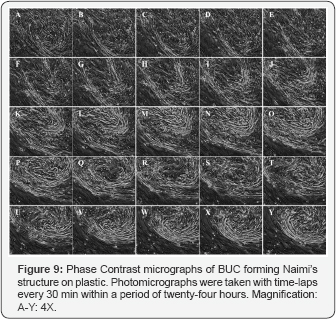
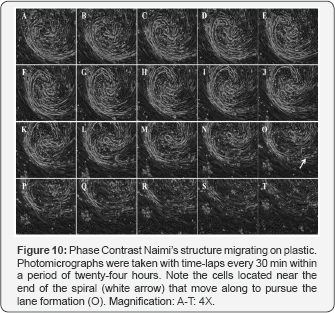
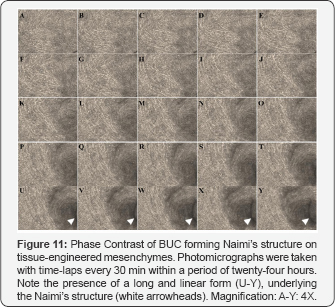
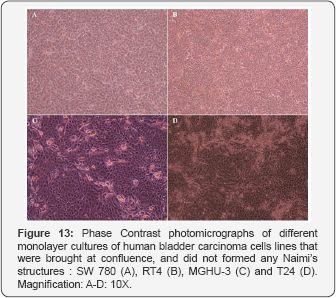
Such BUCs behavior and organization were observed on plastic (Figure 9 & 10), and on reconstructed mesenchymes (Figure 11 & 12). However, on the tissue-engineered mesenchymes, the three-dimensional structure of the matrix allowed visualization of the superposition of BUC at the center of the spiral of cells, and the presence of underlying mesenchymal bundles was previously described (Figure 11U-11Y). In both cases, independent rows of BUC, leading to respective Naimi’s structures, cross each other (Figure 9A-9J & Figure 11A-11E), before inducing the gyratory movement that progressively expand (Figure 9K-9Y & Figure 11F-11Y) and moved laterally (Figure 10 & 12), attracting surrounding cells to follow the flow induced by this major displacement. Interestingly, the cells located near the end of the spiral (Figure 10O & Figure 12P), often moved along to create lanes that joined another center of rotation. More importantly, the spiral organization of the cells was observed on native urothelia, but not on different bladder carcinoma cells lines (Figure 13A-13D), demonstrating that this pattern of cell migration wasn’t an artifact induced in-vitro.

Discussion
Science is based on observations. Trivialization of classical cell culture happens too often, being integrated in a daily routine and presenting several limitations that can’t be denied, when compared to complete native tissues. Living cells in culture have been the source of numerous discoveries associated with major advances in many fields of research. To our knowledge, Naimi’s structure and migration were never described and analyzed, despite such particular pattern of cell organization that can be observed repeatedly in confluent BUC monolayers grown on conventional plastic dishes.
Tissue-engineering still belongs to recent fields of research, as it offers multiple possibilities of culture conditions including many types of substrates combined to multiple threedimensional types of co-cultures [17]. Collagen gel facilitates direct observations of the cells seeded at its surface and within the fibers network. Naimi’s structure and migration of confluent BUC, grown on tissue-engineered mesenchymes, provided the opportunity to perform ultra-structural analyses between the cells and under the spiral made by the cells. Such in-depth observations could not be done with BUC seeded on plastic. Obviously, Naimi’s structure was certainly seen by several other research groups working with BUC in culture, but to our knowledge, none studied this process. Having worked with various types of human and animal cells, including hepatocytes, human skin (keratinocytes and dermal fibroblasts), bronchi (ciliated cells, goblet’s cells, mesenchymal cells, smooth muscle cells), connective tissue cells (ligament fibroblasts, osteoblasts, chondrocytes), blood cells and various immune cell types, and cancer cells of various epithelial and mesenchymal origins, none ever showed such pattern of coordinated migration [15,16,18,20]. These results strongly suggest that Naimi’s structure and migration are tissue-specific, and more precisely urothelia- specific. The typical spiral pattern, that seems to be essential for Naimi’s migration to occur, was observed on native bladder urothelia. This is an important indication that Naimi’s structure and migration represent a natural mode of cell movement that happens in-vivo. The purpose of this particular pattern of structure and migration remains unknown for now. However, pre-terminal (in-vitro) and terminal (in-vivo) differentiation of urothelium, including Naimi’s structure, suggest that it could be a mechanism for vertical urothelial development. The remarkable rearrangement of underlying BMC and collagen matrix into wide matrix bundles also indicates a potential mode of tissue-specific development. The researches to determine the implications of such epithelial and mesenchymal events are pursued. One of the triggering events that stimulate BUC to induce Naimi’s migration is the state of confluence, in accordance with the reported fact that the vertical development requires a complete bladder mesenchyme coverage by urothelial cells in-vivo [13]. We may postulate that tension between BUC, associated with cells high density, stimulates Naimi’s structure formation and migration within the confluent BUC layer. This cellular tension, in addition to a physiological environment supplied by the mesenchymalepithelial interactions, and urine components promoting rapid extension of Naimi’s structures and migration, allowed preterminal differentiation of our tissue-engineered urothelium in only 10 days in culture. The ultrastructural properties of the matrix bundles observed in the mesenchymal layer of Naimi’s structures may play an important role in the maintenance of the spiral-like tissue, especially during its displacement. In fact, such densely packed bundles, oriented in a parallel fashion and changing their direction only in periodic crimps (Figure 7), may be compared to ligament collagen matrix [6,21]. Biomechanical elongation and contraction happen in a cyclic manner in response to bladder wall physiological response to urine collection and excretion. Such cyclic strain demands a fast adaptation of the bladder wall to frequent variation in internal tension. Similarly, ligaments respond to cyclic elongation in-vivo, being subjected to strains induced by the movement of the bones within a joint. It is possible that Naimi’s structures contribute to bladder wall adaptation to the level of diametrical tension associated with urine volume variations. Moreover, the Naimi’s structures gyratory movement, observed under time lapse microscope, involves significant matrix modulation that attracts surrounding BUC, resulting in a wider spiral-like structure bordered by well- aligned cell rows. Work is in progress to assess the function of Naimi’s structure and migration and its modulation in response to external tension applied on tissue-engineered bladder constructs in-vitro.
Conclusion
Cellular alignment into a circular arrangement followed by coordinated gyratory cellular movement were sequentially observed in our in-vitro model and were also confirmed in native bladder. Ultrastructural observations showed the remarkable organization of urothelial cells and the underlying mesenchymal rearrangement. This phenomenon could be involved in the process of urothelium maturation.
References
- Kurosaka S, Kashina A (2008) Cell Biology of Embryonic Migration. Birth Defects Res C Embryo Today 84(2): 102-122.
- Huttenlocher A, Horwitz AR (2011) Integrins in Cell Migration. Cold Spring Harb Perspect Biol 3(9): a005074.
- Li X, Kolega J (2002) Effects of direct current electric fields on cell migration and acting filament distribution in bovine vascular endothelial cells. J Vasc Res 39(5): 391-404.
- Miyakoshi J (2006) The review of cellular effects of a static magnetic field Sci. Technol Adv Mat 7: 305-307.
- Huang D, Chang TR, Aggarwal A, Lee RC, Ehrlich HP (1993) Mechanisms and dynamics of mechanical strengthening in ligament- equivalent fibroblast-populated collagen matrices. Ann Biomed Eng 21(3): 289305.
- Goulet F, Rancourt D, Cloutier R, Germain L, Poole AR, et al. (2007) Tendons and ligaments. In: R. Lanza, R. Langer and J.Vacanti (Eds), Principles of Tissue Engineering (2nd edn), Academic Press Ltd, San Diego, USA, pp. 711-722.
- Apodaca G (2004) The uroepithelium: not just a passive barrier. Traffic 5(3): 117-128.
- Khandelwal P, Abraham SN, Apodaca G (2009) Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297(6): F1477-1501.
- Veranic P, Romih R, Jezernik K (2004) What determines differentiation of urothelial umbrella cells? Eur J Cell Biol 83: 27-34.
- Kreplak L, Wang H, Aebi U, Kong XP (2007) Atomic force microscopy of Mammalian urothelial surface. J Mol Biol 374(2): 365-373.
- Liang FX, Riedel I, Deng FM, Zhou G, Xu C, et al. (2001) Organization of uroplakin subunits: trans membrane topology, pair formation and plaque composition. Biochem J 355(Pt 1): 13-18.
- Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT (2009) Uroplakins in urothelial biology, function, and disease. Kidney Int 75(11): 11531165.
- Erman A, Vidmar G, Jezernik K (2004) Temporal and spatial dimensions of postnatal growth of the mouse urinary bladder urothelium. Histochem Cell Biol 121(1): 63-71.
- Magnan M, Berthod F, Champigny MF, Soucy F, Bolduc S (2006) In-vitro reconstruction of a tissue-engineered endothelialized bladder from a single porcine biopsy. J Pediatr Urol 2(4): 261-270.
- Paquette JS, Tremblay P, Bernier V, Tremblay N, Laviolette M, et al. (2004) A new cultured human bronchial equivalent that reproduce in-vitro several cellular events associated with asthma. Eur Cells Mat 7: 1-11.
- Auger FA, Lopez Valle CA, Guignard R, Tremblay N, Noel B, et al. (1995) Skin equivalents produced using human collagen. In Vitro Cell Dev Biol Anim 31(6): 432-439.
- Bouhout S, Rousseau A, Chabaud S, Morissette A, Bolduc S (2013) Potential of different tissue engineering strategies in the bladder reconstruction. Regenerative Medicine and Tissue Engineering (1st edn), Andrades, JA 23: 573-597.
- Paquette JS, Tremblay P, Bernier V, Auger FA, Laviolette M, et al. (2003) Production of tissue-engineered three-dimensional human bronchial models. In Vitro Cell Dev Biol Anim 39(6): 213-220.
- Wang CS, Goulet F, Auger FA, Tremblay N, Germain L, Tetu B (2001) Production of bioengineered cancer tissue constructs in- vitro: epithelium-mesenchyme heterotypic interactions. In Vitro Cell Dev Biol Anim 37(7): 434-439.
- Tremblay P, Cloutier R, Lamontagne J, Belzil AM, Larkin AM, et al. (2011) Potential of skin fibroblasts for application to anterior cruciate ligament tissue engineering. Cell Transplantation 20(4): 535-542.
- Franchi M, Raspanti M, Dell’Orbo C, Quaranta M, De Pasquale V, et al. (2008) Different crimp patterns in collagen fibrils relate to the sub fibrillar arrangement. Connect Tissue Res 49(2): 85-91.






























