Evaluation, Hallmark, Clinical Relevance and Role of Anti Citrullin Antibody, IgG and IgM Rheumatoid Factor with Serological Parameters of Disease Activity or Both as Overall Sifted Test in Undifferentiated Seronegative Arthropathy with or without Joint Inflammation and Bone Structure Differences. Boost, over Helling or Amplification to Classification Criteria to Rheumathoid Arthritis?
Dejan Spasovski*
Ss Cyryl and Methodius University of Skopje, Macedonia
Submission: November 11, 2016; Published: December 09, 2016
*Corresponding author: Dejan Spasovski, University Clinic of Rheumatology, Ss Cyryl and Methodius University, Skopje, Republic of Macedonia, Tel: +389023147-668; Email: drspovski@yahoo.co.uk
How to cite this article: Dejan S. Evaluation, hallmark, clinical relevance and role of anti citrullin antibody, IgG and IgM rheumatoid factor with serological parameters of disease activity or both as overall sifted test in undifferentiated seronegative arthropathy with or without joint inflammation and bone structure differences. Boost, over helling or amplification to classification criteria to rheumathoid arthritis?. JOJ uro & nephron. 2016; 1(2): 555557. DOI: 10.19080/JOJUN.2017.01.555557
Abstract
Aim: To compare the values and acuracy of the test in anticyclic citrullinated peptides (Anti-CCP /ACPA) antibodies, Rheumatoid factor (RF), C-Reactive Protein (CRP) and disease activity index (PASI) in early diagnosis of untreated Psoriatic arthritis(PsA).
Methods: Using the ELISA method of DIA-STATTM Anti CCP (Axis-Shield Diagnostics), sera of 70 participants were examined (35 untreated patients with PsA and 35 persons from the healthy control group). RF and CRP were determined with the agglutination test (Lateks test) in the same participants. In the same time we determined the sensitivity, specificity, predictive value for positive and negative test and accuracy.
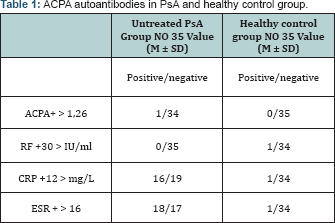
Results: From 35 pts with PsA, 1 patient showed presence of Anti-CCP antibodies (sensitivity test 2.86%), while RF was present in 0 pts (sensitivity test 0%). In the healthy control group positive values for RF, CRP and Erythroid Sedimentation Rate (ESR) were detected in 1 participant.
Conclussion: ACPA antibodies have low sensitivity, but high specificity in PsA.
Keywords: Anti cyclic citrullinated peptide (Anti-CCP); Psoriatic arthritis (PsA); Rheumatoid factor (RF)
Introduction
Anti-cyclic peptide antibodies (CCP/ACPA) are antibodies directed towards synthetic citrullinated peptides and are specific markers in diagnosis of Rheumatoid arthritis (RA). They belong to the group of protein/peptide antibodies. There are several generations of these antibodies in their evolution. Antibodies like APF (Anti-perinuclear factor) and AKA (Antikeratin antibodies) detected by indirect fluorescence using buccal epithelium or rat^s esophagus [1], have great specificity for RA.
Absence of donors for buccal cells limits the use of APF as a routine laboratory test. Antigen for these Anti-filaggrin antibodie (AFA) is identified as an epidermal filaggrin which is intermediary filament involved in the epidermal cornification [2-3]. Profilaggrin, present in keratohyalin granules of the buccal cells is proteolytically released in filaggrin subunits during cell differentiation. In this stadium, the protein is dephosphorylated and some arginine residues are converted in citrullines from enzyme peptidyl-arginine deaminase (PAD) [4]. Their reactivity depends on epitopes which contain amino acid citrulline. In 1998, Schellekers et al. reported about autoantibodies that reacted in linear synthetic peptifdes which contained unusual amino acid citrulline. So two types of CCP assay were developed with peptides A and B. They are present in 76% in RA, with 96% specificity. Antibodies in patients with RA are predominantly IgG type and have relatively high affinity [5]. The ELISA test, based on these cyclic citrullinated peptides (CCP) have superior characteristics in detection of RA [6], with different sensitivity and specificity [7]. Sensitivity of the ACPA test in different populations ranges between 64% and 74%, while specificity ranges between 90% and 99%. There are PsA patients in whom these antibodies are detected. The aim of this study is to determine the diagnostic value of ACPA antibodies in PsA.
Materials and Methods
In patients included in the study the disease diagnosis was based upon revised diagnostic criteria for classification of Psoriatic arthritis from 2005, proposed by the American Association of Rheumatism (ARA) [8]. Clinical evaluation of disease activity and disease diagnosis was performed by subspecialist in the field based upon the diagnostic criteria of Moll-Wright for classification of Psoriatic arthritis [9]. They were dermatologically tested, including examination of the psoriatic changes of the nails, psoriatic areas, disease activity index (PASI) and evaluation of the peripheral and axial joints [10] . Oligoarthritis is taken in consideration when < 5 joints are involved and polyarthritis when > 5 joints are involved. Symmetric arthritis is considered when there is bilateral involvement and when > 50% of joints are seized.
In the study were included 35 pts (18 women, 17 men) with PsA and 35 pts (19 women, 16 men) from the healthy control group. Mean age was 47.18 years (±9.08) (35-65 years) in the group with PsA, while 40.2 years (±9.21) (29-65 years) in the healthy control group. Mean disease duration was 6.27 months (±8.22) (1-36 months). None of them received disease modification drugs. The others nagated drug use before entering the study, especially drugs from the base line such as methotrexate, leflunomide or sulphasalasine. Specimen were collected in the period of 2 years.
Including criteria
In the study were incuded patients with Psoriatic arthritis aged 18-65, newly diagnosed and previously untreated.
Excluding criteria
From the study were excluded patients with diseases or conditions that could influence results directly or indirectly:
- Patient younger than 18 years.
- Patients with previous history of disease of the spleen,thyroid gland, liver, kidneys, hematological, cardiovascular,neurological, autoimmune and lung diseases.
- Patients with diabetes mellitus, febrile conditions, acute infections, neoplasms.
- Patients with uric arthritis, SLE, mixed connective tissue disese, vasculitis.
- Patients with history of blood transfusion and patients with body overweight.
- Patients with history of use of drugs from the base line.
- Patients that in 0 point had increased level of glucose, serum ind urine urea and creatinine, blood hypertension, smokers and blood and enzyme disorders.
- Patients previously treated with salycilates, antibiotics, golden salts or diuretics.
All the patients took part in this study voluntarily, so the ethic criteria for this study are fulfuled.
Laboratory evaluation
For clinical evaluation of the disease, the following parameters were necessary: complete blood count (CBC) and differential, reactants of the acute phase, ACPA antibodies, C-reactive protein (CRP), Rheumatoid factor (RF) and Erythrocyte sedimentation rate (ESR), alkaline phosphatase (AP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase (CK), lactate dehydrogenase (LDH), serum urea and serum creatinine. Serum creatinine was determined with the "Jaffe" method.
Referent values: serum creatinine 45-109 mmol/L; Urine creatinine 7-17mmol/dU.
CRP was determined with the agglutination test (Lateks CRP test) (Bio Systems S.A. Reagens & Instruments Costa Brava 30, Barcelona, Spain). Referent values: < 6 mg/L CRP in serum.
RF was determined with the agglutination test (Lateks CRP test) (Bio Systems S.A. Reagens & Instruments Costa Brava 30, Barcelona, Spain). Referent values: < 8 IU/ml RF in serum. Quantitative method used for for determination of ESR was Westergren method. Referent values: 7-8mm for women, 1116mm for men.
DIA-STATTM Anti CCP (Axis-Shield Diagnostics) test is semi quantitative/qualitative ELISA test, based upon the detection of IgG auto antibodies in human plasma or serum, directed towards synthetic cyclic citrullinated peptides (CCP), which contain modified arginine residues. This test was an additional tool.
Principles of work
The walls of the microtiter have highly purified synthetic cyclic peptide which contains modified arginine residues. During first incubation, the specific auto antibodies from diluted serum or plasma are connected to the surface antigen. It is washed than in order to eliminate the unconnected components. During the second incubation, the conjugate, which is an enzyme of the monoclonal autoantibody for human IgG, is connected to the surface autoantibody. After the second wash, the specific auto antibodies are incubated with the substrate. After adding the stop solution, the reaction is interrupted and this results with the colored end result. The amount of the absorbed conjugate is expressed in absorption units. In the quantitative protocol, the amount of the conjugate connected with the sample is compared with the same connected in the referent control. In the semiquantitative protocol the anti CCP autoantibody concentration could be estimated with interpolation of the curve based on the standard. Fresh serum or plasma is used. Calculation and interpretation of the results for the qualitative protocol are estimated from the absorbed value (optic density) from the positive and negative control as well as for every sample.
Statistical analysis
For testing the significance of differences between two arithmetical means, i.e. proportions the Student-t-test is used to compare the mean parameters of certain numerical parameters between groups, as well as Willcoxon-matched test for independent samples. Sensitivity and predictivity for positive and negative test of the examined markers is determined with the test for sensitivity and specificity. P-value between 0.05 and 0.1 is considered statistically significant. Analysis of the data is performed with the statistical package Statistical 7.0.
Results
From the 35 pts with PsA, 1 patient (2.86%) showed presence of ACPA antibodies, while RF was present in 0 pts (0%). In the healthy control group 0 pts (0%) showed ACPA positivity, while 1 patient (2.86%) had positive RF (Table 1).
Diagnostic value of ACPA autoantibodies in Psoriatic arthritis
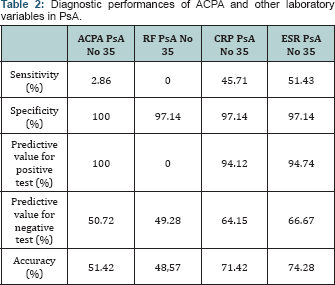
For ACPA autoantibodies and reactants of the acute phase in PsA, sensitivity, specificity, predictive value for positive and negative test and their acurasy are shown on Table 2. ACPA autoantibodies have equal diagnostic performances as RF (sensitivity 2.86% vs 0%, specificity 100% vs 97.14% in detection of untreated PsA.
- Using the Wilcoxon-matched test we found statistical correlation between ACPA in PsA and healthy control group for p<0.05 (p=0.01). In the PsA group there was statistical correlation between ACPA and RF for p< 0.05 (p=0.00); ACPA and CRP (p=0.00);
- Using the Wilcoxon-matched test we found no statistical correlation in the PsA group between ACPA and age, disease diuration in months, PASI index, RF and CRP for p< 0.05: (ACPA vs age p=0.04; ACPA vs disease diuration in months p=0.07; ACPA vs PASI p=0,08; ACPA vs RF p=0,02; AAP vs CRP p=0,05; AAP vs ESR p=0,06).
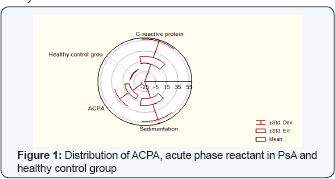
- Using the Wilcoxon-matched test we found statistical correlation in the PsA group between disease diuration in months, PASI index, RF and CRP for p<0.05: (CRP vs age p=0.00; CRP vs disease diuration in months p=0.00; CRP vs PASI p=0,00; CRP vs RF p=0,02; CRP vs ESR p=0,00) (Figure 1).
Discussion
While introducing some new diagnostic method it is necessary to estimate its quality i.e. to find out the utility of information compared to the risk for the patient and the price of the test. This became more interesting recently when due to the development in technology a lot of methods were introduced.Although the subjective estimation of the doctor who is responsible for the patient would be crucial in the choice of the available diagnostic methods, objective quantitative estimation of every method would help him in the most rational approach. The contribution of every method quantitatively expressed would be able to classify the methods according to their efficacy.
ACPA autoantibodies are specific markers in diagnose of RA and have role in the disease pathophysiology. Psoriasis vulgaris is found in 3% of the common population. PsA in Psoriasis vulgaris is found in 7%. ACPA autoantibodies in PsA have greater prevalence of 8% in comparison with the common population [11] . Isolation of ACPA autoantibodies could be from synovial fluid also [12]. Unlike RA, the presence of ACPA autoantibodies in PsA could be explained only as an epifenomenon in this disease [13]. It is noted in the literature that these antibodies have significance in PsA for the evaluation of osteoporosis, erosive changes and bone destructiones - fractures. Data obtained for ACPA autoantibodies are not higher than previously tested kits from other researches [14-19]. The ACPA autoantibody as an isolated laboratory variable does not dominate with its performances in diagnose of early, undiferentiated PsA. However, it should be mentioned that obtained values in this study are equal and do not digress from the values obtained from the producer DIA-STATTM Anti CCP (Axis-Shield Diagnostics) for PsA (sensitivity for anti ACPA 5%, specificity 100%).
Conclusion
ACPA autoantibodies have low sensitivity and high specificity in diagnose of PsA. Every positive result should be interpreted together with the clinical evaluation of the disease and diagnoastic procedures designated for it. It should be mentioned that elevatied values of these antibodies could appear in persons without clinical signs of disease. The concentration of these antibodies not always correlate with the disease severity. They could be applied in pediatric cases with great caution, because they are not designated for them.
References
- Nakamura RM (2000) Progress in the use of biochemical and biological markers for evaluation of rheumatoid arthri-tis. J Clin Lab Anal 14(6): 305-313.
- Simon M, Girbal E, Sebbag M, Gomes-Daudrix V, Vin-cent C, et al. (1993) The cytokeratin filament-aggre- gating protein filaggrin is the target of the so-called "antikeratin antibodies", autoantibodies specific for rheumatoid arthritis. J Clin Invest 92(3): 1387-1393.
- Sebbag M, Simon M, Vincent C, Masson-Bessiere C, Girbal E, et al. (1995) The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies.J Clin Invest 95(6): 2672-2679.
- Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, et al.(1999) The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin auto- antibodies are posttranslationally generated on various sites of (pro) filaggrin by deimination of arginine re- sidues. J Immunol 162(1): 585-594.
- Schellekens GA, Jong BAD, Hoogen FHVD, Putte LBVD, Venrooij WJV (1998) Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 101: 273-281.
- Schellekens GA, Visser H, de Jong BA, Hoo-gen FHVD, Hazes JM, et al.(2000) The diagnostic pro- perties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 43: 155163.
- Van Boekel MAM, Vossenaar ER, van den Hoogen FHJ, van Venrooij WJ (2002) Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res 4(2): 87-93.
- Helliwell PS, Taylor WJ (2005) Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis 64(Suppl 2): ii3-ii8.
- Moll JMH, Wright V (1973) Psoriatic arthritis. Semin Arthritis Rheum 3(1): 55-78.
- Schmitt J, Wozel G (2005) The psoriasis area and severity index is the adequate criteria to define severity in chronic plaque-type psoriasis. Dermatol 210(3): 194-199.
- Cruyssen BV, Hoffman IE, Zmierczak H, Berghe MVD, Kruithof E, et al. (2005) Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis 64(8): 1145-1149.
- Spadaro A, Riccieri V, Scrivo R, Alessandri C, Valesini G (2007) Anti- cyclic citrullinated peptide antibody determination in synovial fluid of psoriatic arthritis. Clin Exp Rheumatol 25(4): 599-604.
- Huynh D, Etzel C, Cox V, Kremer J, Greenberg J, et al. (2013) SAT0268 Anti Citrullinated Peptide Antibody (ACPA) in Patients with Psoriatic Arthritis (PSA): Clinical Relevance. Ann Rheum Dis 72: A673.
- Palazzi C, Olivieri I, Petricca A, Salvarani C (2002) Rheumatoid arthritis or psoriatic symmetric polyarthritis? A difficult differential diagnosis. Clin Exp Rheumatol 20(1): 3-4.
- Helliwell PS (2004) Relationship of psoriatic arthritis with the other spondyloarthropathies. Curr Opin Rheumatol 16(4): 344-349.
- Alenius GM, Berglin E, Dahlqvist SR (2006) Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann Rheum Dis 65(3): 398-400.
- Korendowych E, Owen P, Ravindran J, Carmichael C, McHugh N (2005) The clinical and genetic associations of anti-cyclic citrullinated peptide antibodies in psoriatic arthritis. Rheumatology (Oxford) 44(8): 10561060.
- Inanc N, Dalkilic E, Kamali S, Kasapoglu-Gunal E, Elbir Y, et al. (2007) Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol 26(1): 17-23.
- Abdel Fattah NSA, Hassan HE, Galal ZA, Okda ESEE (2008) Antibodies to cyclic citrullinated peptides in patients with psoriatic arthritis. Egypt J Dermatol Venereol 28: 13-23.






























